“Normal” Creatinine Levels Predict Persistent Kidney Injury and Waitlist Mortality in Outpatients With Cirrhosis
Supported by the Paul B. Beeson Career Development Award in Aging Research (K23AG048337 to J.C.L.) and by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK026743, to the University of California San Francisco Liver Center; T32 DK060414, to G.C.), neither of which played a role in the analysis of the data or the preparation of the manuscript.
Abstract
Acute kidney injury (AKI) is a critical determinant of outcomes in hospitalized patients with cirrhosis, but little is known of the impact of AKI in the outpatient setting. We analyzed 385 adult outpatients with cirrhosis listed for liver transplant at a single center; excluded were those with severe hepatic encephalopathy, with hepatocellular carcinoma, or on hemodialysis. Baseline serum creatinine (bCr) was defined as the lowest value recorded, peak Cr as the highest value, ΔCr as peak Cr minus bCr, AKI as a rise in serum Cr (sCr) by ≥0.3 mg/dL from bCr, persistent kidney injury as elevation of sCR by ≥0.3 mg/dL from bCr on each subsequent clinical assessment. Among 385 outpatients with cirrhosis, bCr was ≤0.70, 0.70-0.97, and ≥0.97 mg/dL in 28%, 38%, and 34%, respectively. At a median follow-up of 16 (range 8-28) months, 143 (37%) had one or more AKI episode, which increased significantly by bCr group (24% versus 37% versus 48%, P = 0.001). Of these 143 with AKI, 13% developed persistent kidney injury. A multivariable Cox regression analysis highlighted that bCr (hazard ratio [HR], 2.96) and ΔCr (HR, 2.05) were the only factors independently associated with the development of persistent kidney injury (P < 0.001). The likelihood of death/delisting increased by bCr group (14% versus 19% versus 28%, P = 0.03). A competing risk analysis demonstrated that each 1 mg/dL increase in bCr was independently associated with a 62% higher risk of death/delisting when accounting for transplantation and adjusting for confounders. Conclusion: AKI is not only common in outpatients with cirrhosis but even “clinically normal” bCr levels significantly impact the risk of persistent kidney injury and waitlist mortality, supporting the need for a lower clinical threshold to initiate monitoring of renal function and implementation of kidney-protective strategies.
Abbreviations
-
- AKI
-
- acute kidney injury
-
- bCr
-
- baseline serum creatinine
-
- CKD-EPI
-
- Chronic Kidney Disease Epidemiology Collaboration
-
- eGFR
-
- estimated glomerular filtration rate
-
- HR
-
- hazard ratio
-
- MDRD
-
- Modification of Diet in Renal Disease Study
-
- MELD
-
- Model for End-Stage Liver Disease
-
- MELD-Na
-
- MELD with serum sodium
-
- NASH
-
- nonalcoholic steatohepatitis
-
- sCr
-
- serum creatinine
-
- SHR
-
- sub-hazard ratio
-
- UCSF
-
- University of California San Francisco
Renal dysfunction is a critical determinant of outcomes in patients with cirrhosis.1, 2 It results from a broad spectrum of pathologies including functional etiologies (transient injury from alterations in perfusion) and structural causes (irreversible damage to the renal parenchyma).3 Regardless of the cause, the development of acute kidney injury (AKI) has a profound impact on survival.4-7 This is particularly true in those with AKI who then progress to persistent kidney injury, where the 30-day mortality rate is nearly 10-fold higher.4, 5, 8 These findings highlight the importance of identifying the predictors of AKI as it is a potentially preventable and reversible condition.9-11
To date, most studies evaluating AKI in patients with cirrhosis have included only those who are hospitalized.5, 12-16 While AKI is a critical determinant of short-term mortality in the inpatient setting, this setting limits the opportunity to implement strategies to prevent progression to persistent kidney injury. The one study that has focused on AKI in nonhospitalized patients with cirrhosis reported an association of AKI and mortality, but this was an observational study with only 90 patients and was underpowered to identify predictors of the development and progression of AKI.17
To address this knowledge gap, we used a well-characterized prospective cohort of outpatients with cirrhosis awaiting liver transplantation to describe the prevalence of AKI, predictors of its persistence, and its impact on survival.
