Khat chewing increases the risk for developing chronic liver disease: A hospital-based case–control study
Potential conflict of interest: Nothing to report.
Supported by grants from the Norwegian Research Council (220622/H10) and Helse Sør-Øst RHF (the South-Eastern Norway Regional Health Authority) (2011068).
Abstract
The chewing of the leaves of Catha edulis (khat) has been implicated in the development of liver disease, but no controlled observations have been undertaken. The objective of the present study was to determine whether khat chewing is associated with development of chronic liver disease (CLD). A case–control study was conducted at two public hospitals in Harar, Ethiopia, between April 2015 and April 2016. A consecutive sample of 150 adult hospital attendees with CLD were included as cases, and 300 adult hospital attendees without clinical or laboratory evidence of CLD were included as controls. Khat consumption was quantified in “khat years”; 1 khat year was defined as daily use of 200 g of fresh khat for 1 year. A logistic regression model was used to control for confounders. There was a significant association between chewing khat and the risk for developing CLD (crude odds ratio, 2.64; 95% confidence interval [CI], 1.56-4.58). In men, this risk, following adjustment for age, alcohol use, and chronic hepatitis B/C infection, increased with increasing khat exposure; thus, compared to never users the adjusted odds ratios were for low khat exposure 3.58 (95% CI 1.05-12.21), moderate khat exposure 5.90 (95% CI 1.79-19.44), and high khat exposure 13.03 (95% CI 3.61-47.02). The findings were robust in a post hoc sensitivity analysis in which individuals with identifiable risk factors for CLD were excluded. Conclusion: A significant association was observed between chewing khat and the risk for developing CLD, and in men the association was strong and dose-dependent, suggesting a causal relationship; as the prevalence of khat chewing is increasing worldwide, these findings have major public health implications. (Hepatology 2018;68:248-257).
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- AOR
-
- adjusted odds ratio
-
- AP
-
- attributable proportion
-
- CI
-
- confidence interval
-
- CLD
-
- chronic liver disease
-
- CYP
-
- cytochrome P450
-
- HBsAg
-
- hepatitis B surface antigen
-
- HCV
-
- hepatitis C virus
-
- OR
-
- odds ratio
The leaves and shoots of the evergreen shrub Catha edulis (khat) are chewed to reduce fatigue, to increase performance, and for the pleasurable effects, which include euphoria, loquacity, and excitement. Khat chewing is common in the Horn of Africa, the Arabian Peninsula, and the East Coast of Africa, where it has been part of the social and cultural heritage for centuries.1 Over the past three decades khat has become increasingly available worldwide and its use perpetuated and even adopted by the wider diaspora.2 The global prevalence of khat chewing is unknown; the proposed figure of 20 million daily users is probably an underestimate.3
Khat leaves, typically 100-300 g, are chewed in sessions lasting several hours; and the juice of the masticated leaves is swallowed. Alkaloids, of which cathinone (aminopropiophenone) is the most important, are extracted during chewing; the buccal mucosa plays a major role in the absorption.4 The state of alertness and euphoria associated with khat usage is most likely induced by cathinone, which exerts effects on the central nervous system similar to those of amphetamine.4, 5
The World Health Organization has defined khat as a drug of abuse as it may lead to health and social problems. Chronic khat use is associated with psychological adverse effects, including psychosis and exacerbation of preexisting psychotic disorders.6 Khat users may also exhibit dependence; however in the majority of users the degree of associated physical dependence is low, although the levels of psychological dependence may be substantial.5
Khat chewing is also associated with a number of somatic health sequelae, including myocardial infarction, systemic hypertension, upper gastrointestinal cancers, cognitive impairment, and impaired fetal growth.7 Khat has also been implicated in the development of both acute hepatitis8-12 and chronic liver disease (CLD)13-16 in several case series. Chapman et al. reported on 6 patients of Somali origin, living in the United Kingdom, in whom khat abuse was implicated in the development of fulminant hepatic failure.12 Stuyt et al. reported unexplained CLD in 6 male immigrants from Somalia and Ethiopia living in The Netherlands and noted their history of chronic khat use.14 However, because khat is illegal in Europe, North America, and Australia and the users are mainly from closed immigrant communities, no controlled observations are available.
