Hypoxia-inducible factor-1α/interleukin-1β signaling enhances hepatoma epithelial–mesenchymal transition through macrophages in a hypoxic-inflammatory microenvironment
Potential conflict of interest: Nothing to report.
Supported by the National Natural Science Foundation of China (81401954, 81472212, and 81300341); the Key Program of Medical Scientific Research Foundation of Zhejiang Province, China (WKJ-ZJ-1410); the Key Program of Administration of Traditional Chinese Medicine of Zhejiang Province, China (2014ZZ007); the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents, the National 973 Program (2014CB542101); and the National Nonprofit Industry Research Subproject (201502014).
*[Correction added on April 4, 2018, after first online publication: The second corresponding author name, Xueli Bai, was left out erroneously. The name has now been added.]
Abstract
The development and progression of hepatocellular carcinoma (HCC) are dependent on its local microenvironment. Hypoxia and inflammation are two critical factors that shape the HCC microenvironment; however, the interplay between the two factors and the involvement of cancer cells under such conditions remain poorly understood. We found that tumor-associated macrophages, the primary proinflammatory cells within tumors, secreted more interleukin 1β (IL-1β) under moderate hypoxic conditions due to increased stability of hypoxia inducible factor 1α (HIF-1α). Under persistent and severe hypoxia, we found that the necrotic debris of HCC cells induced potent IL-1β release by tumor-associated macrophages with an M2 phenotype. We further confirmed that the necrotic debris–induced IL-1β secretion was mediated through Toll-like receptor 4/TIR domain–containing adapter-inducing interferon-β/nuclear factor kappa-light-chain-enhancer of activated B cells signaling in a similar, but not identical, fashion to lipopolysaccharide-induced inflammation. Using mass spectrometry, we identified a group of proteins with O-linked glycosylation to be responsible for the necrotic debris–induced IL-1β secretion. Following the increase of IL-1β in the local microenvironment, the synthesis of HIF-1α was up-regulated by IL-1β in HCC cells through cyclooxygenase-2. The epithelial–mesenchymal transition of HCC cells was enhanced by overexpression of HIF-1α. We further showed that IL-1β promoted HCC metastasis in mouse models and was predictive of poor prognosis in HCC patients. Conclusion: Our findings revealed an HIF-1α/IL-1β signaling loop between cancer cells and tumor-associated macrophages in a hypoxic microenvironment, resulting in cancer cell epithelial–mesenchymal transition and metastasis; more importantly, our results suggest a potential role of an anti-inflammatory strategy in HCC treatment. (Hepatology 2018;67:1872-1889)
Abbreviations
-
- CD68
-
- cluster of differentiation 68
-
- COX-2
-
- cyclooxygenase-2
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- EMT
-
- epithelial–mesenchymal transition
-
- HCC
-
- hepatocellular carcinoma
-
- HIF-1α
-
- hypoxia-inducible factor-1α
-
- HLA
-
- human leukocyte antigen
-
- IL-1β
-
- interleukin-1β
-
- iNOS
-
- inducible nitric oxide synthase
-
- LPS
-
- lipopolysaccharides
-
- MDM
-
- monocyte-derived macrophage
-
- NF-κB
-
- nuclear factor kappa-light-chain-enhancer of activated B cells
-
- p-NF-κB
-
- phosphorylated NF-κB
-
- RFP
-
- red fluorescence protein
-
- siRNA
-
- small interfering RNA
-
- SEM
-
- standard error of the mean
-
- TAM
-
- tumor-associated macrophage
-
- TLR
-
- Toll-like receptor
-
- TNF-α
-
- tumor necrosis factor-α
-
- TRIF
-
- TIR domain–containing adapter-inducing interferon-β
The initiation and progression of solid tumors, including hepatocellular carcinoma (HCC), are closely associated with the local inflammatory microenvironment.1 Various mechanisms by which tumor and inflammation mutually interfere with each other have been discovered.2 Multiple components in addition to inflammatory mediators exist in the tumor microenvironment, and the crosstalk presents an extremely complicated picture; however, this relationship is far less understood. Hypoxia is common in HCC and impacts universally on all cells within tumors, including tumor cells and stromal cells.3 The influence of hypoxia on tumor cells has been widely studied; however, for tumor-associated macrophages (TAMs), which play a critical role in tumor progression,4 the association between hypoxia and inflammation remains poorly understood.
