Liver-specific deficiency of unc-51 like kinase 1 and 2 protects mice from acetaminophen-induced liver injury
Potential conflict of interest: Nothing to report.
Supported by grants from National Natural Science Foundation of China (#31430094, #31690101 and #31600961), the National Key R & D Program of China (2016YFA0502001), the Fundamental Research Funds for the Central Universities (20720160058), and XMU Training Program of Innovation and Entrepreneurship for Undergraduates (2017Y0578).
Abstract
unc-51-like autophagy activating kinase 1 and 2 (Ulk1/2) regulate autophagy initiation under various stress conditions. However, the physiological functions of these Ser/Thr kinases are not well characterized. Here, we show that mice with liver-specific double knockout (LDKO) of Ulk1 and Ulk2 (Ulk1/2 LDKO) are viable, but exhibit overt hepatomegaly phenotype. Surprisingly, Ulk1/2 LDKO mice display normal autophagic activity in hepatocytes upon overnight fasting, but are strongly resistant to acetaminophen (APAP)-induced liver injury. Further studies revealed that Ulk1/2 are also dispensable for APAP-induced autophagy process, but are essential for the maximum activation of c-Jun N-terminal kinase (JNK) signaling both in vivo and in isolated primary hepatocytes during APAP treatment. Mechanistically, APAP-induced inhibition of mechanistic target of rapamycin complex 1 releases Ulk1 from an inactive state. Activated Ulk1 then directly phosphorylates and increases the kinase activity of mitogen-activated protein kinase kinase 4 and 7 (MKK4/7), the upstream kinases and activator of JNK, and mediates APAP-induced liver injury. Ulk1-dependent phosphorylation of MKK7 was further confirmed by a context-dependent phosphorylation antibody. Moreover, activation of JNK and APAP-induced cell death was markedly attenuated in Mkk4/7 double knockdown hepatocytes reconstituted with an Ulk1-unphosphorylatable mutant of MKK7 compared to those in cells rescued with wild-type MKK7. Conclusion: Together, these findings reveal an important role of Ulk1/2 for APAP-induced JNK activation and liver injury, and understanding of this regulatory mechanism may offer us new strategies for prevention and treatment of human APAP hepatotoxicity. (Hepatology 2018;67:2397-2413).
Abbreviations
-
- ActD
-
- actinomycin D
-
- AILI
-
- APAP-induced liver injury
-
- ALT
-
- alanine aminotransferase
-
- ANOVA
-
- Analysis of Variance
-
- APAP
-
- acetaminophen
-
- ASK1
-
- apoptosis signal-regulating kinase 1
-
- Atg
-
- autophagy-related gene
-
- cDNA
-
- complementary DNA
-
- Ctrl
-
- control
-
- CIP
-
- calf-intestinal alkaline phosphatase
-
- CYP2E1
-
- cytochrome P450 2E1
-
- DKD
-
- double knockdown
-
- DMSO
-
- dimethyl sulfoxide
-
- FBS
-
- fetal bovine serum
-
- FIP200
-
- focal adhesion kinase family kinase-interacting protein of 200 kDa
-
- GalN
-
- galactosamine
-
- GCLC
-
- catalytic subunit of glutamate-cysteine ligase
-
- GCLM
-
- modulatory subunit of glutamate-cysteine ligase
-
- GSH
-
- glutathione
-
- HA
-
- hemagglutinin
-
- H&E
-
- hematoxylin and eosin
-
- IP
-
- immunoprecipitation
-
- JNK
-
- c-Jun N-terminal kinase
-
- KI
-
- kinase inactive
-
- LC3B
-
- light chain 3 beta
-
- LDKO
-
- liver-specific double knockout
-
- mTORC1
-
- mechanistic target of rapamycin complex 1
-
- MAPK
-
- mitogen-activated protein kinase
-
- MKK4/7
-
- MAPK kinase 4 and 7
-
- MAP3K
-
- mitogen-activated protein kinase kinase kinase
-
- MKK
-
- mitogen-activated protein kinase kinase
-
- MLK3
-
- mixed-lineage protein kinase 3
-
- NAPQI
-
- N-acetyl-p-benzoquinone imine
-
- NEFA
-
- nonesterified fatty acid
-
- Nrf2
-
- nuclear factor (erythroid-derived 2)-like 2
-
- PBS
-
- phosphate-buffered saline
-
- PCNA
-
- proliferating cell nuclear antigen
-
- PI
-
- propidium iodide
-
- ROS
-
- reactive oxygen species
-
- S6K
-
- S6 kinase
-
- Sab
-
- SH3 domain-binding protein 5 (SH3BP5)
-
- shRNA
-
- short hairpin RNA
-
- TAG
-
- triacylglycerol
-
- TNF-α
-
- tumor necrosis factor alpha
-
- Ulk
-
- unc-51 like kinase
-
- Ulk1/2
-
- unc-51-like autophagy activating kinase 1 and 2
-
- WT
-
- wild type
Acetaminophen (APAP) is one of the most commonly used drugs with analgesic and antipyretic properties.1 When taken at proper therapeutic doses (350-600 mg every 4-6 hours, with a daily maximum of 4 g for an adult), APAP has an excellent safety profile. However, hepatotoxicity occurs when APAP is taken at overdose or misused by at-risk individuals.2-4 In the United States, with approximately 50 million adults taking some form of APAP each week, APAP toxicity has replaced viral hepatitis as the most common cause of acute hepatic failure.5 The mechanism of hepatic injury caused by APAP overdose is complicated.4, 6 A significant number of early studies showed that rapid depletion of intracellular glutathione (GSH) by the APAP reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI), may cause oxidative stress and ultimately lead to hepatic necrosis and liver injury.7-9 In addition to GSH depletion, other events, such as macrophage activation, nitrotyrosine formation, and mitochondrial dysfunction, have also been shown to contribute to the development of liver damage in response to APAP stimulation.9 However, after more than 30 years of intensive research, the precise mechanism of APAP hepatotoxicity is still not well understood.2, 4
