RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2
Potential conflict of interest: Nothing to report.
Supported by the Hong Kong Research Grants Council (RGC) General Research Fund (17142516 and 17115815) to C.M.W., RGC Theme-Based Research Scheme (T12-704/16R) to I.N., National Natural Science Foundation of China General Program (81572446) to C.M.W., and the University of Hong Kong Seed Funding Program for Basic Research (201511159077) to C.M.W.
Abstract
Epigenetic alterations have contributed greatly to human carcinogenesis. Conventional epigenetic studies have predominantly focused on DNA methylation, histone modifications, and chromatin remodeling. Recently, diverse and reversible chemical modifications of RNAs have emerged as a new layer of epigenetic regulation. N6-methyladenosine (m6A) is the most abundant chemical modification of eukaryotic messenger RNA (mRNA) and is important for the regulation of mRNA stability, splicing, and translation. Using transcriptome sequencing, we discovered that methyltransferase-like 3 (METTL3), a major RNA N6-adenosine methyltransferase, was significantly up-regulated in human hepatocellular carcinoma (HCC) and multiple solid tumors. Clinically, overexpression of METTL3 is associated with poor prognosis of patients with HCC. Functionally, we proved that knockdown of METTL3 drastically reduced HCC cell proliferation, migration, and colony formation in vitro. Knockout of METTL3 remarkably suppressed HCC tumorigenicity and lung metastasis in vivo. On the other hand, using the CRISPR/dCas9-VP64 activation system, we demonstrated that overexpression of METTL3 significantly promoted HCC growth both in vitro and in vivo. Through transcriptome sequencing, m6A sequencing, and m6A methylated RNA immuno-precipitation quantitative reverse-transcription polymerase chain reaction, we identified suppressor of cytokine signaling 2 (SOCS2) as a target of METTL3-mediated m6A modification. Knockdown of METTL3 substantially abolished SOCS2 mRNA m6A modification and augmented SOCS2 mRNA expression. We also showed that m6A-mediated SOCS2 mRNA degradation relied on the m6A reader protein YTHDF2-dependent pathway. Conclusion: METTL3 is frequently up-regulated in human HCC and contributes to HCC progression. METTL3 represses SOCS2 expression in HCC through an m6A-YTHDF2-dependent mechanism. Our findings suggest an important mechanism of epigenetic alteration in liver carcinogenesis. (Hepatology 2018;67:2254-2270).
Abbreviations
-
- ALKBH5
-
- alkB homologue 5
-
- CpG
-
- cytosine followed by guanine
-
- CRISPR
-
- clustered regularly interspaced short palindromic repeats
-
- FTO
-
- fat mass and obesity-associated protein
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
-
- m6A
-
- N6-methyadenosine
-
- MeRIP
-
- methylated RNA immune-precipitation
-
- METTL3
-
- methyltransferase-like 3
-
- miRNA
-
- microRNA
-
- mRNA
-
- messenger RNA
-
- NT
-
- nontumor
-
- qRT-PCR
-
- quantitative reverse-transcription polymerase chain reaction
-
- Seq
-
- sequencing
-
- shRNA
-
- short-hairpin RNA
-
- SOCS
-
- suppressor of cytokine signaling
-
- TCGA
-
- The Cancer Genome Atlas
-
- WTAP
-
- Wilms' tumor 1-associating protein
-
- YTH
-
- YT521-B homology
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer and ranks as the fifth leading malignancy worldwide, accounting for more than 700,000 deaths annually.1 The situation is particularly bad in Asia due to the prevalence of hepatitis B virus (HBV)- and hepatitis C virus-induced viral hepatitis, which are the major risk factors for HCC development. Limited clinical treatments are available due to the disease generally being diagnosed at a late stage or the patient having advanced liver cirrhosis.1 Thus, understanding the molecular mechanisms of how HCC develops is essential to advance future diagnostic and therapeutic inventions. Accumulating evidence has revealed that liver carcinogenesis is a multistep process that involves complicated interplays between genetics, epigenetics, and transcriptomic alterations. Recent whole genome- and exome-sequencing analyses have delineated the mutational landscape of HCC and uncovered a number of novel driver mutations.2 Strikingly, epigenetic regulation is among the most prevalent aberrant pathways and may lead to profound gene expression changes to facilitate HCC formation and development.2
Traditionally, epigenetic regulation refers to diverse and reversible chemical modifications of DNA and histones that regulate gene expression in a way independent to genome sequences.3 Previous studies have suggested that HCC acquires extensive changes in DNA methylation, with a background of global DNA hypomethylation and gene-specific focal DNA hypermethylation. Epigenetic silencing often follows when DNA hypermethylation occurs on cytosine followed by guanine (CpG) islands at gene promoter regions. Many well-characterized tumor suppressors, such as deleted in liver cancer 1 (DLC1), tissue factor pathway inhibitor-2 (TFPI-2), cyclin-dependent kinase inhibitor 2A (CDKN2A), and phosphatase and tensin homolog (PTEN), have been shown to be silenced in HCC by this mechanism.4-6 In addition, dysregulation of epigenetic modifying enzymes has also been frequently reported in human malignancies and has profoundly contributed to human carcinogenesis. For instance, we previously demonstrated that histone methyltransferases, enhancer of zeste homolog 2 (EZH2), suppressor of variegation 39H1 (SUV39H1), SET domain bifurcated 1 (SETDB1), and euchromatic histone-lysine N-methyltransferase 2 (G9a/EHMT2) are conspicuously up-regulated in HCC and consequently promote HCC development and metastasis by epigenetic silencing of critical tumor suppressor genes and microRNAs (miRNAs).7-10 Collectively, epigenetic deregulation is now considered as a major driving force of liver carcinogenesis.
