Favorable response to mammalian target of rapamycin inhibition in a young patient with unresectable fibrolamellar carcinoma of the liver
Potential conflict of interest: Nothing to report.
Abbreviations
-
- CT
-
- computed tomography
-
- FLC
-
- fibrolamellar carcinoma
-
- HCC
-
- hepatocellular carcinoma
-
- mTOR
-
- mammalian target of rapamycin
-
- mTORC1
-
- mammalian target of rapamycin complex 1
Fibrolamellar carcinoma (FLC) is a distinct and rare liver cancer typically occurring in young adults in the noncirrhotic liver. Its rarity impedes evidence-based treatment guidelines derived from large clinical trials, and thus case reports describing successful experimental treatments are an important instrument in improving FLC patient care. We report on 1 patient with end-stage FLC at time of diagnosis with a significant reduction in tumor mass and improvement of quality of life upon third-line palliative treatment with the mammalian target of rapamycin (mTOR) inhibitor, everolimus.
Case Presentation
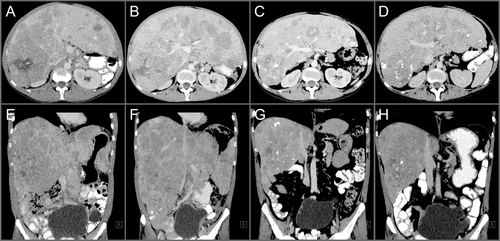
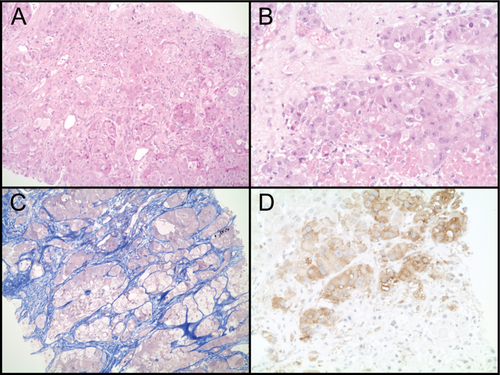
A 28-year-old man presented with fluctuating and diffuse abdominal pain. The patient was tested human immunodeficiency virus and hepatitis C virus negative, was immunized against hepatitis B virus and had no history of alcohol abuse. A computer tomography (CT) scan of the abdomen revealed hepatomegaly with diffuse multifocal infiltration of nodular lesions with extended heterogeneous calcifications and signs of central necrosis in all liver segments (Fig. 1A,E; Supporting Fig. S1A,B). Furthermore, multiple osteolytic bone lesions in the spine and left iliac wing were observed. The definitive diagnosis was established by the histopathological workup of a liver biopsy specimen displaying cytoplasma-rich carcinomatous cells with pale bodies (Fig. 2A,B), separated by lamellar fibrosis (Fig. 2C) surrounded by noncirrhotic normal liver tissue. Together with the clinical data, these findings led to the diagnosis of FLC. Furthermore, the tumor cells were positive for HepPar1, CK20, CD10, and mitochondrial antigen, but negative for CK7, TTF-1, CDX2, and RCC (data not shown). Two lines of palliative therapy, first with sorafenib and subsequently with doxorubicin, were performed with only limited success (Fig. 1B,F). Consistent with the data reporting activation of mammalian target of rapamycin complex 1 (mTORC1) signaling in FLC, reanalysis of the biopsy specimen obtained at time of diagnosis stained positive for pS6 (Fig. 2D), a surrogate marker for mTORC1 activity.1 Thus, a third-line palliative treatment with the mTOR inhibitor, everolimus, was initiated (10 mg once-daily). After 7.5 months of therapy with everolimus, CT demonstrated a marked reduction of the hepatomegaly (Fig. 1C,G). Even more important, the previously grotesquely distended abdomen, forcing the patient to walk with a significant lumbar lordosis, normalized and his quality of life dramatically improved, thus enabling the previously heavily handicapped young man to travel around southern Europe. Unfortunately, after 10 months on everolimus treatment, hepatomegaly relapsed attributed to the extensive infiltration of the liver by the tumor (Fig. 1D,H). The patient's general condition rapidly declined and he eventually developed multiorgan failure. A strategy of best supportive care was adopted and the patient died 23 months after the initial diagnosis, after a total of 14 months on everolimus treatment.
Discussion
Current treatment approaches of to FLC with a potentially curative intention in the resectable stage are based on surgery.2 In the palliative setting, attributed to the rarity of this tumor entity and thus the lack of large clinical trials, treatment approaches are often chosen based on data derived from studies including conventional hepatocellular carcinoma (HCC). However, recent genomic profiling has revealed fundamental mechanistic differences between FLC and conventional HCC, suggesting FLC being a unique tumor entity rather than a subtype of HCC.3 The DNAJB1-PRKACA chimeric transcript containing the functional catalytic domain of protein kinase A (PKA) is relatively specific for FLC.4 Although to date clinically approved inhibitors to PKA are not available, recent work has discovered mTORC1 activity in FLC based on the expression of pS6 and has proposed a causative role of reduced tuberous sclerosis complex 2 levels, an upstream negative regulator of mTORC1.1 Immunohistopathological analysis of the tumor specimen in our case indeed identified immunoreactivity to pS6, and the strong antitumor response by everolimus observed in our patient strongly suggests a central role of the mTOR pathway in certain FLCs.
In summary, we report a substantial clinical and radiological response to the mTOR inhibitor, everolimus, in a patient with end-stage FLC. Inspired by our data, we eagerly await the results of an ongoing multicenter, randomized phase II clinical trial investigating the efficacy of everolimus in patients with unresectable FLC (NCT01642186).
Acknowledgments
We are deeply grateful to the patient and his family. We thank P. Kellmann (Hepatology Unit, Department of Clinical Research, University Hospital Bern) for performing immunohistochemical analysis and N. Breakey-Flückiger (Department of Internal Medicine, Spital Emmental, Burgdorf) for critical comments on the manuscript.
REFERENCES
Author names in bold designate shared co-first authorship.