Decompensated CLD is one of the most frequent reasons for admission to medical wards in eastern Ethiopia; in >50% of the cases the liver disease is “unexplained.”16 Khat chewing is widespread throughout the country but more especially in the eastern regions where khat cultivation predominantly takes place.17, 18 The number of people chewing khat has increased rapidly in recent years with the habit gaining popularity in all segments of the community; hence, the overall prevalence of khat chewing in Ethiopia is estimated at 15.3% but with wide regional variations.18 Although khat chewing is traditionally a male habit, a recent community-based study in pregnant women in eastern Ethiopia reported a prevalence of khat usage of 34.6%.19
The aim of the present study was to determine the association between use of khat and the risk for developing significant CLD in eastern Ethiopia, using a case–control design exercising controls for potential confounding variables.
Participants and Methods
STUDY SETTING AND PARTICIPANTS
This prospective, hospital-based, case–control study was undertaken between April 2015 and April 2016 in Harar, eastern Ethiopia. Inpatients and outpatients attending two large public hospitals, the Jugal Hospital and the Hiwot Fana Specialized University Hospital, were eligible for inclusion. Medical subspecialty services are not available in either hospital; thus, all patients with CLD attend a general medical outpatient clinic and, if admitted, are housed on a general medical ward.
Cases comprised adult (≥18 years of age) outpatients and inpatients with a new diagnosis of CLD defined, for the purposes of this study, as (1) the presence of clinical features suggestive of decompensated liver disease (viz. ascites, jaundice, and/or hepatic encephalopathy) and (2) the presence, on ultrasound, of hepatic parenchyma heterogeneity and/or surface irregularity. Patients presenting with severe acute hepatitis, defined as liver injury of <6 weeks duration, serum alanine aminotransferase (ALT) activity of >100 U/L, and the absence of coarsened echotexture and surface irregularity on ultrasonography, were excluded. Also excluded were patients with a previously established diagnosis of CLD or with liver dysfunction secondary to alternative conditions (viz. congestive cardiac failure, hepatic malignancies, biliary obstruction, and septicemia).
As khat use is associated with a number of adverse medical and psychiatric sequelae, controls could not be selected from among the general medical or psychiatric populations. Controls therefore comprised adult hospital outpatients or inpatients under the care of the ophthalmology, dermatology, or surgical service, none of whom had a history or clinical evidence of CLD or a serum ALT above the upper limit of the laboratory reference range of 40 U/L.
PATIENT ASSESSMENT
All patients were assessed using standardized checklists and semistructured interviews to gather information on predetermined demographic and clinical variables. Interviews were conducted by local nurses fluent in the native language of study participants, and interviewers were blinded to the disease status of interviewees. Neither study subjects nor interviewers were told that the study hypothesis was of a likely association between khat chewing and the development of CLD.
Information on the use of khat was obtained and quantified in grams using a visual analogue scale. The frequency of khat chewing was categorized using the Drug Use Disorders Identification Test.20 The frequency and duration of khat usage were used to classify lifetime khat exposure in “khat years,” with 1 khat year defined by daily use of 200 g of fresh khat for 1 year. Exposed subjects were defined as all past or current khat users, regardless of the amount chewed. Those who had never used khat were defined as “never users.”
Information on the frequency and quantity of previous and current alcohol use was obtained using a frequency/quantity questionnaire; average daily intake was quantified in grams using the following equation:
Alcohol concentration by volume × 0.78 × volume consumed (mL)/100
where alcohol concentration by volume is the percentage alcohol content of the local alcoholic beverages21 and 0.78 is the specific gravity of alcohol. Alcohol abuse was defined as consumption of >20 g/day for women and >30 g/day for men.22 Exposed subjects were defined as past or current users of alcohol, regardless of the amount consumed.
Clinical examination was conducted after the patient interview using a prespecified standard. Particular note was made of any features suggestive of CLD including cutaneous and peripheral liver stigmata and features of hepatic decompensation.