In addition to DNA and histones, cellular RNAs (e.g., messenger RNA [mRNA], transfer RNA, small nuclear RNA) also carry hundreds of distinct posttranscriptional modifications at various sites. These modifications are thought to moderate RNA structure, function, and stability. Not surprisingly, researchers have demonstrated that some of these posttranscriptional RNA modifications are reversible and dynamically controlled, indicating that they might have potential regulatory functions similar to modifications of DNA and histones. In this regard, investigating the landscapes and functions of these reversible RNA modifications is now emerging as a new frontier of research known as RNA epigenetics or epitranscriptomics.11 Although these modifications have been known for decades, deciphering their biological roles remains challenging due to the complexity of RNA structure and function. Recently, empowered by the availability of highly specific antibodies and the accessibility of high-throughput sequencing technologies, mapping of different modifications of the human transcriptome and characterizing their biological effects have become feasible.12 Given the fact that epigenetic alterations contribute immensely to carcinogenesis, we reasoned that RNA modifications might also represent a distinct layer of epigenetic deregulation in cancer progression.
In this study, we focused on N6-methyadenosine (m6A) modification because it is the most prevalent chemical modification in eukaryotic mRNA.13 Installation of m6A is a reversible process regulated by the balanced activities of m6A writer and eraser proteins. The addition of a methyl group to the N6 site of adenine usually happens within the consensus sequence of RRm6ACH (where R = G or A, H = A, C, or U) and is accomplished by a highly conserved mRNA methyltransferase complex, the m6A writer. Methyltransferase-like (METTL)3, METTL14, and Wilms' tumor 1-associating protein (WTAP) are the core components of this complex.14-16 Both METTL3 and METTL14 contain an S-adenosyl methionine-binding motif. They colocalize in nuclear speckles, form a heterodimer, and catalyze the covalent transfer of a methyl group to adenine with the assistance of WTAP. The reversible process is conducted by m6A eraser fat mass and obesity-associated protein (FTO) and alkB homologue 5 (ALKBH5).17, 18 While ALKBH5 catalyzes the direct removal of m6A, FTO can sequentially oxidize m6A to N6-hydroxymethyladeosine and N6-formyladenosine, which are moderately stable and can later be hydrolyzed to adenine. The recently published m6A methylome reveals that m6A preferentially appears in the long internal exons and is enriched around the stop codon, suggesting its fundamental regulatory role in mRNA.12, 13 The current hypothesis suggests that for m6A modification to exert its biological functions, it must first be recognized by m6A reader proteins. YT521-B homology (YTH) domain family proteins are reported to be m6A readers. Among which, YTHDF2 is the first identified and the most studied m6A reader protein that influences mRNA stability.19 YTHDF2 binds to m6A located in the 3′ untranslated region through its C-terminal YTD domain and localizes the targeted mRNA to processing bodies for accelerated degradation through its N-terminal domain.19 Furthermore, previous pull-down experiments have suggested that heterogeneous nuclear ribonucleoprotein (hnRNP) could also serve as a potential nuclear m6A reader to influence mRNA localization and alternative splicing.12 The 5′ untranslated region m6A has also been suggested to promote mRNA translation efficiency in a cap-independent manner through YTHDF1.20
m6A modification has been implicated in controlling circadian rhythm21 and modulating embryonic stem cell fate transition from reprogramming to pluripotency.22-24 Dysfunction of METTL3, the key component of the m6A methyltransferase complex, has shown fundamental biological effects in different living organisms. In Saccharomyces cerevisiae and Drosophila melanogaster, silencing of METTL3 homologs leads to defects in gametogenesis.25 Additionally, Mettl3-knockout mice are embryonic lethal, which is consistent with the observation that Mettl3 inactivation in mouse embryonic stem cells resulted in a loss of self-renewal capabilities.22 Given the essential role of RNA m6A modification in regulating gene expression and various biological processes, it is reasonable to speculate that aberrant m6A modification might also be involved in human carcinogenesis. Currently, our knowledge about the mechanistic link between m6A and human carcinogenesis is limited. Whether deregulation of the m6A machinery could lead to cancer initiation and progression remains an outstanding question. We are only beginning to explore this intriguing hypothesis. To this end, some recent studies have highlighted the role of m6A eraser proteins in regulating cancer stem cell properties. ALKBH5 has been reported to induce breast cancer stem cell phenotypes under hypoxia and maintain tumorigenicity of glioblastoma stem cells,26-28 while the other demethylase, FTO, has been reported to serve as an oncogene in acute myeloid leukemia,29 both through m6A-dependent mechanisms. On the other hand, METTL3 knockdown also leads to apoptosis and modulation of p53 signaling in cancer cells, suggesting that METTL3 might be essential in cancer cell survival.30 Interestingly, METTL3 has been reported to promote protein translation of oncogenes in lung cancer cells, which is surprisingly through a mechanism independent of its methyltransferase activity.31 Whether METTL3 could epigenetically silence tumor suppressor genes in human cancers through an m6A methyltransferase-dependent manner remains to be elucidated. Herein, by thoroughly investigating the role of METTL3 deregulation in HCC, we explore a distinct layer of epigenetic alteration in human malignancy. Studying the function of METTL3 in tumor progression is urgently needed and might shed light on future clinical cancer therapy and drug developments.
