Efficacy and Safety of Peginterferon Alfa-2a (40KD) in Children With Chronic Hepatitis B: The PEG-B-ACTIVE Study
Potential conflict of interest: Dr. Nasmyth-Miller is employed by Roche. Dr. Wat is employed by and owns stock in Roche. Dr. Wirth consults for Roche. Dr. Pavlovic is employed by Roche. Dr. Sokal consults for, advises for, received grants from, and owns stock in Promethera. Dr. Schwarz consults for, advises for, and received grants from Gilead. She consults for and received grants from Roche. She consults for UpToDate. She received grants from Bristol-Myers Squibb. Dr. Huang is employed by Roche. Dr. Frey is employed and owns stock in F. Hoffmann-La Roche.
Supported by F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Abstract
Children with chronic hepatitis B (CHB) represent an area of unmet medical need, attributed to increased lifetime risk of CHB sequelae and limited therapeutic options compared with adult CHB patients. The PEG-B-ACTIVE (NCT01519960) phase III study evaluated peginterferon (PegIFN) alfa-2a treatment in children aged 3 to <18 years with CHB. A total of 161 hepatitis B e antigen (HBeAg)-positive immune-active patients without advanced fibrosis (AF)/cirrhosis were randomized (2:1) to PegIFN alfa-2a (Group A, n = 101) or no treatment (Group B, n = 50); patients with AF were assigned to PegIFN alfa-2a (Group C, n = 10). PegIFN alfa-2a was administered for 48 weeks by body surface area (BSA) category, based on 180 μg/1.73 m2. HBeAg seroconversion rates at 24 weeks posttreatment were significantly higher in Group A (25.7% vs. 6%; P = 0.0043), as were the rates of hepatitis B surface antigen (HBsAg) clearance (8.9% vs. 0%; P = 0.03), hepatitis B virus (HBV) DNA <2,000 IU/mL (28.7% vs. 2.0%; P < 0.001) or undetectable (16.8% vs. 2.0%; P = 0.0069), and alanine aminotransferase (ALT) normalization (51.5% vs. 12%; P < 0.001). Safety, including incidence of ALT flares and neutropenia, was comparable to the established PegIFN alfa-2a profile in HBV-infected adults or hepatitis C virus-infected children. Changes in growth parameters were minimal during treatment and comparable to those in untreated patients. Safety and efficacy outcomes in Group C were in line with Group A. Conclusion: PegIFN alfa-2a treatment of children in the immune-active phase of CHB was efficacious and well tolerated, and associated with higher incidence of HBsAg clearance than in adults. This represents an important advance to the treatment options for children with CHB.
Despite effective vaccination, hepatitis B virus (HBV) chronically infects an estimated 240 million people worldwide1 and is associated with risk of cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC). Approximately 887,000 deaths per year are attributable to chronic hepatitis B (CHB).1 The risk of chronicity is age-dependent; up to 90% for infants and 50% for children <6 years, compared with <5% for adults.1, 2
Children with CHB are at higher lifetime risk of developing CHB sequelae than adults, with a 40% lifetime risk of death from cirrhosis or HCC.3 Although vaccination has been successful in reducing childhood CHB infection,1 many children still develop CHB through vertical transmission at birth because of lack of access to vaccination programs or (less commonly) horizontal transmission during childhood.
Safe and effective CHB treatment for children <12 years remains an unmet medical need.4-7 Except for entecavir, most first-line treatments recommended for adults with CHB (peginterferon [PegIFN] alfa-2a/2b, tenofovir) have not been licensed across the full pediatric age range. Approved treatments for pediatric patients possess significant limitations (e.g., suboptimal efficacy, high rates of resistance, safety concerns, and very frequent subcutaneous administration), and most are no longer recommended as first-line treatment for adults (lamivudine, adefovir, and interferon [IFN] alfa-2b).8-10 IFN alfa-2b is effective in terms of hepatitis B e antigen (HBeAg) seroconversion, with 10% of patients also experiencing hepatitis B surface antigen (HBsAg) clearance versus 1% in untreated patients,11 providing a strong rationale to evaluate PegIFN in pediatric patients.
Entecavir is approved in children ≥2 years with CHB, although virological suppression rates are lower in children than in adults (49% vs. 67% after 48 weeks) with higher rates of resistance (2.6% vs. 0.5% after 2 years).12-14 Tenofovir, which is licensed for adolescents ≥12 years only, has been associated with decreased bone mineral density (BMD) in human immunodeficiency virus (HIV)-infected adult and pediatric patients, decreased gains in BMD in children and adolescent HBV-infected patients,15-18 and renal dysfunction in adult CHB patients.19-21 Neither treatment increases HBsAg loss/seroconversion rates. The need for lifelong treatment to prevent off-treatment relapse, with unknown long-term safety and resistance rates, remains a challenge.