Patients and Methods
PATIENTS
We analyzed data from patients listed for liver transplantation at the University of California San Francisco (UCSF) from March 2012 until December 2016. These patients were enrolled as part of a prospective study of adults (≥18 years) with cirrhosis who were seen as outpatients in the UCSF Liver Transplant Clinics.18, 19 Patients with severe hepatic encephalopathy, as defined by the time to complete a numbers connection test20 of >120 seconds, were excluded because this may impair the patient's ability to provide informed consent. For the purposes of this specific study, patients with hepatocellular carcinoma were excluded as these patients receive transplant at a rate that is not dependent upon their laboratory Model for End-Stage Liver Disease (MELD) score. We excluded patients on hemodialysis as serum creatinine (sCr) does not reflect renal function in this setting.
COMORBIDITIES AND MEDICATIONS
We defined refractory ascites as fluid overload that either was unresponsive to a sodium-restricted diet and high-dose diuretic treatment or recurred rapidly after therapeutic paracentesis and developed anytime during follow-up.21 At the time of study enrollment, medical comorbidities (e.g., hypertension and diabetes) were determined from the patient's electronic health record, while hepatic encephalopathy was classified as none/mild versus moderate based on the patient's numbers connection test score of <60 or >60 seconds, respectively.22 Doses of loop and potassium-sparing diuretics were determined at the time of study enrollment. For the analysis, all doses of either the loop or potassium-sparing diuretic were converted to equivalent doses of furosemide and spironolactone, respectively.
DEFINITION OF Cr VALUES, AKI, AND PERSISTENT KIDNEY INJURY
- Peak Cr (pCr): the highest Cr measured during follow-up
- ΔCr: pCr minus bCr
- AKI: a rise in sCr by ≥0.3 mg/dL or >50% from baseline
- Persistent kidney injury: elevation of sCR by ≥0.3 mg/dL from baseline on each subsequent clinical assessment
- Transient AKI: return of sCr to within 0.3 mg/dL from baseline at any point during follow-up
We defined AKI as any qualifying change in sCr, regardless of time. Episodes of AKI were categorized by International Club of Ascites AKI stage.15 There are no clear consensus guidelines on the optimal estimated glomerular filtration rates (eGFR) in patients with cirrhosis. Given that blood urea nitrogen levels—which are necessary for the Modification of Diet in Renal Disease Study (MDRD)-6 estimation—are not included in the MELD with serum sodium (MELD-Na) score and therefore are not routinely drawn on all patients with every blood draw (only 31% had baseline blood urea nitrogen levels in our cohort), we calculated eGFR using both the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) and MDRD-4 formulas.24, 25
STATISTICAL ANALYSIS
Categorical data are presented as percentages and were compared between groups by the chi-squared test. Continuous variables are presented as medians with respective interquartile ranges and were compared between groups by the Wilcoxon rank-sum or Kruskal-Wallis test, as appropriate.
The primary outcome was persistent kidney injury. The secondary outcome was waitlist mortality, defined as death on the waitlist or delisting for sickness. Unadjusted models assessed the association of all listed covariates with persistent kidney injury and waitlist mortality. All covariates with a P < 0.2 in univariate analysis were considered for inclusion in multivariate models. Those not reaching significance of P < 0.05 were sequentially eliminated (backward selection). Cox regression assessed the risk of AKI occurrence and AKI persistence. Survival was compared between bCr groups using the log-rank test. A competing risks analysis assessed the association between kidney injury and other covariates with waitlist mortality, with liver transplantation as the competing risk.26 We censored patients who were removed from the waitlist for “social” reasons on the date of their removal because these patients do not have the same risk for waitlist mortality or liver transplantation. We censored those who remained alive on the waitlist at the end of follow-up because they remained at risk for transplant or waitlist mortality. We completed all analyses with both sCR and eGFR to ensure that there were no differences in the findings.