c-Jun N-terminal kinase (JNK) is a member of the mitogen-activated protein kinase (MAPK) family that plays major roles in integrating extracellular and intracellular stress signals that affect cell differentiation, proliferation, survival, and migration.10-12 Activated by the upstream MAPK kinase (MKK) 4 and 7 (MKK4/7), the JNK signaling pathway is stimulated by a diverse range of stresses such as ultraviolet radiation, reactive oxygen species (ROS), and pro-inflammatory cytokines, leading to phosphorylation of many specific effectors, including c-Jun, activating transcription factor 2, and the activating protein 1 transcription factors, to regulate diverse biological functions.10 Several studies have also revealed the JNK pathway as a determining signaling route downstream of APAP metabolism to promote hepatotoxicity.3, 13, 14 It was found that JNK was activated by phosphorylation after APAP treatment in mouse liver, and depletion of JNK by short hairpin RNA (shRNA)-mediated knockdown almost completely protects mice from APAP-induced liver damage.13, 14 Interestingly, knockdown or inhibition of JNK in APAP-treated mice did not provide protection against GSH depletion in liver,13 indicating that GSH depletion is likely not the direct cause for APAP toxicity.2-4
Autophagy is an evolutionarily conserved self-digesting process by which cytoplasmic proteins and organelles are sequestered inside double-membrane vesicles (known as autophagosome) and delivered to the lysosome for degradation under nutrient-poor or other stress conditions.15, 16 Dysregulation of autophagy is associated with diverse human diseases, such as cancers and neurodegeneration.17 Genetic delineation of the critical components for autophagy has identified a variety of autophagy-related genes (ATGs).18-20 Among these ATG-encoded proteins, the Atg1/Ulk (unc-51 like kinase) complex is one of the most upstream units for autophagy induction in both yeast and mammalian cells21, 22 by integrating multiple signaling pathways, including mechanistic target of rapamycin complex 1 (mTORC1) and AMP-activated protein kinase.23-25 In mammals, besides Ulk1/2, several other subunits, including the adaptor protein focal adhesion kinase family kinase-interacting protein of 200 kDa (FIP200), ATG101, and ATG13, belong to the Ulk complex, and depletion of any of these subunits would render unc-51-like autophagy activating kinase 1 and 2 (Ulk1/2) inactive.26-28
Likely attributed to functional redundancy of Ulk1 and Ulk2, mice with either Ulk1 or Ulk2 knockout were viable and showed no aberrant phenotypes except that Ulk1–/– mice exhibit a mild autophagy defect in the clearance of mitochondrial and ribosome during reticulocyte maturation.29, 30 However, similar to Atg3, Atg5, Atg7, Atg9, and Atg16l1-knockout mice,31 mice with whole-body double knockout of Ulk1 and Ulk2 die shortly after birth,27 which therefore restricted the study of the physiological functions of these two kinases in adult animals.
Here, by utilizing molecular, biochemical, cell biology, and genetic approaches, we have identified Ulk1/2 as key regulators in APAP-induced liver injury. Our work demonstrated that MKK4/7, upstream activating kinases of JNK,10 are direct phosphorylation targets of Ulk1/2. Phosphorylation of MKK4/7 by Ulk1 strongly increases their kinase activities toward JNK and thereby promotes the induction of necrotic cell death in vivo and in hepatocytes during APAP treatment. Additionally, APAP-inducted activation of JNK and cell death were markedly attenuated in Mkk4/7 double knockdown hepatocytes reconstituted with a MKK7 mutant that can cannot be phosphorylated by Ulk1 compared to those in cells rescued with wild-type (WT) MKK7, illustrating an important mechanism linking APAP administration to liver damage.
Materials and Methods
ANIMAL EXPERIMENTS
Ulk1+/F mice29 and Ulk2+/– mice30 were obtained from Jackson Laboratory, ME, USA (B6.129-Ulk1tm1Thsn/J, stock number: 017976, backcrossed to C57BL/6J mice for at least 10 generations) and MMRRC (stock number: 011678-UNC, C57BL/6J), respectively. Ulk1+/F mice were crossed with Albumin-Cre mice (Albumin-Cre, C57BL/6J; Jackson Laboratory) and Ulk2+/– whole-body knockout mice to generate Ulk1F/FUlk2–/–Albumin-Cre+ (Ulk1/2 LDKO) mice. Mice were fed a normal chow diet and housed with corncob bedding in specific pathogen-free conditions. All animal experiments were approved by the Institutional Animal Care and Use Committee at Xiamen University (Fujian, China). Male mice were used for all the in vivo experiments. For APAP treatment, Ulk1/2 Ctrl (Ulk1F/FUlk2−/−Albumin-Cre− and Ulk1F/+Ulk2−/−) and Ulk1/2 LDKO mice at 6-8 weeks old were either given saline or APAP (500 mg/kg, Cat. A7085; Sigma-Aldrich, Shanghai, China) for 0-24 hours and were sacrificed. For tumor necrosis factor alpha (TNF-α)/galactosamine (GalN) treatment, mice were injected with phosphate-buffered saline (PBS) or GalN (800 mg/kg, Cat. G0500; Sigma-Aldrich) 30 minutes before PBS or TNF-α (12 μg/kg, Cat. CF09-B; Novoprotein, NJ, USA) intraperitoneally and were sacrificed 6 hours later. Whole blood was collected by cardiac puncture and was centrifuged at 4°C to obtain serum. Mouse liver was fixed in 4% paraformaldehyde and paraffin embedded. Sections of 5 μm were used for hematoxylin and eosin (H&E) staining. Serum was used for determination of alanine aminotransferase (ALT) activities using a commercial kit (Cat. A7526; Pointe Scientific, MI, USA). Levels of triacylglycerol (Cat. 290-63701; Wako, Osaka, Japan), nonesterified fatty acid (NEFA; Cat. 294-63601; Wako), ketone body (Cat. 700190; Caymen, MI, USA ), and cholesterol (Cat. 294-65801; Wako) were measured according to the manufacturer's instructions. Blood glucose values were