Cirrhotic Cardiomyopathy After Transplantation: Neither the Transient Nor Innocent Bystander
Abstract
Cirrhotic cardiomyopathy in end-stage liver disease is currently characterized by blunted contractile systolic response to stress with or without diastolic dysfunction in the absence of known heart disease. Since the establishment of the diagnostic criteria of cirrhotic cardiomyopathy in 2005, there have been multiple studies regarding its pathophysiology and pretransplant clinical course. The data regarding the post-transplant course of this entity are sparse. This review addresses the course and prognosis of the elements of cirrhotic cardiomyopathy after liver transplantation (LT). To this end, there is limited compelling evidence demonstrating the reversibility of this entity post-LT. Cirrhotic cardiomyopathy may, in fact, increase the risk of post-transplant complications. This review reveals a need to refine the diagnostic criteria of cirrhotic cardiomyopathy in view of the remarkable progress in the sphere of echocardiographic evaluation of systolic and diastolic dysfunction. The post-transplant course and outcomes related to cirrhotic cardiomyopathy may be better evaluated in the setting of updated diagnostic criteria.
Cirrhotic cardiomyopathy is defined as cardiac dysfunction in patients with cirrhosis characterized by blunted contractile response to stress and/or altered diastolic relaxation with electrophysiological abnormalities, in the absence of known cardiac disease.1 The diagnostic criteria of cirrhotic cardiomyopathy were first coined during the World Congress of Gastroenterology (WCG) in Montreal in 2005 (Table 1). The underlying pathophysiology involves multiple coinciding factors, including volume expansion resulting in hyperdynamic circulation, cytokine alterations, myocyte membrane abnormalities, and autonomic impairment.2 The prominent feature of diastolic dysfunction is thought to relate to increased left ventricular (LV) mass leading to impaired relaxation.3
| Systolic Dysfunction | Diastolic Dysfunction | Supportive Criteria |
|---|---|---|
|
• Blunted cardiac response to exercise or pharmacological stress testing • LVEF <55% |
• Deceleration time >200 msec • Isovolumetric relaxation time >80 msec • E/A <1 |
• Electrophysiological abnormalities • Abnormal chronotropic response • Electromechanical uncoupling • Prolonged QTc interval • Enlarged left atrium • Increased myocardial mass • Increased BNP • Increased proBNP • Increased troponin I |
- Abbreviations: BNP, B-type natriuretic peptide; proBNP, pro-B-type natriuretic peptide.
In this concise report, we specifically aim to characterize the evolution and complications of cirrhotic cardiomyopathy following liver transplantation (LT), based on current diagnostic criteria.
Electrocardiographic Variables
POST-TRANSPLANT EVOLUTION
QT interval prolongation is a supportive criterion for the diagnosis of cirrhotic cardiomyopathy and thus is inadequate, alone, to diagnose cirrhotic cardiomyopathy. However, it is the most frequently evaluated (10 studies) aspect of LT effect on cirrhotic cardiomyopathy (Table 2). All studies showed significant improvement in corrected QT interval (QTc) post-LT, with four of these studies4-7 finding improvement or normalization in >80% of the patients. The median time to improvement in QTc prolongation was 6 (3-57) months. Notably, one study found that although the QTc decreased post-LT, it remained significantly longer than in the age-/sex-matched healthy individuals.8
| Study | N | Mean QTc Pre-LT | Mean QTc Post-LT | P Value | Maximum Interval to EKG (Months) | Improved/Normalized | Worsened (%) | Mean Follow-up (Years) | Post-LT Outcome |
|---|---|---|---|---|---|---|---|---|---|
|
Bal et al.4 (retrospective) |
162 | 450 | 429 | <0.002 | 6 | 28%/55% | 13.3 | 8.9 | Unaffected |
|
Mohamed et al.7 (prospective) |
45 | 478a | 433a | <0.001 | 3 | 100%/— | — | — | Unaffected |
|
Zurich et al.5 (retrospective) |
269 | 449 | 417 | <0.0001 | 12 | 17%/70% | — | — | Unaffected |
|
Patel et al.9 (retrospective) |
51 | 444 | 425 | <0.01 | 12 | — | — | — | Unaffected |
|
Gonzalez et al.10 (retrospective) Cyclosporine group |
37 | 421 | 395 | 0.05 | 3 | — | — | 2 | Unaffected |
| Tacrolimus group | 420 | 393 | 0.0005 | 3 | |||||
|
Carey et al.6 (prospective) |
46 | 451 | 418 | 0.001 | 4 | 48.5%/45% | 6 | — | — |
|
Zahmatkeshan et al.30 (prospective) |
22 | 420 | 390 | 0.04b | 6 | — | — | — | — |
|
Adigun et al.8 (prospective) |
38 | 436 | 421 | 0.02 | 9.5 | — | — | — | — |
|
Dowsley et al.16 (retrospective) |
107 | 415 | 371 | <0.001 | 6 | — | — | 3.2 | — |
|
Sonny et al.11 (retrospective) |
243 | 453 | 442 | 0.001 | 57 | — | — | 5.2 | Impaired morbidity and mortality |
- a Median QTc.