PegIFN alfa-2a is recommended in international guidelines for treatment of adults with CHB and adults/children with chronic hepatitis C (CHC),8-10 having superseded nonpegylated IFNs because of once-weekly subcutaneous injection (vs. three times weekly) and a superior efficacy/safety profile. The advantages of immune-modulatory agents over nucleos(t)ide analogs include a finite duration of treatment, higher rates of HBeAg/HBsAg seroconversion, sustained responses off-treatment, and lack of resistance. Disadvantages include subcutaneous injections, more-frequent side effects, and potential growth inhibition in children. IFN alfa-2b is licensed for pediatric patients with CHB in the United States, but not in the European Union (EU).4-6, 11, 22 The European Society for Paediatric Gastroenterology, Hepatology and Nutrition guidelines highlight that finite duration IFN therapy is the treatment strategy of choice for pediatric patients in the HBeAg-positive immune-active phase with elevated alanine aminotransferase (ALT) levels, and that PegIFN is likely to replace conventional IFN once adequate clinical trial data become available.6 The current study was undertaken to assess the efficacy and safety of PegIFN alfa-2a treatment in pediatric patients with CHB. Based on the results of this study, both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) approved PegIFN alfa-2a for treatment of pediatric patients from 3 to <18 years with CHB on October 13, 201723, 24 and November 10, 2017,25, 26 respectively.
Materials and Methods
STUDY DESIGN
PEG-B-ACTIVE (YV25718, NCT01519960) was a randomized, controlled, open-label, multicenter, phase III study conducted at 37 sites in China, Russian Federation, Ukraine, Australia, Israel, Belgium, Poland, United Kingdom, Bulgaria, Germany, United States, and Italy.
Patients without advanced fibrosis (AF; METAVIR F0-F2 or equivalent) were randomized (2:1) to 48 weeks of PegIFN alfa-2a (Group A) or an untreated control group (Group B; Supporting Fig. S1). Patients with AF, but not cirrhosis (METAVIR F3 or equivalent), were assigned to 48 weeks of PegIFN alfa-2a (Group C), because it was considered clinically and ethically unacceptable to delay treatment in patients at risk of disease progression. Randomization was stratified by HBV genotype (A vs. non-A) and ALT (<5 × upper limit of normal [ULN] vs. ≥5 × ULN) and performed centrally by Interactive Voice/Web Response System (IxRS).
Group B patients who completed the 48-week observation period without HBeAg seroconversion could switch, at investigator and patient discretion, at any time up to 1 year after the observation period, to receive 48 weeks of PegIFN alfa-2a. This accommodated regional differences in clinical practice: in some regions, pediatricians adopt adult treatment strategies for children in the immune-active phase to avoid treatment delays, whereas in other regions a watch-and-wait approach is preferred. All patients entered long-term follow-up, lasting up to 5 years after the end of treatment/principal observation period.
The study was conducted in accord with the principles of the Declaration of Helsinki and Good Clinical Practice, and the study protocol was approved by the relevant health authority and ethical committee at each site. Written informed consent was obtained from the parent/legal guardian of all patients, from the patient if legally considered an adult by national legislation, and assent from the patient, where appropriate. Patient safety data were monitored by an independent data safety and monitoring board on an ongoing basis throughout the study conduct.
PATIENTS
Patients aged 3 to <18 years were eligible if they were HBeAg-positive (≥6 months), had detectable HBV DNA (>2,000 IU/mL), and elevated ALT (>1 × but ≤10 × ULN) at screening. Patients must have had a liver biopsy within 2 years (9 months if they had AF). Liver biopsy was performed and assessed at participating sites, within 6 months of enrollment in 89% of patients (median 18 days). Patients were ineligible if they had cirrhosis (METAVIR F4 or equivalent), tested positive for hepatitis A, C, or D or for HIV, or had a history or evidence of chronic liver disease other than CHB, or suspicion of HCC. Patients with a history of psychiatric disorder, significant chronic pulmonary or cardiac disease, poorly controlled thyroid disease or diabetes, previous solid organ or stem-cell transplant, or evidence or history of malignancy were also excluded.
Patients in Groups A and C received PegIFN alfa-2a subcutaneously once-weekly for 48 weeks, with dosing based on body surface area (BSA) categories previously approved for use in pediatric CHC patients in the EU: 0.51-0.53 m2, 45 μg; 0.54-0.74 m2, 65 μg; 0.75-1.08 m2, 90 μg; 1.09-1.51 m2, 135 μg; and >1.51 m2, 180 μg. This dosing regimen is based upon the formula of (dose = 180 × BSA/1.73 m2), where a BSA of 1.73 m2 is considered the average in adults, and is designed to achieve equivalent PegIFN alfa-2a exposures in children compared with adults while minimizing potential dosing errors. Dose adjustments during the study were allowed for adverse event (AEs) or changes in BSA.
EFFICACY ASSESSMENTS
The primary efficacy endpoint was proportion of patients with HBeAg seroconversion (loss of HBeAg and presence of antibody to hepatitis B e antigen [anti-HBe] antibody) 24 weeks after the end of treatment/observation in Groups A and B. Secondary efficacy endpoints included the proportion of patients with HBeAg clearance, HBsAg clearance, HBsAg seroconversion, HBV DNA <20,000 IU/mL, HBV DNA <2,000 IU/mL, undetectable HBV DNA, and normalized ALT 24 weeks after the end of treatment/observation and at other time points (e.g., end of treatment). Changes in virological and serological biomarkers over time were also assessed as secondary endpoints.