Materials and Methods
HCC SPECIMENS AND CELL LINES
We used primary HCC and their nontumorous liver samples from 120 patients. The use of clinical samples was approved by the institutional review board of The University of Hong Kong and the Hong Kong Hospital Authority. HCC cell lines HepG2 and Huh-7 were obtained from ATCC. MHCC97L was a gift from Dr. Z.Y. Tang (Fudan University, Shanghai, China).
ESTABLISHMENT OF STABLE KNOCKDOWN, KNOCKOUT, AND OVEREXPRESSED CELLS
Stable knockdown of target genes was achieved by lentiviral-based short-hairpin RNA (shRNA) delivery. METTL3 knockout in Huh-7 was accomplished by using the lentiviral-based clustered regularly interspaced short palindromic repeats (CRISPR) gene editing system (lentiCRISPR v2).32 METTL3 activation in MHCC97L and Huh-7 cells was performed by using the CRISPR/dCas9-VP64/MS2-p65-HSF1 gene activation system.33 The shRNAs and single-guided RNAs used in this study are listed in Supporting Table S1.
IN VIVO SUBCUTANEOUS AND ORTHOTOPIC IMPLANTATION MODEL
For the subcutaneous implantation model, 2 × 106 METTL3 stable knockdown Huh-7 cells or METTL3 overexpression MHCC97L cells were injected subcutaneously into BABL/cAnN-nude mice. For orthotopic implantation, wild-type and METTL3 knockout Huh-7 cells were luciferase labelled, and 2 × 106 cells were then injected orthotopically into the left liver lobe of nude mice. After 4 weeks, mice were killed, and in vivo tumor growth and lung metastases were detected with the IVIS 100 Imaging System (Xenogen). Animal experiments were carried out according to the Control of Hong Kong Animals Experiment Ordinance and the Institute's principles on animal works.
m6A-RNA IMMUNOPRECIPITATION ASSAY AND m6A SEQUENCING
Total RNAs were first extracted from stable METTL3 knockdown Huh-7 and HepG2 cells and their corresponding nontarget controls. Chemically fragmented RNA (∼100 nucleotides) was incubated with m6A antibody for immunoprecipitation according to the standard protocol of the Magna methylated RNA immune-precipitation (MeRIP) m6A Kit (Merck Millipore). Enrichment of m6A containing mRNA was then analyzed either through quantitative reverse-transcription polymerase chain reaction (qRT-PCR) or by high-throughput sequencing. For high-throughput sequencing, purified RNA fragments from m6A-MeRIP were used for library construction with the NEBNext Ultra RNA library Prep kit from Illumina and were sequenced with Illumina HiSeq 2000. Library preparation and high-throughput sequencing were performed by Novogene (Beijing, China). Sequencing reads were aligned to the human genome GRCh37/hg19 by Bowtie2, and the m6A peaks were detected by magnetic cell sorting as described.34
STATISTICAL ANALYSIS
Statistical analysis was performed using GraphPad Prism6 software. P < 0.05 was considered statistically significant.
The detailed methodology can be found in the Supporting Materials and Methods.
Results
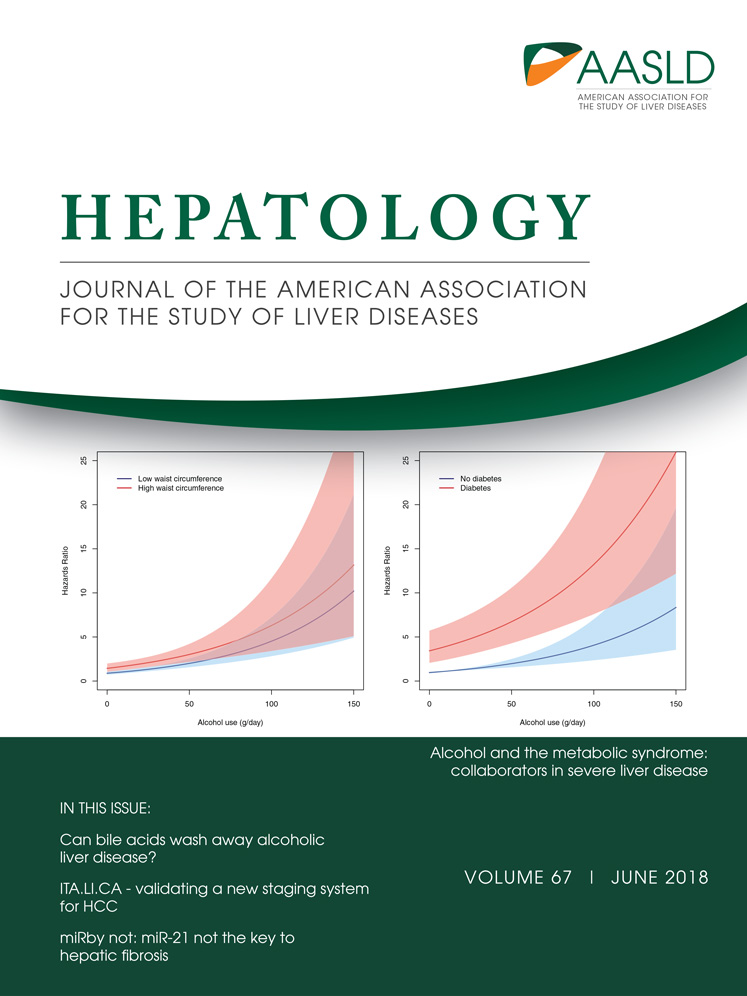
FREQUENT UP-REGULATION OF METTL3 mRNA METHYLTRANSFERASE IN HUMAN HCC
Using transcriptome sequencing (RNA-Seq), we compared the expression of the major m6A modifying enzymes in 16 pairs of HBV-associated HCC and their corresponding nontumor (NT) livers. We identified that METTL3, the core component of m6A methyltransferase, was significantly up-regulated in HCC (Fig. 1A,B; Supporting Fig. S1). Consistent with our findings, METTL3 was also found to be significantly up-regulated in The Cancer Genome Atlas (TCGA) RNA-Seq data set of 50 paired HCC samples (Fig. 1C; Supporting Fig. S2), which further supported our initial observation of METTL3 deregulation in HCC. Next, we validated METTL3 up-regulation in an expanded HCC sample cohort consisting of 104 pairs of primary HCC and NT liver samples by qRT-PCR. Consistently, we demonstrated