- b In lead V1. QTc in AVF and I was not different before and after transplant.
- Abbreviation: EKG, electrocardiogram.
1.2 IMPACT OF QTc CHANGES ON POST-TRANSPLANT COURSE
Five studies assessing post-transplant outcomes did not show impact of prolonged QTc on post-transplant survival.4, 5, 7, 9, 10 However, Sonny et al.11 showed that composite outcomes denoted by mortality, graft failure, and/or major cardiovascular events (CVEs) were associated with prolonged QTc pretransplant during a mean follow-up of 5 years post-transplant. Major CVEs were defined as the presence of coronary artery disease requiring intervention, congestive heart failure (HF), or ischemic stroke.
Persistently prolonged QTc post-transplant can conceptually increase the risk of ventricular arrhythmia in these patients, but data confirming these outcomes are needed. Importantly, baseline prolonged QTc can potentially limit the use of medications that are associated with QTc prolongation. Some of these medications can be instrumental post-LT, such as tacrolimus, quinolones, and fluconazole.
Echocardiographic Variables
POST-TRANSPLANT EVOLUTION
Changes in systolic function post-transplant have been evaluated in six studies (Table 3). Ejection fraction (EF) was normal before and after transplant in all studies, likely reflecting selection criteria for transplant candidacy. Although three of the studies showed statistically significant decline in EF post-transplant, the decline was clinically insignificant. Systolic response to stress before and after LT showed significant improvement 9 months after transplant in one study.3 More recently, a study of the longitudinal systolic strain, a novel surrogate that is more sensitive than EF, showed that although strain values remained within normal range, there was improvement 18 months after transplant in the left ventricle (−18.5% to −21%; P < 0.01) and in the right ventricle (−21% to −23%; P < 0.01).12
| Study | Therapondos et al.14 | Sonny et al.11 | Torregrosa et al.3 | Acosta et al.13 | Dowsley et al.16 | Chen et al.12 | |
|---|---|---|---|---|---|---|---|
| N | 40 | 243 | 15 | 30 | 107 | 41 | |
| Design | Prospective | Retrospective | Prospective | Retrospective | Retrospective | Prospective | |
| Pre-LT systolic function | EF | Normalb | 59 | 73 | 64 | 68 | 66 |
| Response to stress | Absent | Present | |||||
| Post-LT systolic function | EF | Normalb | 57a | 67a | 62 | 65a | 65 |
| Response to stress | Present | ||||||
| Pre-LT diastolic function | E/A | 1.23 | 1.1 | 1.32 | 1.1 | 1 | |
| DT (msec) | 241 | ||||||
| IRT (msec) | 100 | ||||||
| Post-LT diastolic function | E/A | 0.96a | 1 | 1.01a | 1.1 | 0.97 | |
| DT | 232 | ||||||
| IRT | 117a | ||||||
| Pre-LT other echo variables | LA enlargement | 33 mL/m2 (volume index) | 44 mm (diameter) | 35 mL/m2 | |||
| LV wall thickness | 0.9 cm | 93 g/m2 | 115 g/m2 | 1 cm | 99 g/m2 | 1.1 cm | |
| Post-LT other echo variables | LA enlargement | 34.2 mL/m2 | 41a | 34 mL/m2 | |||
| LV wall thickness | 1.07 cma, c | 106 g/m2a | 97 g/m2a | 1 cm | 100 g/m2 | 1.2 cma, c | |
| Mean follow-up post-LT | 57 months | 5.2 years | 9 months | 21 months | 2.6 months | 18 months | |
| Post-LT outcome | 24% had HF post-LT |
- a P < 0.05.