LABORATORY TESTS
Blood was collected by venipuncture, and the serum and plasma were separated within 2 hours and aliquots stored at –20°C until analyzed. Routine blood tests including serum ALT and aspartate aminotransferase activities were analyzed using a semiautomatic biochemistry analyzer, Dirui DR-7000D (DIRUI, Changchun, China) and HumaLyzer 3000 (HUMAN, Wiesbaden, Germany). Hepatitis B surface antigen (HBsAg) screening was undertaken locally using the World Health Organization–approved rapid diagnostic test Determine (Alere, Waltham, MA); hepatitis C virus (HCV) antibody screening was undertaken locally using the World Health Organization–approved rapid diagnostic test SD BIOLINE HCV (Standard Diagnostics, Yongin-si, Republic of Korea). All sera were subsequently transported on ice to Aklilu Lemma Institute of Pathobiology in Addis Ababa for confirmatory testing of HBsAg and HCV antibody using an enzyme-linked immunosorbent assay method (Elisys Uno, HUMAN; or Architect, Abbott Diagnostics, IL). Discrepancies between rapid tests and enzyme-linked immunosorbent assay results were resolved using a second enzyme-linked immunosorbent assay (Architect; or Bio-Rad, Hercules, CA).
ABDOMINAL IMAGING
Abdominal ultrasonography was undertaken, to a predetermined standard, by a local radiologist using a 3.5-MHz convex transducer (Aloka Flexus SSD-1100; Aloka, Tokyo, Japan). The diagnosis of CLD was based on the presence of an irregular liver surface and/or liver parenchyma heterogeneity.23 Other features suggestive of the presence of CLD, such as ascites, splenomegaly, and a collateral circulation, were also noted and recorded.
SAMPLE SIZE CALCULATION
A sample size estimation was performed a priori based on the inclusion of 2 controls per case and the conventional type I error of 5% and power of 80%. Based on an estimated prevalence of daily khat use of 20%24 and the assumption that khat use would be at least twice as common in cases as in controls (odds ratio [OR], 2.00), a minimum of 137 cases and 274 controls would be needed for an adequately powered study. Individuals with other risk factors for CLD were retained in the main analysis because it was assumed that khat chewing could act as either a sole or an adjuvant cause of liver injury.
STATISTICAL METHODS
Categorical variables were summarized as frequencies and continuous variables by the median and interquartile range. Comparisons between cases and controls were performed using the Mann-Whitney U test for continuous variables and the Pearson chi-squared test for categorical variables.
An explanatory strategy investigating the association between khat chewing and CLD was undertaken and quantified as OR with its 95% confidence interval (CI).25 An initial stratified analysis evaluated effect modification (interaction) and confounding by other variables considered in the protocol. A Breslow and Day test of homogeneity between strata was performed to pinpoint effect modification (interaction). Confounding was controlled univariately using the Mantel-Haenszel method. The magnitude of the confounding effect was evaluated by comparing the crude OR and the adjusted Mantel-Haenszel OR. When cells contained zero counts, a value of 0.5 was added to each cell frequency before calculating the stratum-specific OR and the Mantel-Haenszel summary OR. A logistic regression model was used to control for multiple confounders and the presence of effect modification.25 The relationship between levels of khat exposure and the risk for developing CLD was explored by (1) using a chi-squared test for trend with khat exposure categorized by khat year quartiles and (2) using the per unit increase in khat years as a continuous variable in the logistic regression model.
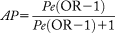
A post hoc sensitivity analysis was performed excluding all cases of CLD with an identifiable etiology after a comprehensive panel of tests, which included parasitology and serological testing for viral, autoimmune, and genetic liver diseases.16 Controls did not undergo the same comprehensive panel of tests or abdominal ultrasound, but all had serum ALT activities <40 U/L and were screened for the risk factors assumed a priori to be the most relevant (viz. alcohol abuse and viral hepatitis); those with a history of alcohol abuse or evidence of chronic hepatitis B and C infection were excluded from the sensitivity analysis.