Two-sided p values < 0.05 were considered statistically significant. Analyses were performed using Stata 15.0 statistical software (College Station, TX). This study was approved by the institutional review board at UCSF.
Results
BASELINE CHARACTERISTICS OF THE ENTIRE COHORT
A total of 385 patients with cirrhosis were followed for a median of 1.3 (range 0.67-2.3) years, with a median of 3 (range 2-4) clinical assessments. Baseline characteristics are shown in Table 1. Women comprised 42% of the cohort, 63% were Caucasian, and 40% were listed with cirrhosis secondary to chronic hepatitis C virus. Median age was 58 (range 50-63) years. Rates of diabetes and hypertension were 24% and 34%, respectively. Eighteen percent had refractory ascites, and 18% had any hepatic encephalopathy. Median MELD-Na score was 18 (range 15-22), and median Child-Pugh score was 8 (range 7-9). Median bCr for the entire cohort was 0.80 (range 0.68-1.04) mg/dL, occurring at the first clinical assessment in 167 (43%) patients.
| Total | <0.7 mg/dL | bCr 0.70-0.97 mg/dL | >0.97 mg/dL | P | |
|---|---|---|---|---|---|
| n (%) | 385 | 108 (28) | 148 (38) | 129 (34) | — |
| Age (years) | 58 (50-63) | 54 (47-60) | 59 (52-63) | 60 (53-64) | <0.001 |
| Female (%) | 160 (42) | 57 (53) | 58 (39) | 45 (35) | 0.02 |
| Race (%) | |||||
| Caucasian | 242 (63) | 54 (50) | 110 (74) | 78 (60) | <0.001 |
| African American | 13 (3) | 0 (0) | 7 (5) | 5 (4) | 0.08 |
| Hispanic | 94 (24) | 40 (37) | 21 (14) | 33 (26) | <0.001 |
| Asian | 13 (3) | 3 (3) | 6 (4) | 4 (3) | 0.84 |
| Others | 24 (6) | 11 (10) | 4 (3) | 9 (7) | 0.05 |
| Etiology (%) | |||||
| HCV | 153 (40) | 42 (39) | 61 (41) | 50 (39) | 0.90 |
| Alcoholic cirrhosis | 122 (32) | 23 (21) | 46 (31) | 53 (41) | 0.01 |
| NASH | 49 (13) | 9 (8) | 18 (12) | 22 (17) | 0.13 |
| Autoimmunea | 63 (16) | 24 (22) | 24 (16) | 15 (12) | 0.09 |
| HBV | 8 (2) | 3 (3) | 2 (1) | 3 (2) | 0.71 |
| Other | 40 (10) | 19 (18) | 16 (11) | 5 (4) | 0.003 |
| Diabetes mellitus (%) | 94 (24) | 19 (18) | 38 (26) | 37 (29) | 0.13 |
| Hypertension (%) | 132 (34) | 28 (26) | 43 (29) | 61 (47) | 0.001 |
| BMI (kg/m2) (IQR) | 28.3 (25.1-33.1) | 27.5 (25.1-31.8) | 27.9 (25.2-32.8) | 28.8 (25.0-35.2) | 0.35 |
| Refractory ascites (%) | 69 (18) | 11 (10) | 24 (16) | 34 (26) | 0.005 |
| Any hepatic encephalopathy (%) | 70 (18) | 19 (18) | 27 (18) | 24 (18) | 0.99 |
| Serum Na (mmol/L) (IQR) | 136 (134-139) | 136 (134-139) | 136 (133-138) | 136 (134-140) | 0.74 |
| Albumin (mg/dL) (IQR) | 3.1 (2.6-3.5) | 3.00 (2.70-3.45) | 3.10 (2.50-3.50) | 3.20 (2.65-3.60) | 0.37 |
| bCr (mg/dL) (IQR) | 0.80 (0.68-1.04) | 0.60 (0.51-0.63) | 0.8 (0.75-0.88) | 1.19 (1.04-1.30) | <0.001 |
| MDRD-4 variable eGFR (mL/min/1.73 m2) | 75.5 (55.2-98.8) | 109.1 (91.2- 134.1) | 78.9 (66.5-91.6) | 51.2 (38.0-60.6) | <0.001 |