determined using OneTouch UltraVue automatic glucometers (Johnson & Johnson, NJ, USA). GSH was measured by using the DTNB-glutathione reductase recycling assay, as described.32 APAP-cysteine adducts were measured by high-pressure liquid chromatography with electrochemical detection, as described.33 Immunohistochemical detection of Ki-67 (Cat. 27309-1-AP; Proteintech, IL, USA) was performed on paraffin-embedded liver tissue sections. To assess the function of JNK inhibitor in APAP-induced liver injury, mice were injected with JNK inhibitor SP600125 (10 mg/kg, Cat. S1460; Selleck, TX, USA) 3 hours before APAP administration. Five percent dimethyl sulfoxide (DMSO) in PBS was used as the solvent for SP00125 (SP00125 was first dissolved in DMSO and then diluted with PBS), as described.13 A vehicle control containing the same percentage of DMSO in PBS, but without the inhibitor was used for all experiments using SP00125. For Ulk1/2 rescue experiments, Ulk1/2 LDKO mice were injected with adenovirus-expressing vector WT-Ulk1 or WT-Ulk2 by caudal vein. Five days later, mice were treated with APAP and sacrificed. Total liver lysates were prepared using a lysis buffer as described.34
ANTIBODIES AND DRUGS
Antibodies to phospho-serine in S*/P-motif (Cat. #9477; used for detection of phospho-S403-MKK7) in the KinomeView Profiling Kit (Cat. #9812), Ulk1 (Cat. #8054), phospho-Ser757-Ulk1 (Cat. #6888), FIP200 (Cat. #12436), ATG13 (Cat. #13273), ATG101 (Cat. #13492), phospho-Thr183/Tyr185-JNK (Cat. #4668), P-S6K (S6 kinase; Cat. #9234), S6K (Cat. #9202), MKK4 (Cat. #9152), MKK7 (Cat. #4172; used for western blotting), phospho-Ser271/Thr275-MKK7 (Cat. #4171), and tubule-associated protein 1 light chain 3 beta (LC3B; Cat. #2775) were purchased from CST, MA, USA. Antibodies to hemagglutinin (HA; F-7), JNK (C-17), and MKK7 (E-7; used for immunoprecipitating endogenous MKK7) were from Santa Cruz Biotechnology, TX, USA. Antibody to p62 (Cat. ab56416) and Ulk2 (Cat. ab56736) were from Abcam, London, UK. Antibodies to Flag (Cat. F2555), Actin (Cat. A8481), and anti-Flag beads (Cat. M8823) were from Sigma-Aldrich. Antibodies to proliferating cell nuclear antigen (PCNA; Cat. 10205-2-A), cytochrome P450 2E1 (Cyp2E1; Cat. 19937-1-AP), modulatory subunit of glutamate-cysteine ligase (GCLM; Cat. 14241-1-AP), catalytic subunit of glutamate-cysteine ligase (GCLC; Cat. 12601-1-AP), and SH3 domain-binding protein 5 (SH3BP5 [Sab]; Cat. 11127-2-AP) were from Proteintech.
ELECTRON MICROSCOPY
Liver tissues were fixed with 2.5% glutaraldehyde in 0.05 M of sodium cacodylate buffer (pH 7.2), followed by 1% OsO4. The average number of autophagosomes from each cell was determined from a randomly selected pool of 20 fields under each condition.
CELL CULTURE, TRANSIENT TRANSFECTION, IMMUNOPRECIPITATION, AND WESTERN BLOTTING
HEK293T were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS), 2 mM of L-glutamine, 100 IU of penicillin, and 100 μg/mL of streptomycin in a humidified incubator with 5% CO2 at 37 °C. Adenoviruses for infection were packaged and amplified in β5 cells using TurboFect transfection reagent (Cat. #R0531; Thermo Scientific, MA, USA). Polyethylenimine at a final concentration of 10 μM was used to transfect HEK293T cells. Transfected cells were harvested at 16 hours after transfection. Cell lysis, immunoprecipitation (IP), and western blotting were carried out as described.34
qRT-PCR
Total RNA was extracted from livers of Ulk1/2 LDKO and their control mice and was reverse transcribed. Complementary DNA (cDNA) was then used as the template for the detection of the different transcripts using GoTaq qPCR Master Mix (Promega, WI, USA). Expression levels were analyzed by Applied Biosystems 7900HT Sequence Detection System v2.3 software. All mRNA transcript levels are expressed as the ratio of expression of the target gene to Actin. The sequences of the primers were as described.33
BACULOVIRUS EXPRESSION SYSTEM
For recombinant Ulk1 expression, Ulk1 was subcloned into pFastBac containing N-terminal His-tag and expressed in Sf21 cells using the Bac-to-Bac expression system from Invitrogen as described.35
PLASMID CONSTRUCTIONS
Full-length cDNAs encoding mouse Ulk1, Ulk2, and human MKK4, MKK7, JNK1, and JNK2 were obtained by PCR using mouse testis cDNA or human cDNA generated from HeLa cells.
PRIMARY HEPATOCYTES
Primary hepatocytes were isolated from mice with a nonrecirculating perfusion of livers with 0.05% Collagenase Type IV (Sigma-Aldrich). Cells were then plated in six-well plates in Williams' E medium (Cat. 12551-032; Gibco, MA, USA; supplemented with 10% FBS and 1 mM of GlutaMAX, [Cat. 35050061; Gibco], 100 IU penicillin and 100 mg/ml streptomycin) for 4 hours for attachment. After that, cells were cultured in the William's E medium without serum overnight before APAP treatment. TNF-α (20 ng/mL)/actinomycin (ActD; 0.5 μg/mL) were used for primary hepatocyte treatment. For generation of Mkk4/7 double knockdown (DKD) cells, attached hepatocytes were infected with adenovirus-expressing control shRNA or shRNAs targeting mouse Mkk4 (targeted sequence 5′-GCAACTGTGAAAGCACTAA-3′) and mouse Mkk7 (targeted sequence 5′-GCCATGACTGCCCTTACAT-3′) for at least 24 hours before APAP treatment. For generation of reconstituted hepatocytes, hepatocytes were infected with adenovirus expressing vector, WT-Ulk1, WT-Ulk2, WT-MKK7 (human), or MKK7 mutants. Experiments using primary hepatocytes were performed 24 to 48 hours after isolation and harvested within 72 hours. All cells were maintained in a 37°C incubator with 5% CO2.