Statistical analyses were performed in STATA 14.0 (StataCorp, College Station, TX). The study was compliant with the Strengthening the Reporting of Observational Studies in Epidemiology statement guidelines.27
ETHICS
The study was conducted in accordance with the Declaration of Helsinki28 and approved by the National Research Ethics Review Committee (ref. no. 3.10/829/07) in Ethiopia and by the Regional Committees for Medical and Health Research Ethics (ref. no. 2014/1146) in Norway. Written informed consent was obtained from all study subjects.
Results
CHARACTERISTICS OF THE STUDY POPULATION
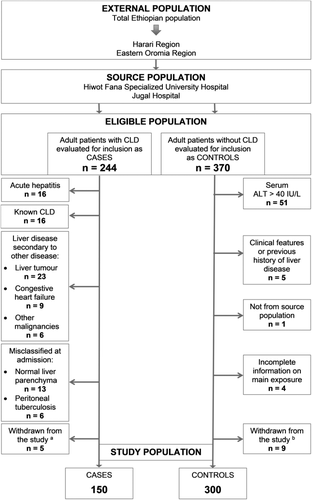
In total 244 potential cases and 370 controls were screened for inclusion. The final study population comprised 150 cases with CLD and 300 controls without liver disease (Fig. 1). Of these, 31 (20.7%) cases and 265 (88.3%) controls were outpatients, while 119 (79.3%) cases and 35 (11.7%) controls were inpatients. Cases were significantly more likely than controls to be male, Muslim, nondrinkers of alcohol, and HBsAg-positive and to use khat (Table 1).
| Characteristic |
Cases (n = 150) |
Controls (n = 300) |
P |
|---|---|---|---|
| Men | 108 (72.0) | 172 (57.3) | 0.002 |
| Age (years) | 30 (25-40) | 30 (24-52) | 0.125 |
| Religion | <0.001 | ||
| Islam | 139 (92.7) | 198 (66.0) | |
| Christianity | 11 (7.3) | 102 (34.0) | |
| History of alcohol consumption | <0.001 | ||
| Never | 139 (92.7) | 233 (77.7) | |
| Current | 6 (4.0) | 52 (17.3) | |
| Stopped | 5 (3.3) | 15 (5.0) | |
| Alcohol abusea | 3 (2.0) | 9 (3.0) | 0.758 |
| History of khat use | 127 (84.7) | 203 (67.7) | <0.001 |
| Khat yearsb | 20 (3-70) | 2 (0-20) | <0.001 |
| Men | 36 (6-75) | 10 (0-40) | |
| Women | 0.6 (0-7) | 0.1 (0-5) | |
| HBsAg-positive | 55 (36.7) | 22 (7.3) | <0.001 |
| HCV antibody–positive | 2 (1.3) | 0 (0.0) | 0.111 |
- Data are presented as number (%) or as median (interquartile range).
- a Defined as consumption of >20 g alcohol/day in women and >30 g/day in men.
- b One khat year is defined by daily use of 200 g of fresh khat for 1 year.
Men were more likely than women to be HBsAg-positive, among both cases (41.7% versus 23.8%; P = 0.042) and controls (9.9% versus 3.9%; P = 0.049). Moreover, the proportion reporting current or previous khat use was significantly higher among men than women (cases, 96.3% versus 54.8%; P < 0.001; controls, 77.9% versus 53.9%; P < 0.001). The median level of khat exposure was also significantly higher in men compared to women (Table 1).
ASSOCIATION BETWEEN KHAT EXPOSURE AND CLD
In univariable analysis, there was a significant association between chewing khat and the risk for developing CLD (crude OR, 2.64; 95% CI, 1.56-4.58; P < 0.001). The magnitude of the risk estimate was different in men and women (Table 2). Chronic hepatitis B virus infection had a considerable confounding effect on the risk estimate (18%), whereas the effects of age (2%) and alcohol consumption (3%) were minimal. The variable HCV infection contained too few positive results to be included in both stratified and multivariable analyses.