| CKD-EPI eGFR (mL/min/1.73 m2) | 81.1 (58.3-99.0) | 104.6 (96.4-112.8) | 85.5 (71.6-97.1) | 55.0 (41.0-63.5) | <0.001 |
| INR (IQR) | 1.4 (1.2-1.6) | 1.4 (1.2-1.6) | 1.4 (1.3-1.7) | 1.4 (1.2-1.6) | 0.31 |
| Equivalent dose of furosemide (mg) (IQR) | 20 (0-40) | 20 (0-40) | 20 (0-60) | 40 (0-40) | 0.049 |
| Equivalent dose of spironolactone (mg) (IQR) | 50 (0-100) | 25 (0-75) | 50 (0-100) | 50 (0-100) | 0.10 |
| Total bilirubin (mg/dL) (IQR) | 2.4 (1.7-3.9) | 2.8 (2.0-3.8) | 2.6 (1.8-4.5) | 2.0 (1.3-3.4) | <0.001 |
| Albumin (g/dL) (IQR) | 3.1 (2.6-3.6) | 3.0 (2.7-3.5) | 3.1 (2.5-3.5) | 3.2 (2.7-3.6) | 0.37 |
| Child-Pugh score (IQR) | 8 (7-9) | 8 (7-9) | 8 (7-9) | 8 (7-9) | 0.57 |
| MELD-Na (IQR) | 18 (15-22) | 17 (15-21) | 19 (14-21) | 19 (16-23) | 0.009 |
- a Combined autoimmune hepatitis, primary sclerosing cholangitis, and primary biliary cholangitis.
- Abbreviations: BMI, body mass index; HCV, hepatitis C virus; HBV, hepatitis B virus; INR, international normalized ratio; IQR, interquartile range.
COMPARISON OF BASELINE CHARACTERISTICS BY Cr STATUS
Categorizing patients by their bCr, 108 (28%) patients had a bCr of ≤0.70 mg/dL, 148 (38%) patients had a bCr of 0.70-0.97 mg/dL, and 129 (34%) patients had a bCr of ≥0.97 mg/dL. Demographics, clinical parameters, and laboratory findings for each of these three groups are listed in Table 1. Compared to the higher bCr groups, patients in the lowest bCr group were younger (54 versus 59 versus 60 years, P < 0.001) and more likely to be female (53 versus 39 versus 35%, P = 0.02) or Hispanic (37 versus 14 versus 26%, P < 0.001). Those in the lowest bCr group were less likely to have alcoholic liver disease (21 versus 31 versus 41%, P = 0.01), hypertension (26 versus 29 versus 47%, P = 0.001), and refractory ascites (10 versus 16 versus 26%, P < 0.001) and to be on any diuretics (67 versus 82 versus 85%, P = 0.003). Those in the lowest bCr group had higher total bilirubin levels (2.8 versus 2.6 versus 2.0 mg/dL, P < 0.001) but lower MELD-Na scores (17 versus 19 versus 19, P = 0.009). There were no significant differences in percentage with any hepatic encephalopathy (18 versus 18 versus 18%), albumin levels (3.0 versus 3.1 versus 3.2 g/dL), or Child-Pugh score (8 versus 8 versus 8) (P > 0.05 for each).
INCIDENCE OF AND RISK FACTORS FOR AKI
Using the International Club of Ascites staging criteria of AKI,15 143 (37%) patients had at least one episode of AKI while on the liver transplantation waitlist. An episode of AKI was identified in all bCr groups, increasing significantly from the lowest to the highest bCr group (24 versus 37 versus 48%, P = 0.001). Of these episodes of AKI, 117 (82%) were defined as AKI stage 1, 18 (13%) were AKI stage 2, and 8 (6%) were AKI stage 3. The median ΔCr during these episodes of AKI was 0.48 (0.38-0.76) mg/dL.