Serum levels of HBeAg, HBsAg, anti-HBe, anti-HBs (by enzyme-linked immunosorbent assay [Siemens Centaur, Siemens Healthcare Diagnostics, Camberley, UK]) and HBV DNA (by real-time quantitative PCR [qPCR]; Roche COBAS TaqMan [Roche Diagnostics, Welwyn, UK] 48; lower limit of detection, 29 IU/mL), were measured centrally at baseline and every 12 weeks thereafter.
SAFETY ASSESSMENTS
Safety evaluations included incidence of AEs, laboratory assessments, growth parameters, and vital signs. Severity of AEs was assessed (mild, moderate, or severe) and causality determined by the investigator. Dose reductions could be made for moderate-severe AEs, and specific dose-adjustment guidance was provided for management of treatment-emergent neutropenia, thrombocytopenia, or elevated ALT (Supporting Table S1).
Safety was assessed at baseline, weeks 1, 2, 4, 8, and 12, and every 6 weeks thereafter during treatment, and as appropriate during follow-up. Standard hematology and blood chemistry were analyzed locally or centrally, while urinalysis was conducted locally. Sex-specific Centers for Disease Control and Prevention Growth Charts were used to calculate height/weight for age percentiles and deviation from the mean (Z-scores).27
SUBSTUDIES
Patients at eight centers could participate in an optional liver elasticity substudy, where transient elastography (TE; FibroScan) assessments were performed at baseline, end of treatment, and week 24 and year 2 of follow-up. Correlation between liver elasticity/liver stiffness (LS) and liver fibrosis (LF) at baseline, and longitudinal changes in LS were assessed.
Patients who received PegIFN alfa-2a (including Group B patients who switched) could participate in an optional pharmacokinetic (PK) substudy. PK blood samples were collected at weeks 1 and 24 (predose and 24-48, 72-96, and 168 hours postdose) and at weeks 4, 8, and 12 (predose). PegIFN alfa-2a concentrations were analyzed using a population PK approach (see Supporting Material).
STATISTICAL ANALYSIS
The primary endpoint was assessed using the Cochran-Mantel-Haenszel test, adjusted for stratification factors of genotype (A vs. non-A) and ALT (<5 × ULN vs. ≥5 × ULN). The Breslow-Day test was used to assess homogeneity of odds ratios (ORs) across strata, and sensitivity analyses were performed using unstratified Pearson’s chi-square and Fisher’s exact tests. Assuming a 32% HBeAg seroconversion rate for Group A and 10% for Group B, a sample size of 145 patients was required to provide ≥80% power at the 0.05 level of significance with a two-sided test.
Descriptive statistics of secondary endpoints were summarized by treatment group. For binary endpoints, P values were calculated by unstratified Fisher’s exact test. Continuous endpoints were summarized descriptively with 95% confidence intervals (CIs). No interim analyses were performed.
Response rates were calculated for all patients who received ≥1 dose of study drug. Patients with missing values were classified as nonresponders, including Group B patients who switched to PegIFN alfa-2a before the primary endpoint at 24 weeks posttreatment. Efficacy and safety endpoints are reported up to 24 weeks’ follow-up. Long-term follow-up and data from Group B patients who switched will be reported separately, once available.
Results
PATIENTS
Of the 162 enrolled patients (June 2011-September 2014), 151 were randomized to Group A (n = 101) and Group B (n = 50), and 10 patients with AF were allocated to Group C. An additional patient, initially allocated to Group C but subsequently found not to have AF, withdrew without receiving any study drug.
Baseline characteristics were well balanced between Groups A and B (Table 1). Most patients (64%) were male, 56% were Asian, and mean age was 10.7 years (range, 3-17) with approximately 15% <5 years. Genotype C (34%) and D (32%) infections were more common, and 13 (9%) patients had normal ALT at baseline (despite having elevated levels at screening).