that the expression of METTL3 was significantly elevated in primary HCCs (Fig. 1D). In this sample cohort, 33% (34/104) of patients with HCC had a remarkable METTL3 up-regulation in HCC (Fig. 1D). Additionally, up-regulation of METTL3 was observed in multiple human HCC cell lines (Supporting Fig. S3). Moreover, METTL3 was also found to be significantly up-regulated in other solid tumors available in TCGA, including breast, colorectal, prostate, and bile duct cancers (Fig. 1E). In line with this observation, elevation of the mRNA m6A level was detected in human HCC compared to the corresponding NT livers (Fig. 1F). Moreover, METTL3 expression was positively correlated with the expression of cell proliferation marker Ki67, which suggested a protumorigenic function of METTL3 in HCC progression (Supporting Fig. S4A). Expression of METTL3 was gradually elevated from grade 1 to grade 3 HCC, but there was no significant difference between grade 3 and grade 4 tumors (Supporting Fig. S4B). METTL3 expression was not correlated with HBV or hepatitis C virus infection, suggesting up-regulation of METTL3 is a general feature of HCC developed from different etiological factors (Supporting Fig. S4C). Further analysis of TCGA survival data revealed that patients with high METTL3 expression were associated with poor overall survival and poor disease-free survival (Fig. 1G). With the above evidence, we concluded that METTL3 is frequently up-regulated in human HCC and may be implicated in pathogenesis and progression of HCC.
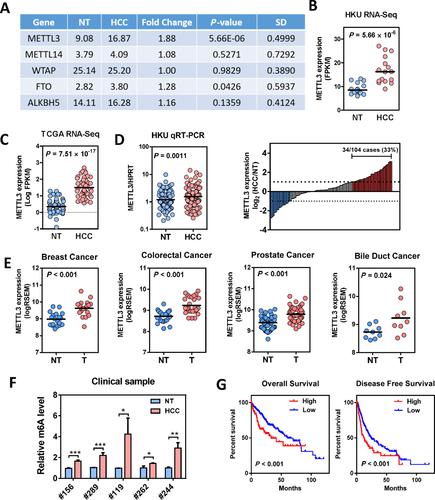
KNOCKDOWN OF METTL3 INHIBITED HCC GROWTH AND METASTASIS
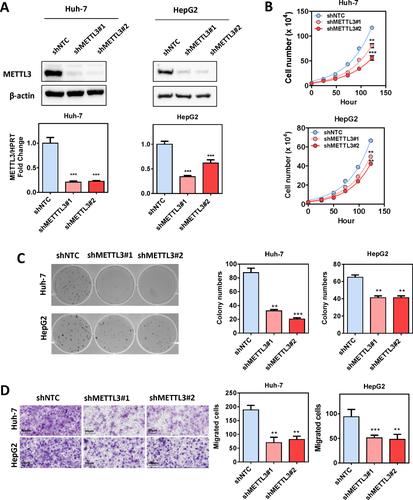
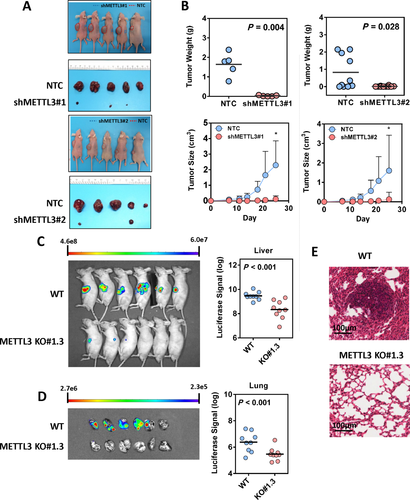
To investigate the functional roles of METTL3 in HCC, we established stable METTL3 knockdown models in Huh-7 and HepG2 cells with two independent shRNA sequences (shMETTL3 #1 and #2). Successful knockdown of METTL3 was confirmed at both mRNA and protein levels (Fig. 2A). As expected, knockdown of METTL3 remarkably reduced the mRNA m6A level in Huh-7 and HepG2 cells (Supporting Fig. S5). Knockdown of METTL3 significantly suppressed HCC cell proliferation (Fig. 2B) and inhibited HCC colony-forming abilities (Fig. 2C). Furthermore, the transwell cell migration assay showed that knockdown of METTL3 dramatically suppressed HCC cell migratory abilities (Fig. 2D). To further verify the oncogenic function of METTL3 in HCC, we performed a subcutaneous implantation experiment in nude mice to test the effect of METTL3 knockdown in HCC tumorigenicity. Through this in vivo experiment, we observed that stable knockdown of METTL3 effectively suppressed tumor growth in nude mice as reflected by the significant reduction of tumor size and weight when compared to the nontarget shRNA control (Fig. 3A,B). Consistent results were obtained from two stable METTL3-knockdown cell lines. To consolidate our findings, we used the CRISPR/Cas9 gene editing system with two independent single-guided RNAs to knockout METTL3 in Huh-7 cells. Knockout efficiency was demonstrated at the protein level through western blotting (Supporting Fig. S6A). Consistent with the knockdown models, knockout of METTL3 significantly suppressed HCC cell proliferation, colony formation and cell migration (Supporting Fig. S6B-D). An in vivo orthotopic implantation experiment was performed to further verify the METTL3 knockout effect in HCC tumorigenicity. We found that knockout of METTL3 dramatically suppressed tumor formation in the orthotopic liver microenvironment (Fig. 3C) and attenuated lung metastasis (Fig. 3D,E) in nude mice. Taken together, our results suggest that METTL3 plays a pivotal role in promoting HCC growth and metastasis.
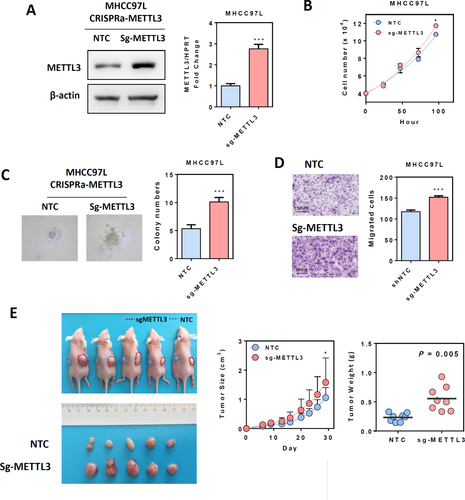
OVEREXPRESSION OF METTL3 PROMOTED HCC PROLIFERATION AND MIGRATION