|
Cases (n = 150) |
Controls (n = 300) |
||||||
|---|---|---|---|---|---|---|---|
| Khat exposure | Khat exposure | OR | ORMH | Breslow and Day test of homogeneity | |||
| Characteristic | Yes | No | Yes | No | (95% CI) | (95% CI) | (P) |
| Crude (n) | 127 | 23 | 203 | 97 | 2.64 (1.56-4.58) | ||
| Sex | |||||||
| Men | 104 | 4 | 134 | 38 | 7.37 (2.52-29.18) | a | 0.001 |
| Women | 23 | 19 | 69 | 59 | 1.04 (0.49-2.22) | ||
| Age | |||||||
| 18-29 years | 47 | 10 | 71 | 57 | 3.77 (1.68-9.07) | 2.57 (1.55-4.28) | 0.177 |
| ≥30 years | 80 | 13 | 132 | 40 | 1.86 (0.91-4.03) | ||
| Alcohol consumption | |||||||
| Yes | 10 | 1 | 42 | 25 | 5.95 (0.75-268.43) | 2.57 (1.54-4.30) | 0.396 |
| No | 117 | 22 | 161 | 72 | 2.38 (1.36-4.26) | ||
| HBsAg | |||||||
| Positive | 50 | 5 | 18 | 4 | 2.22 (0.39-11.51) | 2.16 (1.27-3.67) | 0.966 |
| Negative | 77 | 18 | 185 | 93 | 2.15 (1.19-4.04) | ||
| HCV antibody | |||||||
| Positive | 1 | 1 | 0 | 0 | b | ||
| Negative | 126 | 22 | 203 | 97 | |||
- a Cannot be calculated because sex is an effect modifier of khat use in chronic liver disease.
- b Cannot be calculated since none of the controls are anti-HCV positive.
- Abbreviation: ORMH, Mantel-Haenszel summary OR estimate.
The final multivariable analysis showed that the effect of khat on the risk for developing CLD was dependent on its interaction with sex when adjusting for age, alcohol use, and chronic hepatitis B virus infection (interaction khat × sex; P = 0.013). The effect was strong in men (adjusted odds ratio [AOR], 5.67; 95% CI, 1.85-17.37; P = 0.002) but not evident in women (AOR, 1.04; 95% CI, 0.49-2.19; P = 0.922).
An upward trend in the risk for developing CLD with increasing khat exposure was found after adjusting for hepatitis B virus infection, alcohol use, and age; this was indicative of a dose–response relationship in men but not in women (Table 3). When khat exposure was employed as a continuous variable in the logistic regression model, the findings pointed in the same direction; per 1 khat year increment, the odds of CLD was significantly increased in men (AOR, 1.007; 95% CI, 1.001-1.013; P = 0.019) but not in women (AOR, 1.012; 95% CI, 0.998-1.027; P = 0.102).
|
Men (n = 280) |
Women (n = 170) |
|||||
|---|---|---|---|---|---|---|
|
Cases (n = 108) |
Controls (n = 172) |
AOR (95% CI)a |
Cases (n = 42) |
Controls (n = 128) |
AOR (95% CI)a | |
| Khat years, by quartilesb | ||||||
| Q1: 0 | 4 | 40 | 1.0 (reference) | 19 | 61 | 1.0 (reference) |
| Q2: 0.1-5.0 | 21 | 40 | 3.58 (1.05-12.21) | 11 | 37 | 1.21 (0.48-3.06) |
| Q3: 5.1-40.0 | 34 | 51 | 5.90 (1.79-19.44) | 7 | 22 | 0.98 (0.32-3.00) |
| Q4: 40.1-250 | 49 | 41 | 13.03 (3.61-47.02) | 5 | 8 | 1.97 (0.46-8.45) |
| P for trend | <0.001 | 0.801 | ||||
- a Adjusted for the confounding effect of age, alcohol consumption, and chronic hepatitis B infection.
- b One khat year is defined by daily use of 200 g of fresh khat for 1 year.
PROPORTION OF CLD ATTRIBUTABLE TO KHAT EXPOSURE
Assuming that the relationship between khat exposure and the risk for developing CLD is causal, more than half of the CLD cases in this study population were attributable to khat usage (AP, 52.6%; 95% CI, 33.1-72.0). This effect showed a marked difference in relation to sex; in men, the AP was 83.2% (95% CI, 66.4-100), while in women it was only 1.9% (95% CI –35.6 to 39.3).