- b The exact values were not provided by the original article
- c The difference was noted in interventricular septal wall thickness only. No difference in posterior wall thickness.
- Abbreviations: DT, deceleration time; IRT, isovolumetric relaxation time.
The aforementioned six studies also examined post-transplant changes in diastolic function (Table 3). Diastolic function worsened in three studies11, 13, 14 and improved in one study post-transplant.3 Mean pretransplant E/A ratio, which is the peak velocity flow in early diastole (E wave) to peak velocity flow in late diastole (A wave), was normal in all five studies evaluating it. Two studies illustrated worsening in E/A post-transplant at 314 and 21 months.13 Peak filling rate refers to the rate of LV filling during diastole, which was shown to significantly decrease post-transplant from 2.9 to 2.56 (end-diastolic volume/second; P < 0.05) at 21 months.15 Conversely, another report found increased peak filling rates from 49% to 93% (P = 0.04) 9 months after transplant. They also showed that although mean deceleration time (time taken from maximum E point to baseline) did not normalize post-transplant, it demonstrated a nonstatistically significant downtrend.3 Therapondos et al.14 showed that isovolumetric relaxation time (i.e., time from the closure of aortic valve to the opening of mitral valve) was increased (>80 msec), before and after transplant with further increase 3 months after transplant (Table 3). E/e′ ratio, with e′ being the early diastolic mitral annular velocity reflecting myocardial relaxation, is a surrogate to best measure filling pressure that was assessed in two studies. Whereas Dowsley et al.16 showed increase in E/e′ 2.6 months post-LT from 7.5 to 8.6 (P = 0.04), Chen et al.12 did not reveal significant change in E/e′ ratio 18 months post-transplant (P = 0.3).
Left atrial (LA) enlargement is a supportive diagnostic criterion. Changes in LA enlargement in relation to transplant were investigated in three studies. Two studies11, 16 showed that the mean LA volume index (LAVI) was borderline high(33-35) (normal <34 mL/m2) before and after transplant without significant changes after transplant at 41 and 2.6 months, respectively. The third study3 evaluated LA diameter and showed significant decrease at 9 months post-transplant, which is expected if stroke volume decreases post-transplant.
LV muscle thickness is another criterion that can relate to diastolic dysfunction. Of six studies assessing LV muscle thickness post-transplant, three studies11, 12, 14 showed increase at 3-41 months after transplant and one study showed a decrease in thickness at 9 months.3 However, only one study accounted for development of post-transplant systemic hypertension in their analysis.12
IMPACT OF ECHOCARDIOGRAPHIC CHANGES ON POST-TRANSPLANT COURSE
The post-transplant echocardiographic mean follow-up period ranged from 3 months14 to 5.2 years.11 Dowsley et al.16 showed that worsening pretransplant LAVI and E/e′ were associated with post-transplant systolic HF in 24% of their cohort within a mean interval of 2.6 months. Furthermore, during long-term follow-up, patient survival among LT recipients with pretransplant LAVI <40 mL/m2 was 82% at 1 year and 71% at 5 years compared with 54% 1-year and 50% 5-year survival in patients whose LAVI was >40 mL/m2.