In univariable analysis, the factors associated with the development of AKI were refractory ascites (hazard ratio [HR], 1.95; P = 0.001), Child-Pugh score (HR, 1.14 per point; P = 0.009), MELD-Na (HR, 1.08 per point; P < 0.001), equivalent dose of furosemide at study enrollment (HR, 1.20 per 25 equivalent mg; P < 0.001), equivalent dose of spironolactone at study enrollment (HR, 1.10 per 25 equivalent mg; P = 0.01), and bCr (HR, 1.63 per 1 mg/dL; P < 0.001). Relative to the group with the lowest bCr, the middle and higher categories of bCr had a higher risk of AKI (HR, 2.32 for bCr 0.70-0.97 mg/dL; P < 0.001; HR, 2.93 for bCr >0.97 mg/dL; P < 0.001). The final multivariable model for the development of AKI included bCr (HR, 1.70 per 1 mg/dL; P < 0.001), equivalent dose of furosemide (HR, 1.21 per 25 mg; P < 0.001), and presence of refractory ascites (HR, 1.91; P = 0.001).
PREVALENCE OF AND RISK FACTORS FOR PERSISTENT KIDNEY INJURY
Of the 143 subjects with acute kidney injury in our cohort, 18 (13%) developed persistent kidney injury. Persistent kidney injury was identified in all bCr groups but occurred predominantly in the highest bCr group (4% versus 4% versus 24%, P = 0.001). Those with AKI who developed persistent kidney injury compared to those with AKI who did not develop persistent kidney injury had significantly higher ΔCr (1.09 versus 0.45 mg/dL, P < 0.001). The factors associated with persistent kidney injury in univariable analysis were etiology of nonalcoholic steatohepatitis (NASH; HR, 4.07; P = 0.008), diabetes mellitus (HR, 3.03; P = 0.02), bCr (HR, 3.00 per 1 mg/dL; P < 0.001), and ΔCr (HR, 2.11 per 1 mg/dL; P < 0.001). The final multivariable model included bCr (HR, 2.96 per 1 mg/dL; P < 0.001) and ΔCr (HR, 2.05 per 1 mg/dL; P < 0.001). The complete univariable and multivariable analyses for development of persistent kidney injury are demonstrated in Table 2. A survival analysis was completed looking at the primary outcome of persistent AKI (Fig. 1). This demonstrates that the likelihood of persistent kidney injury increases from the lowest to the highest bCr group (P < 0.001).
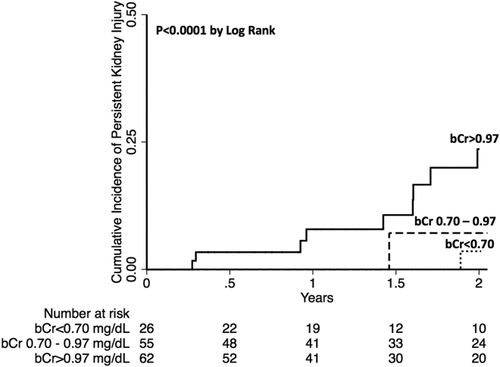
Cumulative incidence curve for persistent kidney injury.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age per year | 1.02 | 0.96-1.09 | 0.46 | |||
| Female sex | 1.25 | 0.48-3.25 | 0.65 | |||
| Autoimmunea | 0.35 | 0.05-2.67 | 0.31 | |||
| NASH | 4.07 | 1.44-11.47 | 0.008 | |||
| BMI per 1 kg/m2 | 1.05 | 0.97-1.13 | 0.24 | |||
| Refractory ascites | 2.20 | 0.80-5.99 | 0.13 | |||
| Any hepatic encephalopathy | 1.89 | 0.67-5.35 | 0.23 | |||
| Hypertension | 2.12 | 0.84-5.39 | 0.11 | |||
| Diabetes mellitus | 3.03 | 1.19-7.71 | 0.02 | |||