SENSITIVITY ANALYSIS
Seventy (46.7%) of the 150 cases and 31 (10.3%) of the 300 controls had documented risk factors for CLD other than khat use (Supporting Fig. S1). The remaining 80 (53.3%) cases and 269 (89.7%) controls had no identifiable risk factors for CLD except that 66 (82.5%) and 178 (66.2%), respectively, were regular khat users (Supporting Table S1).
The significant association between khat use and the risk for developing CLD was robust in this subpopulation of 80 cases and 269 controls (crude OR, 2.41; 95% CI, 1.25-4.90; P = 0.005). The sex differences were slightly more pronounced than in the total cohort (Supporting Table S2). No other significant confounders were identified.
The upward trend in the risk for developing CLD with increasing khat exposure observed in the primary analysis persisted in the sensitivity analysis, with findings indicative of a dose–response relationship in men but no such relationship in women (Supporting Table S3). Similarly, the findings from the primary analysis when khat exposure was analyzed as a continuous variable were reproduced in the sensitivity analysis; per 1 khat year increment, the odds of CLD was significantly increased in men (AOR, 1.008; 95% CI, 1.001-1.015; P = 0.034) but not in women (AOR, 1.014; 95% CI, 0.997-1.031; P = 0.108).
Discussion
A strong and significant association was observed in the present study between chewing khat and the risk for developing CLD. No risk factors for liver injury were identified in 53% of the cases with CLD despite extensive investigations, except that >80% of them used khat. Additionally, although confined to men, a clear dose–response relationship was observed between khat exposure and the associated risk for CLD. In previous case reports, resolution of the liver injury has been recorded following cessation of khat, while relapse following reexposure has also been documented.9, 12, 13 Moreover, khat has been shown to be hepatotoxic in animals, manifesting as a spectrum of liver injury.29-31 Evidence from the present study, together with previous case reports and animal studies, supports a strong association and suggests a causal relationship between khat chewing and the development of CLD.
The mechanism of the khat-related hepatotoxicity is unknown, but several plausible biological explanations have been proposed. First, although there were no compelling features of an autoimmune process in the cases in the present study, previous case reports have documented low titers of autoantibodies and histological features reminiscent of an autoimmune hepatitis in patients suspected of having khat-related liver injury.9, 10, 32 Second, the khat-related alkaloids are metabolized extensively in the liver by the enzyme cytochrome P450 2D6 (CYP2D6) and have short half-lives.4, 33 The finding in one case series of very high concentrations of khat alkaloids in a sample of explanted liver many weeks after the last exposure to khat suggests that accumulation of khat and/or its metabolites may be important.12 Thus, polymorphisms in the gene controlling CYP2D6 may play a role in determining individual susceptibility to khat-related liver injury, as may possession of variants in other genes implicated in the risk for developing CLD per se.34 Third, the possibility that the liver injury might relate not to khat itself but to contaminants such as herbicides and pesticides or to contamination with heavy metals or toxogenic fungi has to be considered but is thought to be unlikely.35, 36 Rodents given diets containing uncontaminated khat leaves showed elevated liver enzymes and necroinflammatory change on histology, supporting the contention that the natural substances contained in khat leaves are responsible for the hepatic injury.29, 30 Finally, khat has been shown to trigger generation of reactive oxygen species in human cells in vitro, resulting in hepatocyte apoptosis.37 Similar histological features have been described in humans following ingestion of ecstasy, another amphetamine-related drug.38
There is no obvious explanation for the observed sex differences in the susceptibility to khat-related liver injury observed in the present study. Khat usage was generally lower in women than men, but even women with moderate-level or high-level khat exposure did not seem to be at significantly increased risk of CLD. This apparent differential susceptibility to khat-related hepatotoxicity could be explained by one or more of the following: (1) the levels of exposure in women may not reach the threshold for toxicity; (2) there are sex differences in chewing habits which might influence the duration of exposure, with men spending concentrated blocks of recreational time chewing khat—and hence the duration of exposure is prolonged—while women tend to chew khat intermittently so that the duration of overall exposure is much shorter; (3) exposure to certain dietary or environmental agents may result in either induction or inhibition of CYP2D6 activity, causing changes in khat metabolism; sex-specific differences in exposure to these agents may, therefore, play a role. It is also possible that there are sex-specific differences in the number of copy variants of CYP2D6 or differences in the frequency of variant single-nucleotide polymorphisms. None of these possibilities, however, can be addressed using the data from the present study.