Next, we overexpressed endogenous METTL3 in MHCC97L cells using the CRISPR/dCas9-VP64/MS2-p65-HSF1 gene activation system, with a specific single-guided RNA targeting the promoter region of the METTL3 gene. Up-regulation of METTL3 was confirmed at both the mRNA and protein levels (Fig. 4A). Overexpression of METTL3 moderately but significantly increased the HCC cell proliferation rate under low-serum (1%) culture conditions and promoted HCC anchorage-independent growth in soft agar (Fig. 4B,C). In addition, overexpression of METTL3 also accelerated HCC cell migration in the transwell assay (Fig. 4D). Similar findings were also observed in Huh-7 cells (Supporting Fig. S7). We further subcutaneously injected nude mice to investigate the effect of METTL3 overexpression on HCC growth in vivo. Compared to the nontarget control group, overexpression of METTL3 significantly promoted HCC growth in nude mice. The size and weight of tumors were dramatically increased upon METTL3 overexpression (Fig. 4E). Results from our knockdown/knockout and overexpression models led us to conclude that METTL3 may act as an oncogene that promotes HCC proliferation and metastatic growth.
TRANSCRIPTOME SEQUENCING AND m6A SEQUENCING IDENTIFIED SOCS2 AS A METTL3 DOWNSTREAM TARGET
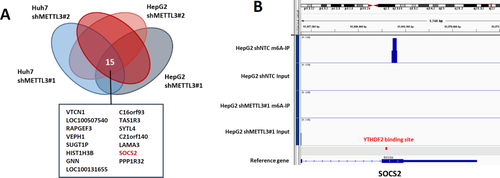
To delineate the functional implications of METTL3 and identify its downstream targets in HCC, we performed transcriptome sequencing to interrogate the expression changes in METTL3 stable knockdown cells. We observed good consistency between the two independent shRNAs. In total, we identified 515 and 320 genes that were consistently up-regulated by two independent shRNAs in Huh-7 and HepG2 cells, respectively (Fig. 5A; Supporting Fig. S8). Gene ontology analysis revealed that differentially expressed genes were significantly enriched in gene sets involved in cell differentiation, cell adhesion, response to external stimulus, extracellular matrix organization, cell migration, and cell proliferation, suggesting that m6A may have profound impacts on cancer biology (Supporting Fig. S9). We also noted that the METTL3 up-regulated gene sets are highly cell-line specific. There was only minimal overlapping between Huh-7 and HepG2 cells (Supporting Fig. S8). Fifteen genes were found to be consistently up-regulated by two independent shRNAs in both HepG2 and Huh-7 cells (Fig. 5A). Among these, we selected suppressor of cytokine signaling 2 (SOCS2) as a candidate target of METTL3-mediated m6A modification for further investigation. We also performed m6A-Seq to map the m6A modification in HepG2 cells. In agreement with previous reports, we found that the m6A signal was enriched around the stop codon of mRNAs12, 13 (Supporting Fig. S10). We identified 4,029 m6A-modified transcripts in HepG2 cells. Pathway analysis revealed that m6A-modified transcripts in HepG2 cells were enriched in many biological processes (such as protein ubiquitination, histone modification, and chromatin organization) and cancer-related pathways (such as Ras and Wnt signaling pathways). In addition, m6A-modified genes were also involved in regulating mRNA stability, splicing, and translation, which are directly related to the functional consequence of m6A modification (Supporting Fig. S11). Importantly, SOCS2 was identified as a direct target of m6A modification in our m6A-Seq. An m6A peak was detected around the stop codon of SOCS2 mRNA in nontarget control shRNA HepG2 cells and was diminished upon METTL3 knockdown (Fig. 5B). Consistent with our findings, previously reported transcriptome-wide m6A profiling data also identified SOCS2 mRNA as a potential target of m6A modification.12, 13, 35 In conclusion, SOCS2 was identified as a METTL3 downstream target.