|
PegIFN alfa-2a (Group A) n = 101 |
Untreated (Group B) n = 50 |
PegIFN alfa-2a allocated (Group C) n = 10 |
|
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 10.41 (4.57) | 11.20 (5.01) | 6.70 (3.27) |
| Median | 11.0 | 13.0 | 6.0 |
| Min-Max | 3.0-7.0 | 3.0-17.0 | 3.0-12.0 |
| Age group, years 3-4 | 14 (14) | 9 (18) | 4 (40) |
| 5-11 | 39 (39) | 11 (22) | 5 (50) |
| 12-17 | 48 (47) | 30 (60) | 1 (10) |
| Sex male | 64 (63) | 32 (64) | 8 (80) |
| Race Asian | 56 (55) | 33 (66) | 7 (70) |
| BSA, m2 0.54-0.74 | 9 (9) | 9 (18) | 2 (20) |
| 0.75-1.08 | 31 (31) | 9 (18) | 6 (60) |
| 1.09-1.51 | 30 (29) | 12 (24) | 1 (10) |
| >1.51 | 31 (31) | 20 (40) | 1 (10) |
| ALT × ULN, mean (SD) | |||
| ALT group, × ULN <1 | 7 (7) | 5 (10) | 0 |
| 1-<2 | 41 (41) | 19 (38) | 3 (30) |
| 2-<5 | 43 (42) | 17 (34) | 7 (70) |
| 5-<10 | 8 (8) | 9 (18) | 0 |
| ≥10 | 2 (2) | 0 | 0 |
| HBV genotype A | 9 (9) | 3 (6) | 1 (10) |
| B | 21 (21) | 6 (12) | 1 (10) |
| C | 34 (33) | 23 (46) | 6 (60) |
| D | 31 (31) | 18 (36) | 2 (20) |
| Othera | 6 (6) | 0 | 0 |
| Mean (SD) ALT, × ULN | 2.78 (2.48) | 2.89 (2.00) | 2.80 (1.12) |
| Mean (SD) HBV DNA, log10 IU/mL | 8.09 (0.99) | 8.06 (0.99) | 7.87 (0.97) |
| Mean (SD) HBeAg, log10 IU/mL | 2.74 (0.50) | 2.57 (0.65) | 2.34 (0.98) |
| Mean (SD) HBsAg, log10 IU/mL | 4.32 (0.69) | 4.38 (0.72) | 4.23 (0.53) |
- a Includes patients with HBV genotypes A and B (n = 1), A and D (n = 1), and E (n = 4).
Most patients in Group A (98%; 99 of 101) completed 48-week treatment and 24-week follow-up; 2 patients did not complete treatment (Supporting Fig. S2). In Group B, 12% of patients withdrew and 58% switched to PegIFN alfa-2a before 24-week follow-up.
EFFICACY
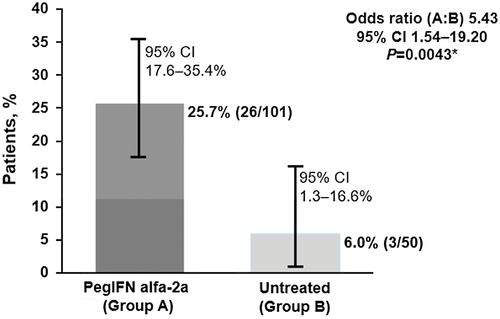
After 24 weeks of follow-up, the percentage of patients with HBeAg seroconversion was significantly higher in Group A (25.7%; 26 of 101) versus Group B (6% [3 of 50]; OR, 5.43; 95% CI, 1.54-9.20; P = 0.0043; Fig. 1). The Breslow-Day test (P = 0.3732) demonstrated homogenous ORs across strata, and the stratified result was supported by the unstratified Pearson’s chi-square (P = 0.0038) and Fisher’s exact (P = 0.0038) tests.
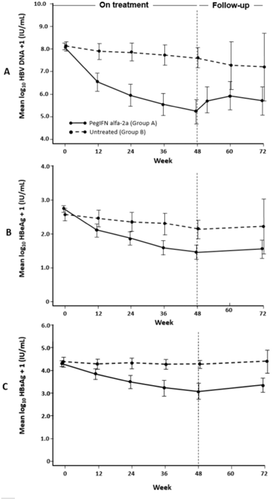
A higher proportion of patients in Group A versus Group B achieved secondary efficacy endpoints, including HBsAg clearance (8.9% vs. 0%; P = 0.03), HBV DNA <2,000 IU/mL (28.7% vs. 2%; P < 0.001), and ALT normalization (51.5% vs. 12%; P < 0.001; Table 2). Levels of HBV DNA, HBeAg, and HBsAg decreased during treatment in Group A while remaining stable in Group B (Fig. 2).
| Patients, n (%) |
PegIFN alfa-2a (Group A) n = 101 |
Untreated (Group B) n = 50 |
P Value |
|---|---|---|---|
| Loss of HBeAg | 26 (25.7) | 3 (6.0) | 0.004 |
| HBsAg seroconversion | 8 (7.9) | 0 (0.0) | 0.053 |
| Loss of HBsAg | 9 (8.9) | 0 (0.0) | 0.030 |
| HBV DNA <20,000 IU/mL | 34 (33.7) | 2 (4.0) | <0.001 |
| HBV DNA <2,000 IU/mL | 29 (28.7) | 1 (2.0) | <0.001 |
| HBV DNA undetectable | 17 (16.8) | 1 (2.0) | 0.007 |
| Normal ALT | 52 (51.5) | 6 (12.0) | <0.001 |
| HBeAg SC and HBV DNA <20,000 IU/mL | 23 (22.8) | 2 (4.0) | 0.003 |
| HBeAg SC and HBV DNA <2,000 IU/mL | 20 (19.8) | 1 (2.0) | 0.002 |
- Abbreviation: SC, seroconversion.