This study had a number of strengths. First, data were collected over a 1-year period, thereby controlling for possible seasonal variations in khat availability and hence exposure. Second, by using newly diagnosed cases, the exposure–disease relationship was less likely to be influenced by altered risk habits, lifestyle change, or other interventions based on previous medical advice.25 Finally, there were no missing data either for the main exposure variables or for the potential confounders.
The study also had its limitations. First, selection bias cannot be excluded; an unknown proportion of patients with CLD may not have been seen by the recruiting medical services for a variety of practical, cultural, and socioeconomic reasons. In addition, the decision to use hospital patients as controls might have introduced Berkson's bias25 as their attendance could have been affected by both exposure and disease. An attempt was made to minimize this risk by the selection of control subjects from a range of hospital departments and not from specialties dealing with illnesses known to be associated with khat usage.7, 39, 40 However, this may inadvertently have resulted in introduction of possible bias as the majority of the controls were outpatients and the majority of the cases inpatients, and we cannot be sure whether this potential bias goes toward or away from the null.
Second, as in any case–control study, information bias cannot be excluded. Underreporting or denial of alcohol consumption or other recreational drugs is common in observational studies and may result in an underestimation of the degree of confounding. The fact that the cases in the present study reported less exposure to alcohol may suggest information bias, but it more likely reflects the fact that the majority of the cases were Muslims compared with only two thirds of the controls. Of note, the 2016 Ethiopian Demographic and Health Survey reported a prevalence of abstinence from alcohol of 86.6% in the Harari region,41 and thus the high abstinence rate in the present study seems representative. Although data from Ethiopia are scarce, the small proportion of people with alcohol-related CLD in the present study is in line with previous findings.42, 43 The use of khat in eastern Ethiopia, on the contrary, is legal and socially accepted, and its usage is less likely to be underreported in this context. Because neither the study subjects nor the interviewers were informed about the primary aim of this study, any misclassification of khat exposure is likely to be nondifferential, so the observed effect of khat on the development of CLD would, if anything, be underestimated.25
Third, abdominal ultrasound was not performed in the controls, and hence, some may have had undiagnosed CLD. However, undiagnosed cirrhosis is rare in the general population, usually <1% of adults in population-based studies,44, 45 so it is unlikely that this would have affected our results significantly.
Fourth, power deficiency might be one explanation why women appeared less susceptible to khat-related liver injury as relatively few women reported high-level khat exposure.
Finally, we cannot exclude residual confounding by factors not accounted for in our analysis, such as cigarette smoking, coffee intake, the use of traditional herbal remedies, and exposure to dichlorodiphenyltrichloroethane and other potentially hepatotoxic pesticides through consumption of unwashed khat leaves.46, 47
In conclusion, a strong and significant association was observed between khat chewing and CLD, strengthening the hypothesis that khat is implicated in the development of CLD. In men, the association was strong and dose-dependent, suggesting a causal relationship. This study identified khat-associated CLD that may be responsible for a significant proportion of the liver disease observed in countries where khat use is widespread. As the prevalence of khat chewing is expanding within the wider diaspora, these findings have important public health implications.
Acknowledgment
We acknowledge the help, support, and expertise of the hospital staff at the Jugal Hospital and the Hiwot Fana Specialized University Hospital in Ethiopia, in particular the laboratory technicians, radiologists, and physicians. We also thank the staff at the Harari Health Research and Regional Laboratory and the Aklilu Lemma Institute of Pathobiology in Ethiopia and the Department of Medical Biochemistry at Drammen Hospital and the Department of Virology at the Norwegian Institute of Public Health in Norway for their help and dedication. We are grateful for the support received from the Harari Regional Health Bureau and the Haramaya University College of Health and Medical Sciences in Ethiopia. Finally, we are indebted to the patients who participated so willingly in the study.