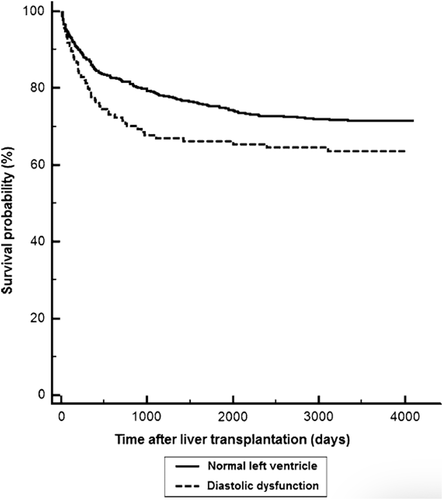
The unfavorable impact of cirrhotic cardiomyopathy on post-transplant outcomes was illustrated in another study by Mittal et al., where they evaluated 970 transplant patients over a mean of 5.3 years. Fifteen percent of patients had diastolic dysfunction pretransplant, and these patients had significantly higher risk for acute cellular rejection (hazard ratio [HR], 10.56; P = 0.0001), graft failure (HR, 2.09; P = 0.007), and all-cause mortality (HR, 1.52; P = 0.01).17 Notably, risk increased with worsening degree of diastolic dysfunction. The impact on survival occurred within 12-18 months after transplant (Fig. 1). A rationale for the association with rejection is that diastolic dysfunction could be associated with up-regulation of proinflammatory cytokines leading to enhanced antigen presentation to the T cells along with elevated venous pressures in patients with diastolic dysfunction possibly resulting in decreased clearance of proinflammatory cytokines.17
Diastolic dysfunction has been associated with increased risk of atrial fibrillation (AF).18 Pretransplant AF was associated with pretransplant increased LAVI, which is a marker for cirrhotic cardiomyopathy and diastolic dysfunction, and was also associated with more complicated intra- and postoperative course.19 Pretransplant AF is an independent risk factor for major CVEs post-LT, which has unfavorable impact on survival.20
PHYSIOLOGICAL EXPLANATIONS FOR THE POST-TRANSPLANT COURSE
Cirrhotic cardiomyopathy has a multifactorial underlying etiology that involves hyperdynamic circulation, myocyte membrane changes, and autonomic impairment, which can explain the blunted systolic response to stress, in addition to the structural myocardial changes that are more related to diastolic dysfunction. Hyperdynamic status resolves and the autonomic impairment improves in most transplant recipients.21, 22 The improvement of QTc prolongation can be reflective of resolution of the myocyte membrane changes, especially in relation to potassium and calcium conductance across the myocyte membrane. More important, at the structural level, diastolic dysfunction pretransplant has been attributed to increased LV stiffness resulting from myocardial hypertrophy, fibrosis, and subendothelial edema. Sodium retention has been thought to be associated with myocardial hypertrophy.23 It is unclear, however, whether this change reverses when sodium retention status is normalized post-LT. It is conceivable that these changes are time dependent, with long-standing cardiomyopathy resulting in fibrosis that does not reverse post-transplant, as opposed to less chronic changes resulting from edema, which may improve. A time-dependent increase in fibrosis may explain our observations from the aforementioned studies showing no definite improvement in diastolic function post-transplant, at least in short-term follow-up.
Are the Current Diagnostic Criteria Obsolete?
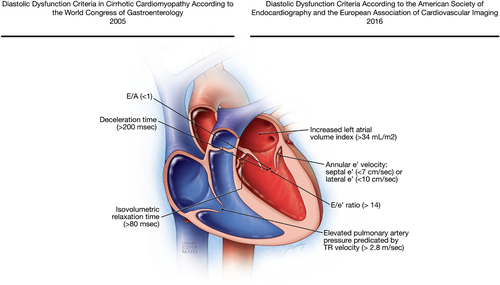
The criteria by the WCG in 2005 defined diastolic dysfunction, which is a hallmark of cirrhotic cardiomyopathy, as decreased E/A ratio, increased deceleration time, and increased isovolumetric relaxation time. These changes usually indicate that there is delayed relaxation, which is a non-specific phenomenon that can occur in any condition impacting the cardiac muscle such as hypertension or even aging. E/A ratio is mainly preload dependent and, therefore, it can change with fluid status. Thus, E/A ratio (0.8-2.0) and its configuration (with deceleration time 200 ± 20 msec) at the time of grade 2 diastolic dysfunction with mild-to-moderate increase in diastolic filling pressure appear the same (“pseudo-normalized”) as those of truly normal diastolic function.24 Therefore, there is a U-shaped phenomenon in E/A ratio. A similar U-shaped curve is observed in isovolumetric relaxation time because it increases during an early stage of diastolic dysfunction (grade 1), but gets shortened with worsening of diastolic dysfunction and increased filling pressure and may be underappreciated.
