Proton pump inhibitors, Enterococcus, and the liver, oh my!
Potential conflict of interest: Dr. Carr received grants from Intercept.
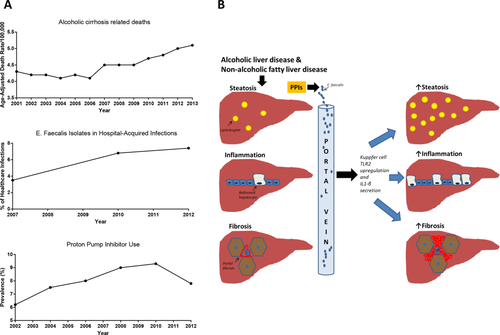
Many gastroenterologists are familiar with the goal of Healthy People 2020 to reduce deaths from colorectal cancer and increase screening by the year 2020, but fewer are likely aware that the same initiative aims to reduce deaths from cirrhosis by 10%.1 Although overall trends in this regard have been promising since 2007 (the baseline year against which cirrhosis metrics for Healthy People 2020 were set), deaths from alcoholic liver disease (ALD) have been stable to slightly increasing over the past 10 years2 (Fig. 1A). In addition, over that same time period, infections from Enterococcus faecalis have increased3-5 and proton pump inhibitor (PPI) use has only recently been declining (Fig. 1A).6 In the October 2017 issue of Nature Communications, Llorente et al. propose a possible mechanistic link among these epidemiologic trends.7 Through a series of novel experiments, the authors demonstrate that proton pump inhibition and gastric achlorhydria promote intestinal dysbiosis and subsequent hepatic inflammation in alcohol-fed mice.
Using Atp4aSl/Sl mice (a genetic model of gastric achlorhydria8), the authors examined if low gastric pH contributes to the pathogenesis of ALD. Atp4aSl/Sl mice have a point mutation in the gene encoding the gastric H+K+-adenosine triphosphatase α subunit, thus preventing intragastric acid secretion from parietal cells. Despite normal ethanol intestinal absorption and metabolism, Atp4aSl/Sl mice develop significantly more hepatic steatosis, inflammation, and fibrosis in response to ethanol compared with control-fed Atp4aSl/Sl mice and wild-type mice fed control or experimental diets. These findings occur concomitantly with an increase in intestinal Enterococcus spp. and reduction in Prevotella spp.
Compared with ethanol-fed wild-type mice, there is an abundance of enterococci in the mesenteric lymph nodes and livers of ethanol-fed Atp4aSl/Sl mice and in ethanol-fed wild-type mice receiving chronic PPI therapy, a model of gastric hypochlorhydria. In addition, the authors convincingly demonstrate that enterococci contribute to the pathogenesis of ALD. Specifically, E. faecalis collected from the feces of Atp4aSl/Sl mice and chronically instilled into the digestive tract of alcohol-fed wild-type mice increases lymphatic and hepatic E. faecalis concentrations and exacerbates ethanol-induced hepatic steatosis, inflammation, and fibrosis. Because of similar fecal albumin levels between the groups, the authors conclude that increased intestinal permeability is not the mechanism by which enterococci migrate from the intestinal lumen to the mesenteric lymph nodes. However, because of the size of albumin, differences in intestinal tight junction integrity may have been underestimated.
In order to establish the hepatic mechanisms that facilitate liver injury, the authors fed either alcohol or control diets to irradiated wild-type mice that underwent bone marrow transplants with bone marrow derived from either toll-like receptor 2 (TLR2)–deficient or myeloid differentiation primary response 88/TIR-domain-containing adapter-inducing interferon-β (MYD88/TRIF)–deficient mice. TLR2 is a cell surface receptor responsible for recognizing gram-positive bacterial cell wall components, while MYD88/TRIF is an intracellular adaptor protein complex critical for TLR signaling. Irradiation destroys Kupffer cells, and bone marrow transplantation reconstitutes this cell population with either TLR2-deficient or MYD88/TRIF-deficient Kupffer cells. Both groups of bone marrow chimeric mice are resistant to the hepatic steatosis, inflammation, and fibrosis observed with the combination of a chronic alcohol diet and chronic enterococcal gavage. Notably, MYD88/TRIF chimeras have significantly more E. faecalis bacteremia than either wild-type or TLR2 chimeras, suggesting that the mechanism by which reduced innate immunity is hepatoprotective in ALD relates to Kupffer cell function rather than reduced bacterial burden in the systemic circulation. Indeed, primary Kupffer cells isolated from Tlr−/− mice and stimulated with E. faecalis have reduced inflammatory gene expression of Il1-β, Cxcl1, and Ccl2 compared with cells isolated from wild-type mice. In addition, incubation of mouse hepatocytes with this conditioned media and ethanol increases cytotoxicity, an effect ameliorated by an anti-interleukin-1β neutralizing antibody and reproduced in vivo in mice treated with Anakinra, an interleukin-β receptor antagonist. These data deserve further exploration, however, as neither TLR2-deficient nor MYD88-deficient mice were protected from alcohol-induced liver injury in a 5-week Lieber-DeCarli alcohol pair-feeding model.9
The study by Llorenti et al. supports the concept of a gut–liver axis; however, the proposed mechanism does not appear to be specific to alcohol. For example, Atp4aSl/Sl mice on either a high-fat diet or a nonalcoholic steatohepatitis–inducing, choline-deficient, L-amino acid–deficient diet have an increase in intestinal Enterococcus spp. and reduction in Prevotella spp. along with significant hepatic steatosis, inflammation, and fibrosis compared with both control-fed Atp4aSl/Sl mice and wild-type mice fed a control or an experimental diet. Moreover, in control-fed mice, both hepatic steatosis (as measured by oil red O) and macrophage infiltration (as measured by F4/80 immunofluorescence) increase to a similar degree as in ethanol-fed mice in response to E. faecalis oral gavage.
Regardless of the specificity of their findings, the mechanistic paradigm the authors propose (summarized in Fig. 1B) is supported by epidemiologic data from a Danish health care database that includes patients who chronically abuse alcohol. Based on their analysis of these data, the authors determined that the 10-year risk of developing ALD is highest for those actively using PPIs (20.7%) and lowest for those who have never used PPIs (12.4%). Prior PPI users have an intermediate risk of developing ALD (16.1%). This dose–response relationship between PPI use and ALD development is further bolstered by the greater numbers of Enterococcus in the feces of patients who abuse alcohol and are on PPIs compared with those who are not on PPIs.
Overall, this study advances our mechanistic understanding of how hypochlorhydria promotes liver injury, but where do we go from here? Increasingly, both preclinical and clinical data demonstrate that providers should exhibit that providers exhibit caution when prescribing PPIs to patients with cirrhosis. Until we know more, perhaps the best strategy is to ensure appropriate indications for PPI use and use the lowest PPI dose possible for the shortest duration of time in patients with chronic liver disease of any etiology.
-
Rotonya M. Carr, M.D.
-
Division of Gastroenterology
-
University of Pennsylvania
-
Philadelphia, PA
REFERENCES
Author names in bold designate shared co-first authorship.