LONG-LIVED PROTEIN DEGRADATION ASSAY
Degradation of long-lived proteins from primary hepatocytes was measured as described.36 Briefly, cells were incubated with a medium containing 0.2 μCi/mL of L-[14C]Valine (Cat. NEC291EU050UC; PerkinElmer, MA, USA) for 20 hours. After washing and incubation with a medium containing 10 mM of unlabelled Valine for 1 hour, the medium was replaced with fresh medium with or without 10 mM of APAP, and the incubation was continued for the indicated durations. The medium was then precipitated by 10% trichloroacetic acid, and the release of [14C]Valine from protein degradation was calculated as the percentage of the total cell radioactivity.
CELL DEATH DETERMINATION
To determine the cell death rate of hepatocytes, primary hepatocytes seeded in six-well plates were treated with 10 mM of APAP, TNF-α (50 ng/mL), or TNF-α (20 ng/mL)/ActD (0.5 μg/mL, Cat. A9415; Sigma-Aldrich) for 0-6 hours. Cell death and morphological changes were analyzed at different time points with propidium iodide (PI) staining (1 μg/mL, 30 minutes).
IN VITRO KINASE ASSAYS
In vitro kinase assays using Ulk1 as the kinase were performed as described.34 For kinase assays using MKK as the kinase, recombinant JNKα1 (Cat. J2455, Sigma-Aldrich) was incubated with Flag-tagged MKK4 or MKK7 in a kinase buffer (20 mM of HEPES [pH 7.4], 5 mM of MgCl2, 0.5 mM of ethylene glycol tetraacetic acid, 1 mM of dithiothreitol) supplied with 100 μM of ATP. The mixtures were maintained at 30 °C for 30 minutes and stopped by addition of sodium dodecyl sulfate sample buffer.
STATISTICAL ANALYSIS
For experiments with only two groups, the Student t test was used for statistical comparisons. Analysis of variance (ANOVA) with Tukey's post-test (one-way ANOVA for comparisons between groups; two-way ANOVA for comparisons of magnitude of changes between different groups from different cell lines or treatments) was used to compare values among different experimental groups using the SPSS Statistics (version 17.0; SPSS, Inc., Chicago, IL) program or GraphPad Prism (version 6; GraphPad Software Inc., La Jolla, CA) program. P < 0.05 was considered as statistically significant (*) and P < 0.01 as highly significant (**).
Results
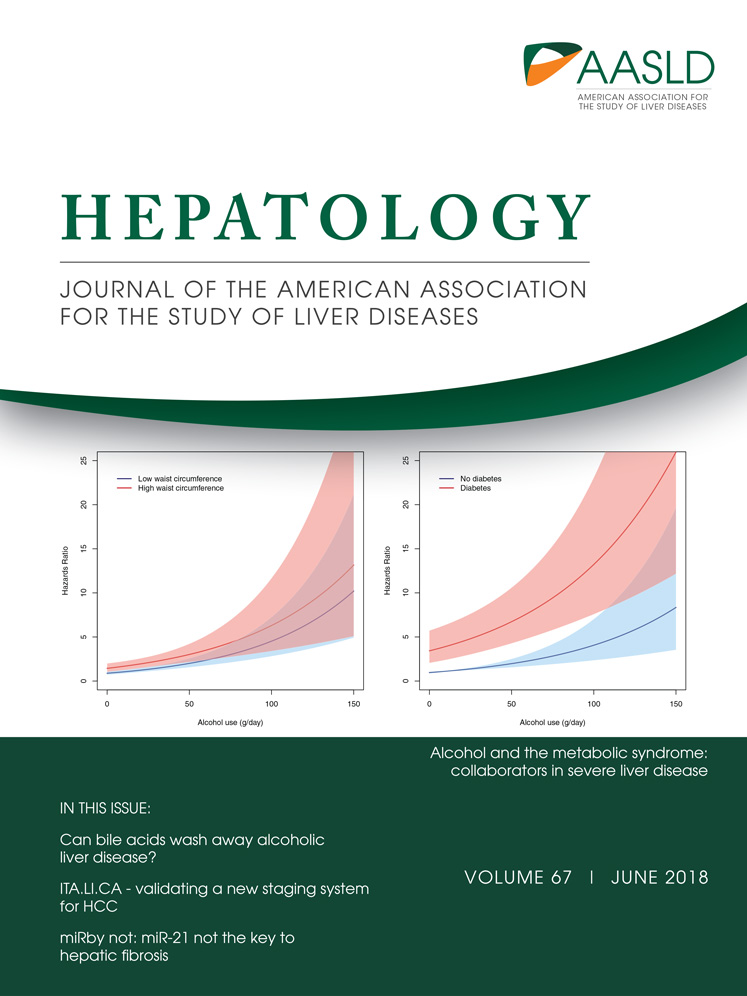
HEPATIC DELETION OF ULK1/2 DEMONSTRATES HEPATOMEGALY BUT NORMAL AUTOPHAGIC ACTIVITY IN THE LIVER
To study the physiological functions of Ulk1 and Ulk2 in the liver, we conditionally deleted Ulk1 in the Ulk2–/– background by crossing Ulk1+/FUlk2−/− mice with albumin-Cre transgenic mice, which specifically express the Cre recombinase in hepatocytes. Ulk1F/FUlk2−/−Albumin-Cre+ mice (Ulk1/2 LDKO mice) were born at the expected Mendelian frequency and did not demonstrate any apparent phenotypes compared to control littermates (Ulk1F/FUlk2−/−Albumin-Cre− and Ulk1+/FUlk2−/− Albumin-Cre+ mice, designated as Ulk1/2 Ctrl mice). Ulk1/2 LDKO mice also showed similar body weights compared to Ulk1/2 Ctrl mice up to 18 weeks of age (Fig. 1A), but displayed significantly larger and heavier liver (hepatomegaly) at as early as 3 weeks of age (Fig. 1B,C). However, histological analysis revealed no evident pathological changes in liver sections of Ulk1/2 liver-specific double knockout (LDKO) mice in comparison to those of WT mice (Supporting Fig. S1A). The protein levels of other Ulk complex components, including FIP200, ATG13, and ATG101, were increased in liver cells from Ulk1/2 LDKO mice (Fig. 1D), perhaps attributed to a compensatory response to the absence of Ulk1/2, similar to the phenomenon in hepatocyte-specific FIP200 deficiency mice.37 Surprisingly, we detected similar autophagic activities in either fed or overnight fasted Ulk1/2 LDKO mice liver compared to those in Ulk1/2 Ctrl mice liver as revealed by accumulation of the levels of p62, lipidation of LC3B and autophagosome formation (Fig. 1D,E). We also analyzed various metabolic parameters in plasma and liver of Ulk1/2 LDKO mice, including glucose, triacylglycerol (TAG), NEFA, ketone body, liver TAG, and cholesterol. It was found that all of the parameters tested were similar between control and Ulk1/2 LDKO mice (Supporting Fig. S1B-G).