With the application of tissue Doppler imaging, which can assess the status of LV relaxation by measuring mitral annulus velocity, the evaluation of diastolic function has remarkably evolved since 2005 and additional determinants for diastolic function have been implemented. E/e′ ratio, e′ velocity, tricuspid regurgitation velocity, and LAVI have become cornerstones in the evaluation of diastolic dysfunction because of their ability to grade the diastolic dysfunction and quantify the elevation of LV filling pressure. Consequently, the most recent joint American and European echocardiography guidelines state that the four recommended variables for identifying diastolic dysfunction are annular e′ velocity: septal e′ (<7 cm/sec) or lateral e′ (<10 cm/sec), average E/e′ ratio (>14), and LAVI (>34 mL/m2) and/or elevated pulmonary artery pressure predicated by tricuspid valve regurgitation (TR) velocity (>2.8 m/sec).24 If ≥3 variables are abnormal, advanced diastolic dysfunction is present with increased filling pressure and E/A ratio determines its severity or grading: grade 3 when E/A ≥2 and grade 2 when E/A<2. In patients with mild or grade 1 diastolic dysfunction, e′ is always abnormal, and LAVI is usually, but not always, increased, but the other two parameters are normal. None of these criteria were included in the definition of cirrhotic cardiomyopathy in 2005 (Fig. 2). Thus, the diagnostic criteria for cirrhotic cardiomyopathy may be obsolete, and studies using these new criteria should be strongly encouraged.
Echocardiographic deformation imaging with LV strain measurement is a noninvasive modality that can aid in early detection of systolic and diastolic dysfunction.24 Speckled imaging determines the extent of myocardial shortening or lengthening. The most clinically useful strain measurement is global longitudinal strain (normal value is –20% ± 4%), which is more sensitive for systolic function assessment than LV ejection fraction (LVEF)25, 26 and is reduced in most patients with diastolic dysfunction. In patients with grade 1 diastolic dysfunction with normal filling pressure despite delayed myocardial relaxation, diastolic exercise test may uncover increased LV filling pressure in response to exercise.27 Exercise echocardiography will be useful to assess diastolic as well as systolic response of patients with cirrhosis. Having a thorough noninvasive method to assess cardiac function in pre-LT patients would be critical for peri- and post-operative risk prediction and prevention.
Summary
Although it is often believed that cirrhotic cardiomyopathy resolves post-LT,28, 29 the data, albeit limited, do not support this postulation. In fact, the data suggest that diastolic function may not improve post-transplant and may actually worsen. Improvement in systolic function was suggested by only two of six studies that evaluated that characteristic. The only criterion of cirrhotic cardiomyopathy that has been shown to improve post-LT is QTc prolongation, which is merely a supportive criterion. More importantly the presence of the diastolic dysfunction may be associated with adverse outcomes post-transplant. Closer follow-up and more study of cardiac function post-transplant are warranted.
Future Research Directions
With the remarkable developments in cardiac imaging, a critical revision of the criteria that define cirrhotic cardiomyopathy may be warranted. A multidisciplinary approach between cardiac imaging specialists, anesthesiologists, and hepatologists is needed to better describe unique cardiac imaging features in patients with cirrhosis compared to the general population, based on the recent advancements in echocardiography. Detailed prospective assessment of this newly defined entity will help identify patients at risk for worse outcomes post-transplant.
Potential conflict of interest
Nothing to report.
REFERENCES
Abbreviations
-
- A
-
- the peak velocity flow in late diastole
-
- AF
-
- atrial fibrillation
-
- CVEs
-
- cardiovascular events E, the peak velocity flow in early diastole
-
- EF
-
- ejection fraction
-
- HF
-
- heart failure
-
- HR
-
- hazard ratio
-
- LA
-
- left atrial
-
- LAVI
-
- LA volume index
-
- LT
-
- liver transplantation
-
- LV
-
- left ventricular
-
- QTc
-
- corrected QT interval
-
- WCG
-
- World Congress of Gastroenterology