| Albumin per 1 mg/dL | 0.95 | 0.69-1.31 | 0.74 | |||
| bCr per 1 mg/dL | 3.00 | 1.75-5.13 | <0.001 | 2.96 | 1.62-5.44 | <0.001 |
| ΔCr per 1 mg/dL | 2.11 | 1.67-2.68 | <0.001 | 2.05 | 1.61-2.62 | <0.001 |
| bCr group | 5.61 | 2.16-14.57 | <0.001 | |||
| MDRD-4 eGFR per 1 mL/min/1.73 m2 | 0.96 | 0.94-0.99 | 0.001 | |||
| CKD-EPI eGFR per 1 mL/min/1.73 m2 | 0.95 | 0.93-0.97 | <0.001 | |||
| MELD-Na per 1 point | 1.14 | 1.06-1.24 | 0.001 | |||
| Child-Pugh score | 1.25 | 0.97-1.61 | 0.09 | |||
| Serum Na per 1 mmol/L | 0.96 | 0.85-1.09 | 0.54 | |||
| Total bilirubin per 1 mg/dL | 1.04 | 0.88-1.23 | 0.62 | |||
| INR per 1 unit | 0.86 | 0.23-3.19 | 0.82 | |||
| Equivalent dose of furosemide per 25 mg | 0.89 | 0.64-1.26 | 0.52 | |||
| Equivalent dose of spironolactone per 25 mg | 1.07 | 0.86-1.34 | 0.55 | |||
- a Combined autoimmune hepatitis, primary sclerosing cholangitis, and primary biliary cholangitis.
- Abbreviations: BMI, body mass index; CI, confidence interval; INR, international normalized ratio.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| SHR | 95% CI | P | SHR | 95% CI | P | |
| Age per year | 1.03 | 1.00-1.06 | 0.03 | |||
| Female sex | 0.96 | 0.62-1.51 | 0.87 | |||
| Autoimmunea | 1.16 | 0.67-2.02 | 0.60 | |||
| NASH | 2.27 | 1.34-3.84 | 0.002 | 2.24 | 1.33-3.78 | 0.003 |
| Refractory ascites | 1.30 | 0.75-2.24 | 0.35 | |||
| Any hepatic encephalopathy | 1.55 | 0.94-2.57 | 0.09 | |||
| Hypertension | 1.02 | 0.65-1.61 | 0.93 | |||
| Diabetes mellitus | 1.18 | 0.74-1.90 | 0.48 | |||
| bCr per 1 mg/dL | 1.60 | 1.17-2.20 | 0.004 | 1.62 | 1.17-2.25 | 0.004 |
| ΔCr per 1 mg/dL | 1.41 | 1.21-1.64 | <0.001 | |||
| Persistent AKI | 1.72 | 0.84-3.50 | 0.14 | |||
| MELD-Na per 1 point | 1.05 | 1.01-1.09 | 0.01 | |||
| Child-Pugh score | 1.16 | 1.03-1.31 | 0.01 | |||
- a Combined autoimmune hepatitis, primary sclerosing cholangitis, and primary biliary cholangitis.
- Abbreviation: CI, confidence interval.
WAITLIST MORTALITY AND AKI
By the end of follow-up, 178 (46%) were still active on the waitlist or removed from the waitlist for social reasons, 79 (21%) either died on the waitlist or were delisted for sickness, and 127 (33%) received a liver transplant. Those in the highest bCr group experienced significantly increased waitlist mortality (14% versus 19% versus 28%, P = 0.03). There was no difference in mortality in those patients with and without an episode of AKI (20% versus 22%, P = 0.64). There was no difference in transplant rates between the three bCr groups (28% versus 35% versus 35%, P = 0.43). Of those who received a liver transplant, the rates of simultaneous liver–kidney transplant were greatest in the highest bCr group (4% versus 0% versus 22%, P = 0.002).