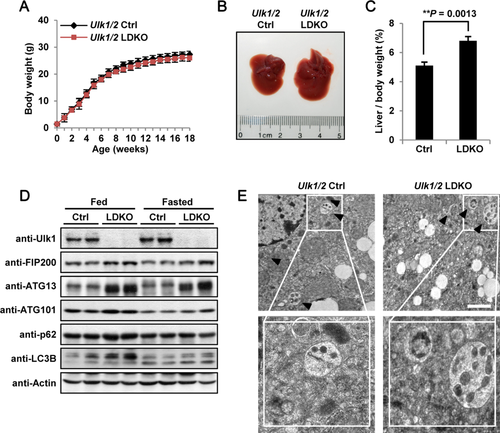
ULK1/2 LDKO MICE ARE RESISTANT TO APAP-INDUCED LIVER INJURY INDEPENDENTLY OF THE AUTOPHAGIC PROCESSES
Previous studies showed that autophagy alleviates APAP-induced liver injury, and depletion of autophagy-related genes such as Atg7 in the liver makes mice become more vulnerable to APAP-induced liver injury (AILI).38, 39 However, another study demonstrated that mice with hepatic deletion of Atg5, another autophagy-essential gene, became more resistant to APAP-induced hepatic damage.33 We therefore tested the role of Ulk1/2 in APAP-stimulated liver injury. It was found that, in Ulk1/2 control (Ctrl) mice, typical centrilobular necrosis, as demonstrated by cellular vacuolization, cell swelling, and nuclear disintegration, was induced after APAP treatment (Fig. 2A), as described.4 However, necrotic cell death was markedly attenuated in APAP-treated Ulk1/2 LDKO mice (Fig. 2A). Of note, single knockout of either Ulk1 or Ulk2 did not protect mice from AILI (Supporting Fig. S2). Meanwhile, we found that APAP-induced increase of serum ALT levels was remarkably attenuated in Ulk1/2 LDKO mice (Fig. 2B), indicative of reduced liver injury in these mice. Unexpectedly, we detected similar induction of autophagy processes in the liver of control and Ulk1/2 LDKO mice (Fig. 2C,D), indicating that autophagy was induced through a mechanism independent of Ulk1/2 after APAP treatment. Together, these results show that AILI was strongly blocked in mouse livers with loss of Ulk1/2 independently of autophagy indication.
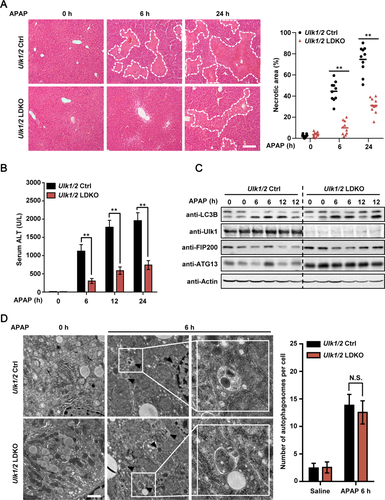
HEPATIC ULK1/2 ARE ESSENTIAL FOR JNK-MEDIATED LIVER INJURY IN RESPONSE TO APAP TREATMENT
APAP is metabolized mainly by cytochrome P450 2E1 (CYP2E1) in the early hours in hepatocytes, which generates the reactive metabolite, NAPQI, that depletes intracellular GSH and covalently binds to proteins.4-6 It was found that loss of Ulk1/2 did not affect the protein level of CYP2E1, GSH depletion, or covalent binding of APAP to proteins after APAP treatment (Supporting Fig. S3A-C). Activation of JNK is another hallmark of AILI.6, 13, 14 Indeed, we found that treatment of Ulk1/2 Ctrl mice with APAP for 6 hours strongly induced activation of JNK in liver (Fig. 3A). Interestingly, consistent with markedly reduced liver injury in APAP-treated Ulk1/2 LDKO mice (Fig. 2A,B), Ulk1/2 LDKO mice were strongly resistant to APAP-stimulated JNK activation in the liver (Fig. 3A). Furthermore, cotreatment of Ulk1/2 Ctrl mice with JNK inhibitor SP600125 almost completely blocked APAP-induced-JNK activation in liver as well as APAP-stimulated increase of plasma ALT and liver injury (Fig. 3B-D), confirming that JNK activation is an essential event for AILI.13, 14
It has been reported that robust oxidative stress response may play protective roles in AILI. 33 We therefore measured the expression levels of nuclear factor (erythroid-derived 2)-like 2 (Nrf2)-targeting genes, including GCLM, GCLC, and NAD(P)H quinone dehydrogenase 1, by either western blotting or RT-PCR. It was found that loss of Ulk1/2 did not affect Nrf2 signaling under both basal and APAP treatment conditions (Supporting Fig. S3C,D). Liver regeneration is also considered to be important for survival after AILI. 2-4, 33 We thus investigated the proliferation rate of Ulk1/2 Ctrl and LDKO liver cells by using Ki-67 immunostaining. It was found that the number of Ki-67-positive cells was significantly elevated in Ulk1/2 LDKO livers under both basal and APAP-treated conditions (Supporting Fig. S4A). Moreover, increased protein levels of cell proliferation marker PCNA were detected in Ulk1/2 LDKO mouse livers compared to those in Ulk1/2 Ctrl mice (Supporting Fig. S4B). These results suggest that the proliferation rate of liver cells of the Ulk1/2 LDKO mouse is higher than that from the Ulk1/2 Ctrl mouse, which may contribute to the hepatomegaly found in Ulk1/2 LDKO mice and accelerate tissue recovery from AILI.