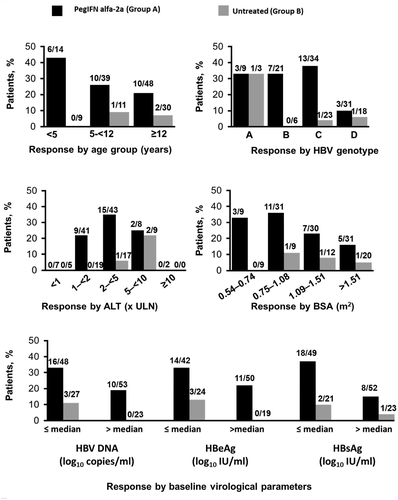
Subgroup analyses, although limited by small patient numbers, suggested higher treatment-induced HBeAg seroconversion in younger patients, those with genotypes B and C, and patients with lower HBV DNA, HBeAg, and HBsAg at baseline (Fig. 3). Multiple logistic regression analyses identified PegIFN alfa-2a therapy, lower baseline HBeAg levels, and HBV-DNA levels as significant predictors of HBeAg seroconversion (Supporting Table S2). Notably, baseline age and HBV genotype (A vs. non-A) were not identified as significant predictors. Of the 9 patients who achieved HBsAg loss, 6 (67%) were <12 years, whereas 4 had genotype B infection, 4 genotype C, and 1 genotype A.
Because 69% of Group B patients (35/51) were imputed as nonresponders attributed to withdrawal (n = 6) or switching to PegIFN alfa-2a (n = 29), additional sensitivity analyses were conducted for the primary endpoint. Using a prespecified last-observation-carried-forward approach, the difference between groups remained significant (27 of 101 [26.7%] vs. 4 of 50 [8.0%]; P = 0.008). Similarly, extrapolating the Group B HBeAg seroconversion rate, from 6% at the end of the principal observation period to 9% at follow-up week 24, still resulted in a considerably lower rate than that observed in Group A. Furthermore, in a post-hoc analysis of Group B data at follow-up week 24 regardless of whether patients switched to PegIFN alfa-2a, the difference between groups remained significant (26 of 101 [25.7%] vs. 4 of 50 [8.0%]; P = 0.011).
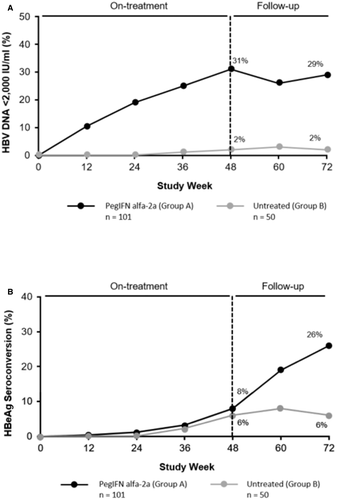
The proportion of patients with HBeAg seroconversion during PegIFN alfa-2a treatment was 8% compared with the 6% in untreated patients. However, the HBeAg seroconversion rate increased to 26% during the 24 weeks posttreatment in Group A while remaining steady in Group B. Virological response (VR; HBV DNA <2,000 IU/mL) increased to 31% during treatment and remained steady during the 24 weeks posttreatment (Fig. 4). Quantitative levels of HBV DNA, HBsAg, and HBeAg declined during treatment, and the decline was significantly more pronounced (as early as week 12) in patients who achieved HBeAg seroconversion (Supporting Fig. S3). These findings are similar to adult data and suggest a potential for response-guided therapy.
TE
Seventy-five patients from Groups A (n = 44), B (n = 25), and C (n = 6) participated in the liver elasticity substudy (Supporting Table S3). There was a modest correlation between LS and LF at baseline, and an accurate cutoff for significant fibrosis (SF) was not identified (Supporting Table S4; Supporting Fig. S4). The cutoff recommended by the European Association for the Study of the Liver and Asociación Latinoamericana para el Estudio del Hígado guidelines for excluding SF (<6.0 kilopascals [kPa])28 showed poor diagnostic performance (sensitivity, 0.65; specificity, 0.67; positive predictive value, 0.55; negative predictive value, 0.76) and wrongly excluded SF in 24% (9 of 37) of patients.
PegIFN alfa-2a treatment was associated with a modest decrease in LS. Mean LS scores from baseline to the end of treatment/principal observation period decreased by 0.49 and 1.52 kPa in Groups A and C, respectively, with an increase of 0.38 kPa in Group B (Supporting Fig. S4; Supporting Fig. S5). Within Group A, the decline in mean LS scores during treatment was similar between patients with/without HBeAg seroconversion at 24 weeks posttreatment (0.55 vs. 0.46 kPa, respectively).