METTL3 REDUCED SOCS2 mRNA STABILITY THROUGH AN m6A-YTHDF2-DEPENDENT PATHWAY
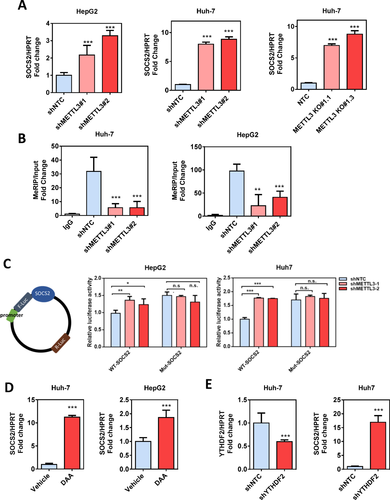
The up-regulation of SOCS2 with METTL3 inactivation was validated by qRT-PCR, and we confirmed that SOCS2 was remarkably up-regulated in stable METTL3 knockdown cells and in METTL3 knockout cells (Fig. 6A). To prove that METTL3 targets SOCS2 mRNA for m6A modification, we validated the m6A-Seq data by MeRIP qPCR.12 The results showed that m6A-specific antibody significantly enriched SOCS2 mRNA compared with the immunoglobulin G pull-down control. As expected, knockdown of METTL3 dramatically reduced the m6A level of SOCS2 mRNA (Fig. 6B). To further address the effect of m6A modification on SOCS2 expression, we constructed both wild-type and mutant SOCS2 reporter minigenes. For the mutant form of SOCS2, the adenosine bases in m6A consensus sequences (i.e., RRACH) were replaced by cytosine, thus abolishing m6A modification. Relative luciferase activity of the wild-type or mutant SOCS2-fused reporter was compared in Huh-7 and HepG2 cells. When compared to the wild-type, mutation on the m6A consensus sequences increased the expression of SOCS2 (Fig. 6C). Luciferase activity of the wild-type SOCS2-fused reporter was also significantly augmented upon METTL3 silencing. Knockdown of METTL3 showed no effect on the expression of the mutant SOCS2-fused reporter, suggesting the modulation of SOCS2 expression was under the control of METTL3-associated m6A modification. In addition, we found that treatment of HCC cells with 3-deazaadenosine, the global methylation inhibitor, substantially increased the expression of SOCS2 (Fig. 6D). Analysis of previously reported photoactivatable ribonucleoside-enhanced crosslinking and immunoprecipitation sequencing (PAR-CLIP-Seq) data identified direct binding between the 3′ end of the SOCS2 transcript and the m6A reader protein YTHDF2.19 The YTHDF2 binding site is close to the m6A modification site as revealed by our m6A-Seq (Fig. 5B). Consistently, we showed that knockdown of YTHDF2 strongly augmented SOCS2 expression in Huh-7 cells (Fig. 6E). Together, our findings suggest that METTL3-mediated m6A modification repressed SOCS2 expression through YTHDF2-dependent mRNA degradation.