ULK1/2 DEFICIENCY PROTECTS HEPATOCYTES FROM APAP-INDUCED JNK ACTIVATION AND NECROTIC CELL DEATH
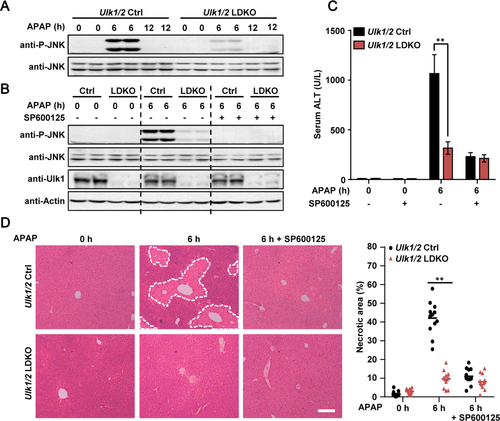
To confirm a pivotal role of Ulk1/2 in APAP-induced necrosis and liver injury, primary hepatocytes were isolated from both Ulk1/2 Ctrl and LDKO mice and treated with APAP. Consistent with the in vivo results (Fig. 3A,B), we found that depletion of Ulk1/2 strongly impaired activation of JNK in response to APAP in primary hepatocytes (Fig. 4A). In contrast, TNF-α, another classical activator of JNK signaling,10 robustly induces JNK phosphorylation in both Ulk1/2 LDKO and Ctrl hepatocytes (Fig. 4B). However, depletion of Ulk1/2 did not affect expression of Sab (SH3BP5; Supporting Fig. S5), which is critical in promoting mitochondrial ROS release that helps sustain JNK activation through activation of MAP kinase kinase kinase (MAP3K), such as apoptosis signal-regulating kinase 1 (ASK1).40 Importantly, in agreement with previous findings on inhibition of mTORC1 by APAP,39 we found that mTORC1-mediated phosphorylation of Ulk1 at Ser757, which suppresses activity of Ulk1,24 was markedly decreased during APAP treatment, but not during TNF-α stimulation (Fig. 4A,B), indicating that de-inhibition of Ulk1 after Ser757 dephosphorylation may be a critical step for APAP-induced JNK activation. Of note, in line with the normal induction of autophagy in liver of Ulk1/2 LDKO mice (Fig. 2C,D), autophagy was effectively induced in Ulk1/2 LDKO hepatocytes after APAP treatment, through assaying p62 degradation, long-lived protein degradation or LC3B levels with or without lysosome inhibitor treatment (Fig. 4A,C,D). By quantifying PI-positive cells with intact nuclei as necrotic cells, we found that APAP-induced necrotic cell death was remarkably decreased in Ulk1/2 LDKO hepatocytes compared to that in Ulk1/2 Ctrl hepatocytes (Fig. 4E). Collectively, these results reveal that Ulk1/2 affect APAP-induced JNK activation and necrosis in a cell-autonomous manner.
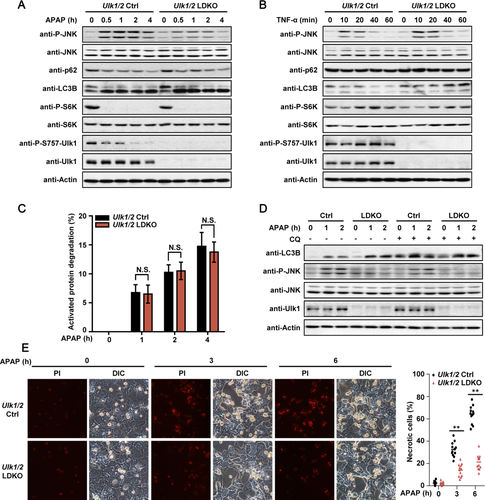
To determine whether the role of Ulk1/2 in liver injury is specific to APAP, TNF-α/GalN (in vivo) or TNF-α/ActD (in vitro) was also used to induce liver injury in Ulk1/2 Ctrl and LDKO mice/hepatocytes.41 Similar extent of liver injury (in vivo) or cell death (in vitro) in Ulk1/2 Ctrl and LDKO mice/hepatocytes treated with TNF-α/GalN or TNF-α/ActD was detected (Fig. 5A-C). Consistently, in the hepatocytes of both mouse strains, JNK signaling was comparably activated (Fig. 5D,E). In addition, contrary to the markedly decreased Ser757-Ulk1 phosphorylation after APAP treatment (Fig. 4A), combined treatment of TNF-α and ActD did not affect Ser757 phosphorylation of Ulk1 (Fig. 5E), similar to the data from TNF-α single-treatment condition (Fig. 4B). These results suggest that hepatic depletion of Ulk1/2 did not affect TNF-α/GalN- or TNF-α/ActD- induced liver injury/cell death in mice/hepatocytes, and that activation of Ulk1 after Ser757 dephosphorylation is a unique step for AILI.