To assess the possible confounding effect of inflammation on LS measurements, a linear regression analysis was performed (Supporting Fig. S6). In Group A, changes in LS scores from baseline to 24 weeks posttreatment were not associated with changes in ALT (correlation coefficient, –0.1392). Further details are provided in the Supporting Material and have been presented elsewhere.29
PK
Thirty patients from Groups A (n = 16), B (n = 9; after switching to PegIFN alfa-2a), and C (n = 5) participated in the PK substudy (Supporting Table S5). PK data were best described by a one-compartment disposition population model with a sequential zero–first-order absorption (Supporting Fig. S7). Estimated mean PegIFN alfa-2a exposures (area under the curve) in children was 3,484 h.ng/mL, which is consistent with exposures estimated in adults (Supporting Figs. S8 and S9). Exposures in pediatric patients were also comparable across BSA dosing categories (Supporting Fig. S9). Five of 21 patients in Groups A and C who participated in the PK substudy achieved HBeAg seroconversion at follow-up week 24. No relationship between response and PegIFN alfa-2a exposure could be identified in this small sample. Further details are provided in the Supporting Material and have been presented elsewhere.30
SAFETY
Consistent with previous reports of IFN alfa therapy, the most common AEs included pyrexia, headache, and abdominal pain (Table 3). Most AEs were mild or moderate and rarely resulted in treatment discontinuation (1%; 1 patient for elevated ALT and aspartate aminotransferase [AST]). No notable difference in safety was observed between younger and older children. Seven serious AEs (SAEs), which resolved without sequelae, were experienced by 6 patients in Group A (progression of hepatitis B, tonsillitis, scalp microsporum infection, latent tuberculosis, elevated AST, elevated ALT, and osteochondrosis). In Group B, 1 patient experienced an SAE (suicidal ideation) and withdrew from the study.
| Patients, n (%) |
PegIFN alfa-2a (Group A) n = 101 |
Untreated (Group B) n = 50 |
|---|---|---|
| Any AE | 87 (86) | 27 (55) |
| Treatment-related AE | 81 (80) | 1 (2) |
| SAE | 6 (6) | 1 (2) |
| AE leading to dose modificationa | 6 (6) | — |
| AE leading to treatment discontinuation | 1 (1) | — |
| AE leading to study withdrawal | 0 | 1 (2) |
| Deaths | 0 | 0 |
| Common AEs (incidence >10%) | ||
| Pyrexia | 49 (49) | 5 (10) |
| Headache | 30 (30) | 2 (4) |
| Abdominal pain | 19 (19) | 0 |
| Influenza-like illness | 15 (15) | 1 (2) |
| Cough | 14 (14) | 3 (6) |
| Vomiting | 14 (14) | 0 |
| Grade 3-4 laboratory abnormalitiesb | ||
| ALT increases (>5 × ULN) | 61 (60) | 14 (29) |
| AST increases (>5 × ULN) | 25 (25) | 3 (6) |
| Hyperbilirubinemia (bilirubin >2.5 × ULN) | 1 (1) | 0 |
| Neutropenia (<750 cells/μL) | 19 (10) | 0 |
| Thrombocytopenia (<50,000 platelets/μL) | 1 (1) | 0 |
|
ALT flares >5× ULN but ≤10 × ULN |
||
| During treatment | 34 (34) | 11 (22) |
| Posttreatment | 15 (15) | 2 (4) |
| >10 × ULN | ||
| During treatment | 19 (19) | 3 (8) |
| Posttreatment | 6 (6) | 0 |
- a Two laboratory abnormalities (elevated AST and low neutrophil count) led to dose modifications.
- b Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (v.1, 2004).
Depression or depressed mood, a potential concern of IFN alfa therapy, was reported infrequently. Twelve patients (12%) in Group A and 1 (2%) in Group B reported a neuropsychiatric disorder during the study. All events in Group A were mild or moderate, with the most common being insomnia (3 patients; 3%). Depression and depressed mood were reported by 1 patient each. In Group B, 1 participant reported suicidal ideation as described above.
Laboratory abnormalities during treatment were common but manageable with dose modifications. Treatment-emergent decreases in neutrophils, leukocytes, hemoglobin, and platelets reversed shortly after cessation of therapy. Neutropenia in Group A was moderate (0.50-0.75 × 109 cells/L) in 16 patients (16%) and severe (<0.5 × 109 cells/mL) in 3 patients (3%; none <0.25 × 109 cells/mL). Of these 19 patients, 7 reported an AE of infection, only one of which was severe or serious. This patient, with a lowest neutrophil count (0.75 × 109 cells/L) on study day 119, was diagnosed with severe and serious acute tonsillitis on study day 129. This was unrelated to study treatment, and the dose was not modified. Following penicillin treatment, the event resolved on study day 137. In the remaining 6 patients, the start of the infection AEs did not correlate with the timing of their lowest neutrophil counts, and the AEs were considered unrelated to study treatment, resolving without dose modification.
Incidence of ALT flares (>5 × ULN and 2× baseline) was higher during (37%) versus posttreatment (16%); most were associated with decreasing HBV-DNA levels (>1 log10 IU/mL); none was accompanied by elevated bilirubin (>2 × ULN) nor resulted in hepatic decompensation. Two patients had AEs of increased thyroid-stimulating hormone in Group A. These were mild and had resolved by the end of follow-up. No patient in Group A reported AEs of hypothyroidism or hyperthyroidism or required dose modification for thyroid function abnormalities. In Group B, 1 case of hypothyroidism was reported (the patient did not switch to PegIFN alfa-2a).