SOCS2 TUMOR SUPPRESSOR FUNCTION IN HCC
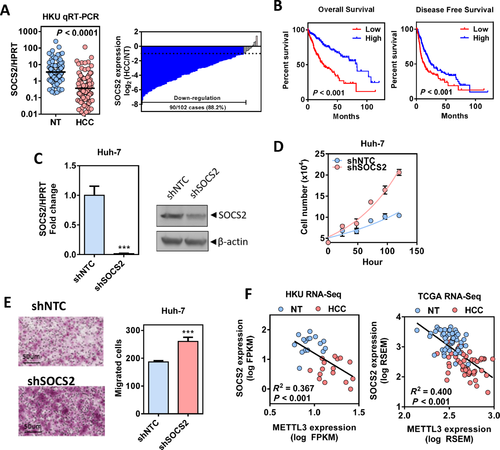
SOCS2 is a member of the suppressor of cytokine signaling family, which serves as a cytokine-inducible negative regulator of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway.36 We found that knockdown of METTL3 increased SOCS2 protein expression and consequently inhibited signal transducer and activator of transcription 5 (STAT5) phosphorylation in Huh7 cells (Supporting Fig. S12A). Next, we validated SOCS2 deregulation in HCC using qRT-PCR in a sample cohort consisting of 102 pairs of HCC and their corresponding NT liver tissues. In this sample cohort, we found that SOCS2 was frequently down-regulated in HCC (Fig. 7A), and 88.2% (90/102) cases showed a significant down-regulation of SOCS2 by more than 2-fold (Fig. 7A). Moreover, lower expression of SOCS2 (<median) significantly correlated with poor overall and disease-free survival of patients with HCC (Fig. 7B). To further characterize the tumor suppressor function of SOCS2, we established stable SOCS2 knockdown Huh-7 cells. SOCS2 knockdown efficiency was confirmed at both the mRNA and protein levels (Fig. 7C). Knockdown of SOCS2 significantly enhanced HCC proliferation under low-serum conditions (Fig. 7D) and increased the HCC migration rate as shown by the transwell migration assay (Fig. 7E). Moreover, we showed that knockdown of SOCS2 by small interfering RNA can partially rescue the proliferation rate of METTL3 knockdown Huh-7 cells (Supporting Fig. S12B,C). More importantly, SOCS2 expression was negatively correlated with METTL3 expression in both TCGA HCC data set and our HCC cohort (Fig. 7F; Supporting Fig. S12D). Together, these data indicate that SOCS2 is a downstream target of METTL3 and serves as a tumor suppressor in HCC.
Discussion
Traditional models hold that genetic information flows from DNA to RNA and then to protein. Diverse chemical modifications of DNA and proteins are well studied, and their biological and pathologic functions have been extensively demonstrated.3 Analogous to DNA and proteins, cellular RNAs also carry more than 100 types of cotranscriptional and posttranscriptional modifications, which have been known for decades. Nevertheless, the biological meanings of these RNA modifications remain largely unexplored due to technical limitations. Among these distinct chemical modifications decorating RNAs, m6A is the most prevalent one for human mRNA. Recently developed, antibody-based, high-throughput sequencing technology allows researchers to precisely map the exact m6A sites and gain further insights into its biological functions.12 Although m6A has shown its potential in regulating mRNA decay, translation, and processing, limited studies have addressed the pathologic implications of m6A deregulation in disease models. In this study, we first unveiled that the major m6A writer METTL3 was significantly up-regulated in different solid tumors and served as an oncogene in human liver carcinogenesis. We functionally demonstrated the essential role of METTL3 in promoting HCC growth and migration through lentiviral-based gene knockdown, CRISPR/Cas9 gene knockout, and the CRISPR/dCas9-VP64 gene activation system in both in vitro and in vivo models. More importantly, we identified a prominent tumor suppressor, SOCS2, as a direct downstream target of METTL3-mediated m6A modification. Collectively, our findings suggest that deregulation of METTL3 and its relevant m6A modification promote liver carcinogenesis through regulating the expression and stability of critical tumor suppressor genes at the posttranscriptional level. Thus, our findings add a new layer of epigenetic alterations that contribute to the progress of liver cancer development.
While we have focused on METTL3 and its epigenetic silencing function on tumor suppressor genes in HCC, Zhang et al.26 reported that deregulation of ALKBH5, an m6A eraser, affected breast cancer differentiation and reprogramming. In their study, hypoxia-induced ALKBH5 expression and subsequent removal of m6A from NANOG mRNA enhanced NANOG mRNA stability, thereby promoting breast cancer stem cell renewal. m6A has been reported to be essential for cell state transition. However, findings of m6A functions in differentiation and reprogramming may sometimes seem controversial. For instance, knockdown of Mettl3 resulted in reduced self-renewal abilities in mouse embryonic stem cells,22 whereas a later study proposed a crucial role of Mettl3 in embryonic priming from naive pluripotency toward a more differentiated lineage.24 A reasonable explanation for this paradox could be attributed to the differences in cellular content and m6A modification status on pluripotency-maintaining genes and cell differentiation-promoting genes. For instance, in cells where pluripotency-maintaining genes are preferentially enriched with m6A modification, inactivation of Mettl3 would decrease the methylation of these transcripts, leading to enhanced mRNA stability and increased gene expression, which eventually restricts the cells in their pluripotency state. In contrast, in cells where cell differentiation-promoting genes are particularly methylated, silencing of Mettl3 would drive cell state transition while elevating m6A level would lead to cell reprogramming.11 Because m6A-modified gene repertoires vary substantially among different cell types and disease states, it would be interesting to investigate the role of m6A modification in the formation and maintenance of liver cancer stem cells. Moreover, when we interrogate the transcriptome changes of METTL3 knockdown in Huh-7 and HepG2 cells, the variations between two shRNAs in the same HCC cell line are much smaller than that of one shRNA between two different HCC cell lines. This evidence supports the idea of cancer heterogeneity and highlights the differences in m6A target gene repertoires among cancer cells. However, it is unclear what determines target specificity and cellular heterogeneity of m6A modification in normal and cancer cells; future experiments will be necessary to shed light on this fundamental question.