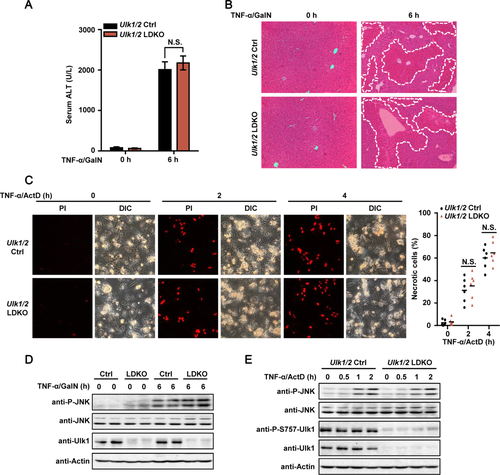
MKK4/7 ARE DIRECT TARGETS OF ULK1/2 AND MEDIATE AILI
Next, we asked the molecular mechanism by which Ulk1/2 participate in JNK signaling. A co-immunoprecipitation assay was performed to test whether Ulk1 would specifically interact with JNK1 and JNK2 or their direct upstream kinases, MKK4 and MKK7.42 We found that MKK4 as well as MKK7, but not JNK1 or JNK2, strongly interacted with Ulk1 (Fig. 6A). We next tested whether MKK4 and MKK7 could be the direct substrates for the serine/threonine kinase, Ulk1. It was found that coexpression of WT, but not the kinase-inactive (KI) form of Ulk1, with MKK4 or MKK7 resulted in different electrophoretic mobility shifts in gels containing Phos-tag that retards the mobility of phosphorylated proteins (Fig. 6B,C).43 Similar results were obtained using Ulk2 as the kinase (Supporting Fig. S6). Treatment of immunoprecipitated MKK4 or MKK7 with calf-intestinal alkaline phosphatase (CIP) effectively diminished the shifts (Fig. 6B,C). In vitro kinase assays using His-tagged Ulk1 expressed and purified from insect cells further confirmed that Ulk1 could directly phosphorylate MKK4 and MKK7 (Fig. 6D). To explore the functional consequences of Ulk1-mediated phosphorylation of MKK4 and MKK7, we tested whether their kinase activities toward JNK were affected by Ulk1-mediated phosphorylation. Intriguingly, we found that coexpression of Ulk1 with MKK7 significantly increased its kinase activity toward JNK by more than 2-fold, whereas the activity of MKK4 was increased by around 30% after Ulk1 coexpression (Fig. 6E), suggesting that MKK7 may play a major role in Ulk1/2-dependent JNK activation. We therefore focused on the phosphorylation event of MKK7 in our following experiments.
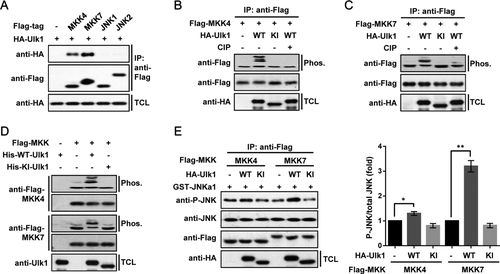
ULK1/2-DEPENDENT MKK7 PHOSPHORYLATION IS CRITICAL FOR APAP-INDUCED JNK ACTIVATION AND CELL DEATH
In vitro phosphorylated MKK7 was then subjected to mass spectrometry; four phosphorylated serine residues (Ser35, Ser378, Ser392, and Ser403) were identified as candidate sites targeted by Ulk1 (Fig. 7A), distinct from the conventional activation sites by MAP3Ks.42 Mutants carrying alterations of these serine residues to alanine singly or in combination were constructed. It was found that combined mutation of the four serine residues to alanine (4SA) almost completely blocked the phosphorylation of MKK7 by Ulk1, as indicated by the disappearance of mobility shifts in Phos-tag-containing gels (Fig. 7B). In addition, by using a motif-dependent antibody kit, we found that Ulk1-mediated phosphorylation of Ser403 on MKK7 happened to be recognized by a context-dependent antibody (Phospho-CDK substrate motif [(K/H)pSP] antibody, referred here as P-S403-MKK7 antibody; Fig. 7B). Further work revealed that the activities of Ulk1-nonphosphorylatable 4SA mutant of MKK7 were no longer affected by Ulk1 coexpression (Fig. 7C). Endogenous phosphorylation of MKK7 after APAP treatment was also detected in Ulk1/2 Ctrl, but not in LDKO mice hepatocytes (Fig. 7D). In addition, interaction between endogenous Ulk1 and MKK4/7 was strongly increased in liver of APAP-treated mice (Fig. 7E), supporting a dynamic response of Ulk1/2 in regulating MKK4/7 after APAP treatment.

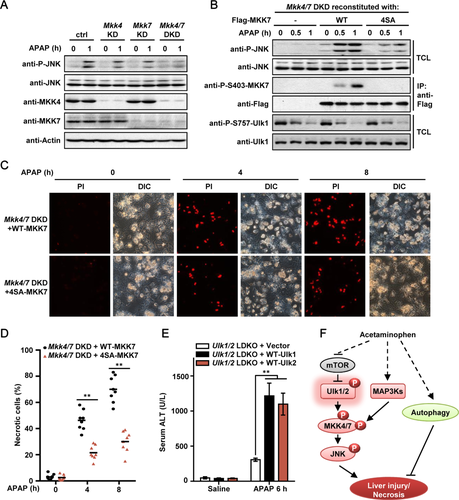
By infecting primary hepatocytes with adenovirus expressing control shRNA or shRNAs targeting Mkk4 and/or Mkk7, we found that DKD of Mkk4 and Mkk7 (Mkk4/7 DKD) strongly impaired APAP-induced JNK phosphorylation and toxicity (Fig. 8A and Supporting Fig. S7). Importantly, reconstitution of WT-MKK7, but not 4SA-MKK7, effectively rescued the activation of JNK after APAP treatment in Mkk4/7 DKD hepatocytes (Fig. 8B). Finally, Mkk4/7 DKD hepatocytes reconstituted with WT-MKK7 showed severely increased necrotic cell death than that of cells reconstituted with the 4SA-MKK7 mutant (Fig. 8C,D). Several MAP3Ks, including ASK1 and mixed-lineage protein kinase 3 (MLK3), have been shown to be critical in AILI by acting as direct upstream kinases of MKK4/7 and thereby activating JNK signaling.46-48 We found that MAP3Ks-mediated phosphorylation on MKK7-Ser271/Thr275 does not alter Ser403 phosphorylation by Ulk1/2, but Ulk1/2-mediated MKK7 phosphorylation is required for a robust phosphorylation of MKK7 on Ser271/Thr275 by MAP3Ks after APAP treatment (Fig. 7D and Supporting Fig. S8). Using adenovirus-mediated protein expression methods, we have also reconstituted the expression of either Ulk1 or Ulk2 both in vivo in Ulk1/2 LDKO mice and in isolated Ulk1/2 DKO hepatocytes. It was found that reconstitution of either Ulk1 or Ulk2 could significantly restore APAP-induced toxicity in Ulk1/2 LDKO mice/hepatocytes (Fig. 8E and Supporting Fig. S9), confirming that Ulk1/2 play key roles in AILI. Together, these results provide strong evidence showing that Ulk1/2-dependent MKK7 phosphorylation is a critical step for the activation of JNK and the induction of necrotic cell death during APAP treatment.