Short-term effects on growth appeared minimal. By the end of treatment, mean change in height-for-age Z-scores was –0.10 and proportion of patients with >15% drop in height-for-age percentile was 6% (Table 4; Supporting Fig. S10). Similar changes were observed in Group B untreated patients (–0.01 and 2%, respectively) and also in weight-for-age Z-scores. Patients will continue to be monitored up to 5 years after the end of treatment to assess any long-term effects.
|
PegIFN alfa-2a (Group A) n = 101 |
Untreated (Group B) n = 49 |
|
|---|---|---|
| Height | ||
| Patients with >15% decrease in height-for-age percentile from baseline, n/N (%) | 6/98 (6) | 1/47 (2) |
| Mean change in height-for-age Z-score | –0.099 | –0.013 |
| Weight | ||
| Patients with >15% decrease in weight-for-age percentile from baseline, n/N (%) | 12/96 (13) | 4/47 (9) |
| Mean change in weight-for-age Z-score | –0.214 | –0.082 |
EFFICACY AND SAFETY OUTCOMES IN GROUP C
Although limited by small cohort size, the efficacy and safety outcomes in 10 patients with AF, 4 (40%) of whom were aged <5 years (Table 1), were broadly consistent with Group A. At follow-up week 24, 3 patients (30%) achieved HBeAg seroconversion, 3 (30%) had undetectable HBV DNA, 7 (70%) HBV DNA <2,000 IU/mL, and none achieved HBsAg clearance (Supporting Table S6). LS decreased, with a mean decrease in LS score of 1.52 kPa, by the end of treatment. No SAEs, or AEs leading to study or treatment discontinuation, were reported in Group C.
Discussion
This randomized, controlled, open-label study was conducted in children aged 3 to <18 years with HBeAg-positive CHB in the immune-active stage. PegIFN alfa-2a administered per the BSA category-based dosing regimen provided comparable exposure across BSA categories, showed an acceptable safety profile, and was efficacious in patients with and without AF. The primary efficacy endpoint was supported by consistent findings from secondary endpoints and subgroup analyses (including by genotype).
The study design aimed to minimize potential confounding using stratified randomization and stratified analysis for the primary endpoint. The established predictors of HBeAg seroconversion in adults with CHB, HBV genotype, and baseline ALT levels were used as stratification factors. Overall, we did not identify evidence of confounding with respect to the primary endpoint results. First, the Breslow-Day test showed homogeneity of ORs across strata, and the stratified result was supported by the unstratified tests. Second, the baseline characteristics of randomized Groups A and B were well balanced, including HBeAg and HBV-DNA levels, which were identified as significant predictors of HBeAg seroconversion in the multiple logistic regression analysis.
Additional sensitivity analyses were undertaken to assess the robustness of the primary efficacy endpoint, which confirmed the significantly higher HBeAg seroconversion rate in patients treated with PegIFN alfa-2a compared with the spontaneous HBeAg seroconversion rate observed in untreated patients.
Although direct cross-trial comparisons cannot be made, the efficacy profile of PegIFN alfa-2a in pediatric patients was consistent with the extensive data available in adults.31, 32 In our study, the rate of HBsAg loss (8.9%) was higher than that observed in adults (2.3%-3.3%), and younger children had a higher rate of HBeAg seroconversion (30.2% in patients aged <12 years vs. 20.8% in those ≥12 years). One possible explanation is that defects in the innate and adaptive immune system (weak or absent virus-specific T-cell reactivity, poor effector cytotoxic activity, impaired cytokine production, and sustained expression of multiple inhibitory receptors), characteristic in adults with CHB, may be less pronounced in younger patients.33-36
Sustained HBsAg clearance is considered the ideal endpoint in CHB because it is associated with complete remission, lack of disease progression, and reduced risk of HCC.37, 38 HBsAg clearance is, however, difficult to achieve in practice,39, 40 and spontaneous HBsAg clearance is considered a rare event in childhood (0%-1%/year).38, 41, 42 In our study, no patients in Group B achieved spontaneous HBsAg loss or seroconversion.
The proportion of pediatric patients who achieved HBeAg seroconversion was numerically lower (26%) compared with studies in adults (32%-36%),31, 32 which may be attributed to differences in HBV-genotype subgroups; in our study, 31% of patients had genotype D infection,43 whereas in the adult studies only 3%-9% of patients were infected with genotype D.31, 32 The HBeAg seroconversion rate in our study was lower in genotype D patients (9.7%), consistent with findings in adult studies.31, 32 Although genotype C is a difficult-to-treat genotype, the HBeAg seroconversion rate in children with this genotype was 38.2% in Group A and 4.3% in Group B, consistent with response rates in adult studies (27.6%-39.0%).31, 32 Interpretation of results from the genotype A subgroup was limited by low patient numbers (9 in Group A, 3 in Group B).
Histological improvements have been demonstrated using paired pretreatment and posttreatment biopsies in adult patients with CHB treated with PegIFN alfa-2a.31, 44 Reversal of fibrosis has been reported in patients with CHC who achieved a sustained VR after IFN treatment45, 46 and in patients with CHB treated with nucleos(t)ide analogs.47 Data from pediatric patients treated with IFN alfa-2b also showed improvement in histology activity index with paired biopsies.11 In the present study, efficacy and safety results from patients with AF were consistent with data in adult patients, and children with AF require immediate treatment considering their higher risk of disease progression to cirrhosis and HCC. Data from this study demonstrated a steady decline in LS scores during treatment, and, although paired biopsies were not available, the decline observed was not associated with changes in ALT and thus may be indicative of improvement in fibrosis stage.