A competing risks analysis was completed to determine if there was an association between baseline renal function and episodes of AKI with waitlist mortality. In univariable analyses, the following were significantly associated with waitlist mortality: etiology of NASH (sub-hazard ratio [SHR], 2.27; P = 0.002), bCr (SHR, 1.60 per 1 mg/dL; P = 0.004), age per year (SHR, 1.03; P = 0.03), ΔCr per 1 mg/dL (SHR, 1.41; P < 0.001), Child-Pugh Score per 1 point (SHR, 1.16; P = 0.01), and MELD-Na per 1 point (SHR, 1.05; P = 0.01). In the final multivariable model, only an etiology of NASH (SHR, 2.24; P = 0.003) or bCr (SHR, 1.62 per 1 mg/dL; P = 0.004) was significantly associated with waitlist mortality.
Discussion
In this study evaluating the prevalence and impact of AKI in outpatients with cirrhosis listed for liver transplantation, we aimed to identify predictors of AKI, determinants of persistent kidney injury, and the impact of baseline renal function on waitlist survival. Confirming prior reports,12, 27 we observed that higher bCr, refractory ascites, and dose of diuretics were associated with a higher risk of developing AKI. We further identified that this risk begins at lower bCr levels than previously appreciated—a 232% increased risk for those with a bCr of 0.70-0.97 mg/dL and a 293% increased risk for those with a bCr of >0.97 mg/dL. Furthermore, we report two determinants of persistent kidney injury—degree of renal injury (ΔCr) and baseline renal function (bCr). Lastly, we demonstrate that bCr levels >0.7 mg/dL, a threshold lower than what is currently used in the MELD score (>1 mg/dL), have a significant impact on waitlist mortality.
What might explain these findings? Patients with cirrhosis have clear reasons for a lower sCr. First, they have decreased hepatic synthesis of creatine, the precursor of creatinine.28 Second, they have increased renal tubular secretion of creatinine.29 Third, they are known to have decreased muscle mass.28, 30 We propose that these variations in biology lead to lower sCr levels. This provides the basis of our findings that relatively low levels and subtle fluctuations in sCr have a significant impact on not only the development of persistent kidney injury but also waitlist mortality.
We acknowledge the following limitations to this study. First, the timing of laboratory assessments differed among patients, so it is possible that patients experienced episodes of AKI (that resolved spontaneously in between blood draws) that were not detected in our analyses. However, given that our laboratory assessments were collected as part of routine clinical care, our analyses reflect the information that would be available to a provider in clinical practice for real-life decision-making, making our results more generalizable to the outpatient practice setting. Second, the outpatient design of this study limited the information that could be collected regarding the etiology (e.g., infection, hypovolemia) of these episodes of AKI, the phenotype of these episodes of AKI (e.g., hepatorenal syndrome; prerenal, intrinsic acute kidney injury), and how these episodes were managed (e.g., vasopressors, diuretic withdrawal). Third, we used sCr as the marker for renal function, which may be inaccurate in patients with cirrhosis1, 31; quantification of eGFR may serve as a more accurate assessment of renal function. To test for this possibility, we performed sensitivity analyses of our regression models using eGFR (calculated with CKD-EPI and MDRD-4), but neither changed the qualitative interpretation of our analyses as bCr and degree of change of renal function remained the strongest predictors of persistent kidney injury in these adjusted models.
Despite these limitations, our study describes renal outcomes in patients with cirrhosis listed for liver transplantation in the outpatient setting. Our finding that risk for persistent kidney injury is elevated even in outpatients with cirrhosis with bCr levels as low as 0.70 mg/dL—what would be considered “normal” based on laboratory reference ranges—has important implications for clinical practice. Specifically, our analyses identified patients listed for liver transplantation who should have closer monitoring of renal function or implementation of kidney-protective strategies (i.e., diuretic adjustment, earlier fluid resuscitation). Furthermore, our data support previous work that sCr levels not accounted for in the MELD score have important implications on waitlist outcomes.32 Lastly, our data suggest that clinicians should prepare their patients for the possibility of listing for simultaneous liver–kidney transplantation earlier in their disease course. Whether interventions to lower sCr levels will reduce rates of persistent kidney injury or the need for simultaneous liver–kidney transplantation warrants further study. Our data, focusing on patients with cirrhosis in the outpatient setting, when intervention is possible, provides the foundation for such investigation.
Potential conflict of interest
Nothing to report.