Discussion
Autophagy has been thought to play a protective role against APAP hepatotoxicity through the removal of damaged organelles and alleviation of oxidative stress, and pharmaceutical inhibition of autophagy by leupeptin or chloroquine would render hepatocytes more sensitive to APAP-induced necrotic cell death.38, 39 Consistent with these findings, depletion of autophagy-related gene Atg7 or SQSTM1/p62 in the liver strongly exaggerates AILI.38, 44 However, probably attributed to the multiple functions of different autophagy-related genes besides their roles in autophagy,27, 31, 34 liver-specific knockout of Atg5 induced a paradoxically reduced liver injury during APAP treatment, which may be caused from a persistent activation of the Nrf2 antioxidant signaling pathway and increased hepatocyte proliferation during Atg5 loss.33
In the present study, we found that after APAP stimulation, autophagy was effectively induced in Ulk1/2-depleted hepatocytes as well as in control cells. However, compared to Ulk1/2 Ctrl mice, necrotic liver injury was remarkably attenuated in Ulk1/2 LDKO mice (Fig. 2A,B), which raises a possibility that Ulk1/2 may utilize a mechanism independently of autophagy to affect AILI. By combining in vitro reconstitution assays, phospho-specific antibodies, and genetic manipulations, we identified MKK4 and MKK7 as direct substrates of Ulk1/2 kinases during APAP treatment, downstream of mTORC1 signaling. Phosphorylation of MKK4/7 by Ulk1 leads to dramatic increases in their kinase activities and thereby induces the activation of JNK and necrotic cell death of hepatocytes (Fig. 8F). In parallel, MAP3Ks, such as ASK1 and MLK3, phosphorylate MKK4/7 at the conventional activation sites.46-48 Both of the two phosphorylation events are likely required for the efficient activation of MKK and JNK during APAP treatment. Meanwhile, activation of autophagy may, to some extent, protect hepatocytes against APAP-induced toxicity (Fig. 8F).
By using a mouse model with combined embryonic liver-specific deletion of Jnk1 and global Jnk2 deletion (JnkΔhepa mice), Cubero et al. reported that hepatic JNK dampens AILI because the JnkΔhepa mice are not protective against AILI.45 However, a large body of other evidence supports an amplifying role of JNK signaling in APAP-induced liver toxicity, demonstrated through a variety of approaches such as different small molecule JNK inhibitors,13, 14 shRNA-mediated knockdown of JNK1 and JNK2,13 global knockout of MLK3 46 and ASK1, 47 ASK1 inhibitor,48 MKK4 knockdown,41 and liver-specific knockdown and knockout of Sab.40 Our data clearly demonstrated that Ulk1/2 and MKK4/7 are required for the effects of APAP on liver injury and mediate the effect of APAP on JNK activation in the liver (Figs. 2A and 3A-D) as well as primary hepatocyte (Figs. 4A,D,E and 8A and Supporting Fig. S7). Admittedly, it is still possible that Ulk1/2 and MKK4/7 may affect AILI through other mechanisms independently of JNK activation, considering the controversy on the specificity of JNK inhibitor, SP600125.45
MKK4 has been shown to play a dominant role in APAP-induced JNK activation and toxicity in liver of C57B/6N mouse strain.41 However, in our study, we consistently found that significant amount of MKK7 was detected in mouse hepatocytes (Figs. 7D,E and 8A), and knockdown of either Mkk4 or Mkk7 singly has only minor effects on APAP-induced toxicity and JNK activation (Fig. 8A and Supporting Fig. S7). Meanwhile, combined knockdown of Mkk4 and Mkk7 significantly blocks necrotic cell death and JNK activation in response to APAP treatment in hepatocytes, indicating that both MKK4 and MKK7 contribute to JNK activation-mediated APAP toxicity. Considering that a C57BL/6J substrain was used in our studies, it is possible that different genetic backgrounds rely on different MAP2 kinases for JNK activation and APAP-induced toxicity.
Unexpectedly, our results indicate that induction of autophagy during APAP treatment may go through a mechanism independent of Ulk1/2, whereas Atg7 is essential.38 Interestingly, a previous study revealed that during ammonia stimulation or long-term glucose starvation, the autophagy response in Ulk1/2–/– DKO cells is also comparable to that observed in WT cells.49 Furthermore, in chicken DT40 cells, Ulk1 and Ulk2 are even not required for nutrient starvation-induced autophagy, although Atg13 and FIP200 are essential.50 These results highlight the fact that cells are able to utilize distinct pathways for induction of autophagy in response to different stimuli.35, 36
In sum, we have uncovered a critical mechanism controlling AILI, mediated by Ulk1/2-dependent regulation of JNK signaling, and may thus open new avenues for the alleviation of APAP hepatotoxicity and its associated complications.
REFERENCES
Author names in bold designate shared co-first authorship.