Baseline factors that predict HBeAg seroconversion with PegIFN alfa-2a in adults (lower baseline HBV DNA, HBeAg, and HBsAg levels) were generally associated with numerically higher response rates in Group A. Although higher baseline ALT level is a predictor of HBeAg seroconversion with PegIFN alfa-2a, in the present study patients with a baseline ALT 2 to <5 × ULN had higher response rates than patients with higher ALT levels. However, subgroup analyses should be interpreted with caution because of the small sample size.
One observed difference between this pediatric population and previous studies in adults was in the timing of HBeAg seroconversion; whereas 26% of patients achieved seroconversion after 24-week follow-up, only 8% had seroconverted by the end of treatment. This may be explained by the approximately 4-fold higher baseline mean HBeAg levels observed in children (2.87 log10 IU/mL) compared with adults (2.25 log10 IU/mL),16 whereby longer periods may be required to reach undetectable HBeAg levels and seroconversion in children. In addition, the percentage of patients with anti-HBe response increased both during treatment and follow-up. VR (HBV DNA <2,000 IU/mL) and declines in HBV DNA, HBeAg, and HBsAg levels were observed during treatment.
No new safety issues were identified and the safety profile of PegIFN alfa-2a in children with CHB was consistent with previous data in HBV-infected adults, and HCV-infected children (a patient population in which PegIFN alfa-2a combined with ribavirin [RBV] is currently approved in Europe, the United States, and other countries).48 PegIFN alfa-2a was well tolerated with low rates of discontinuation (1%) and dose modifications (26%). Laboratory abnormalities, including neutropenia and ALT flares, were common but clinically manageable, generally lower than observed in children with CHC, and similar to that observed in adults with CHB. No patient discontinued study treatment for neutropenia. Depression and/or depressed mood, class effects of IFNs, were rare compared with treated HBV-infected adults or hepatitis C virus-infected children, occurring in 2% of treated as well as untreated patients.
One safety concern of IFN use in children is the class effect of growth inhibition. Although the underlying mechanism is unknown, the primary concern is potential impairment on linear growth of body height, which could affect final adult height. In the IFN alfa-2b study, negative growth changes were observed during treatment; after 6 months of treatment, patients (n = 70) showed a mean change in height-for-age Z-scores of –0.16 compared with +0.06 in untreated patients.49 However, this change was transient, with catch-up growth observed posttreatment. There was little evidence of a treatment effect on growth in the current study, which is surprising as previous IFN studies in children, including the PEDS-C study of PegIFN alfa-2a in children with CHC, consistently showed growth impairment during treatment, with catch-up growth posttreatment.50 An important limitation of the PEDS-C study, however, was the lack of an untreated control arm to allow within-study comparison of growth data, as well as RBV coadministration in approximately 50% of patients.
In conclusion, the results of this phase III study demonstrate a positive benefit/risk profile of PegIFN alfa-2a treatment in pediatric CHB patients. This represents an important therapeutic advance to the treatment options available for children with CHB, and FDA and EMA approvals were received in October and November 2017, respectively.
Acknowledgments
The authors thank the patients, their families, and the study investigators who took part in this study. In addition to the authors, the following investigators participated in the study: Yuriy Antipkin, Sanjay Bansal, Valentyna Berezenko, Xin Yue Chen, Ruth De Bruyne, Feng Fang, Zhi Liang Gao, Miglena Georgieva, Anna Gorczyca, Evelyn Hsu, Maureen Jonas, Deirdre Kelly, Sergiy Kramarov, Daniel Leung, Shumei Lin, Yuri Lobzin, Ewa Majda-Stanislawska, Giorgina Mielei-Vergani, David Moore, Assy Nimer, Malgorzata Pawlowska, Alexander Potapov, Rifaat Safadi, Ron Shaoul, Tatiania Strokova, Gabriella Verucchi, Xiaozhong Wang, Zhaoxia Wang, Gareth Tudor-Williams, and Christo Zhelev. Support for third-party writing assistance, furnished by John Carron, Ph.D., of Health Interactions, was provided by Roche Products Ltd, Welwyn Garden City, UK.
REFERENCES
Abbreviations
-
- AE
-
- adverse event
-
- AF
-
- advanced fibrosis
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- BSA
-
- body surface area
-
- CHB
-
- chronic hepatitis B
-
- CHC
-
- chronic hepatitis C
-
- CI
-
- confidence interval
-
- HBeAg
-
- hepatitis B e antigen
-
- HBsAg
-
- hepatitis B surface antigen
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
-
- IFN
-
- interferon
-
- kPa
-
- kilopascals
-
- LF
-
- liver fibrosis
-
- LS
-
- liver stiffness
-
- OR
-
- odds ratio
-
- PegIFN
-
- peginterferon
-
- PK
-
- pharmacokinetic(s)
-
- SAE
-
- serious adverse event
-
- SF
-
- significant fibrosis
-
- TE
-
- transient elastography
-
- ULN
-
- upper limit of normal
-
- VR
-
- virological response