In addition to mRNA, N6-adenosine methylation has been found on pre-miRNA and plays a promoting role in miRNA maturation.37 Recently, Ma et al.38 reported that METTL14 was suppressed in HCC and impaired primary miRNA processing to promote HCC metastasis. However, as shown in Fig. 1A and Supporting Fig. S1, the expression of METTL14 was unchanged in our transcriptome sequencing analysis and was less abundantly expressed than METTL3 in HCC tumor tissues. In line with our data, we observed the same expression pattern of m6A regulators in TCGA data set of 50 patients with HCC (Supporting Fig. S2). In addition, two recently identified m6A potential writers, RBM15B and KIAA1429,11, 39 are dramatically up-regulated in human HCC (data not shown). Together, the up-regulation of m6A writers METTL3, RBM15B, and KIAA1429 support our conclusion that METTL3-associated m6A machinery is activated in human HCC. Similarly, an earlier study found that m6A modification level was elevated upon cellular transformation.28 Furthermore, we observed a significant increase in mRNA m6A levels in primary HCC samples, which further support the up-regulation of m6A modification in liver cancers. To further address the role of METTL14 in HCC, we stably knocked down METTL14 with two independent shRNA sequences and demonstrated that the knockdown of METTL14 significantly suppressed Huh-7 proliferation, migration, and colony-formation abilities (Supporting Fig. S13A-D). Additionally, we activated endogenous METTL14 expression in MHCC97L using the CRISPR-dCas9-VP64 gene activation system. Overexpression of METTL14 modestly yet significantly promoted HCC cell proliferation. At the same time, overexpression of METTL14 dramatically increased HCC migration (Supporting Fig. S13E-G). Therefore, we concluded that METTL3 and METTL14 are both required for HCC growth and metastasis. The discrepant findings between the two studies underscore the complicity of m6A modification and its regulatory enzymes in human cancers. Further investigations will be required to better address this controversy.
Using RNA-Seq and m6A-Seq, we identified SOCS2 as a downstream target of METTL3-mediated m6A modification. This finding was further supported by the MeRIP assay, luciferase, and qRT-PCR in control and METTL3-knockdown HCC cells. m6A-Seq is a powerful tool to identify m6A-modified transcripts on a genome-wide scale. This approach, a combination of chromatin immunoprecipitation sequencing (ChIP-Seq) and RNA-Seq, involves immunoprecipitation of m6A-modified RNA fragments with specific antibodies. However, there are some limitations related to this experiment, including high-input materials, low resolution, relative high background, and bioinformatic challenges.34 Therefore, although the output of m6A-seq is robust, extensive validations are necessary to confirm the m6A-seq results.
In this study, we demonstrated that METTL3 epigenetically silenced SOCS2 through an m6A-YTHDF2-dependent mechanism. The binding between SOCS2 mRNA and YTHDF2 was revealed by analysis of previously reported YTHDF2 PAR-CLIP-Seq data.19 The identified YTHDF2 binding sequence is located near the m6A enrichment regions around the stop codon. Moreover, knockdown of YTHDF2 extended the half-life of the SOCS2 transcript.19 We demonstrated in this study that knockdown of YTHDF2 augmented SOCS2 expression in HCC cells. Thus, multiple lines of evidence supported our notion that the SOCS2 transcript is a direct target of YTHDF2. It should be noted that YTHDF2-mediated mRNA decay might not be the only mechanism underlying m6A functions in cancer progression. Because m6A modification relies on reader proteins to exert its biological functions, mRNA transcripts might be sorted into different groups based on their readers, which together may form a network to influence cellular functions. For example, YTHDF1 has recently been suggested to promote mRNA translation in an m6A-dependent manner.40 In addition, a recent study proposed that METTL3 could recruit the translational initiation complex to promote mRNA translation in a subset of oncogenes, including epidermal growth factor receptor (EGFR), TAZ, and DNA (cytosine-5)-methyltransferase 3A (DNMY3A), in lung cancer. Interestingly, this mechanism is independent of the catalytic activity of METTL3 and additional m6A reader proteins.31 Thus, it would also be interesting to compare the proteomic changes between tumorous and nontumorous samples upon METTL3 manipulation in future studies.
SOCS family proteins have long been known to be essential tumor suppressors in different cancer types. Compared to SOCS1, which has well-defined inhibitory effects on human malignancy and DNA methylation has been identified as the major mechanism leading to its inactivation in cancers,36 SOCS2 has only been reported to inhibit proliferation, migration, or stemness in different cancer types, including leukemia, oral squamous carcinoma, and most recently HCC.41 Limited information is available to explain how SOCS2 is actually down-regulated in cancers. In this study, we demonstrated that SOCS2, an important member of the SOCS family, is under the regulation of METTL3-dependent m6A modification. We demonstrated that besides hypermethylation in the CpG island at the DNA level, cancer cells also develop mRNA-level m6A hypermethylation to inhibit tumor suppressor gene expressions. Our findings on the implication of METTL3-mediated m6A modification in epigenetic silencing of SOCS2 in HCC exemplified the critical role of m6A epitranscriptomic change in human carcinogenesis.
Acknowledgment
We thank the Core Facility and Center for Genomic Sciences of the Li Ka Shing Faculty of Medicine for their technical support and the Laboratory Animal Unit for animal housing.
REFERENCES
Author names in bold designate shared co-first authorship.