Epithelial–mesenchymal transition (EMT) contributes to HCC metastasis and results in a poor prognosis.5 Several EMT inducers for HCC, including hypoxia and TAMs, have been identified.6, 7 Previous studies investigated the mutual influence between cancer cells and TAMs, primarily under normoxic conditions7; however, in patients, TAMs are exposed to hypoxia of different severities. The distribution and function of TAMs are sensitive to hypoxia-related stress. For instance, TAMs tend to accumulate in the hypoxic areas of tumors8, 9 and have a stronger capacity for promoting angiogenesis in such a scenario.10 Moreover, hypoxia-inducible factor 1α (HIF-1α), which plays a key role in the function of TAMs,11 is stabilized in hypoxia12 and leads to distinct properties of TAMs. Therefore, the mutual interplay between tumor cells and TAMs under hypoxia requires further investigation to deepen our understanding of its role in HCC progression.
We previously found that HIF-1α induced EMT in HCC cells and that interleukin 1β (IL-1β) was secreted by TAMs with different phenotypes.6, 13 While IL-1β can enhance the transcription of HIF-1α, this cytokine appears to be critical in the hypoxic-inflammatory tumor microenvironment where the degradation of HIF-1α has already been blocked. Here, we report that a HIF-1α/IL-1β signaling loop, which involves the interaction of tumor cells and TAMs under hypoxia, mediates TAM-induced HCC progression by promoting EMT of cancer cells.
Materials and Methods
CELL CULTURE AND REAGENTS
Cell culture methods are described in the Supporting Information. For macrophage differentiation, THP-1 cells were stimulated overnight with 200 nM phorbol 12-myristate 13-acetate (Sigma-Aldrich, St. Louis, MO). Human monocyte-derived macrophages (MDMs), mouse bone marrow–derived macrophages, and mouse peritoneal macrophages were obtained as described in the Supporting Information. For Toll-like receptor 4 (TLR4) signaling inhibition, Pepinh-TIR domain–containing adapter-inducing interferon-β (TRIF), Pepinh-MYD, CLI-095, and antihuman TLR4 antibody were purchased from InvivoGen (San Diego, CA).
GENERATION OF NECROTIC DEBRIS AND CONDITIONED MEDIUM
To generate cancer cell necrotic debris, Huh-7 cells were cultured in hypoxia for 48 hours after reaching 100% confluence. For apoptotic cell debris, cells were treated with 6 mM cisplatin (Sigma-Aldrich) for 24 hours. Cells and supernatants were collected and centrifuged (3000 g, 15 minutes). Cell debris was then washed three times with phosphate-buffered saline to completely remove soluble components. The debris was stored at −30°C and resuspended in Roswell Park Memorial Institute 1640 when used. The induction efficiency of necrotic and apoptotic debris was confirmed by flow cytometry with fluorescein isothiocyanate–annexin V/propidium iodide staining (BD Biosciences). The conditioned medium was harvested after cells were cultured for 48 hours, followed by centrifugation (3000 g, 10 minutes) and filtered through 0.2-μm pore filters to remove debris. Before use, the conditioned medium was mixed 1:1 with complete medium. All samples were tested for the presence of bacterial endotoxin using the Limulus amebocyte lysate test (Genscript, Nanjing, China) for qualification (<0.02 EU/mL).
TRANSWELL ASSAYS
Migration and invasion capacities of cells were detected by transwell assays as described in the Supporting Information.
TRANSFECTION, RNA INTERFERENCE, AND LUCIFERASE REPORTER ASSAYS
Small interfering RNA (siRNA) transfection was performed as reported.6 For the luciferase reporter assays, cells were seeded into a 96-well plate and transfected with hypoxia-response element–luciferase reporter plasmids (a gift from Navdeep Chandel14; Addgene plasmid 26731). Luciferase activities were measured using the Luciferase Reporter Assay System (Promega, Madison, WI). Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) reporter plasmids were a gift from Prof. Qingqing Wang at Zhejiang University School of Medicine.
IMMUNOBLOTTING, IMMUNOFLUORESCENCE, AND IMMUNOHISTOCHEMISTRY
Immunoblotting and immunofluorescence were performed as described.6 Paraffin-embedded HCC tissue samples were cut into 5-μm sections and processed for immunohistochemistry. Detailed procedures are shown in the Supporting Information.
ENZYME-LINKED IMMUNOSORBENT ASSAY
Supernatants were collected and stored at −80°C after removal of cells and debris by centrifugation. Concentrations of IL-1β, IL-6, and tumor necrosis factor-α (TNF-α) were detected using specific enzyme-linked immunosorbent assay (ELISA) kits (Biolegend, San Diego, CA, and R&D Systems, Minneapolis, MN). For IL-1β detection, 5 mM adenosine 5′-triphosphate (Sigma-Aldrich) was added for 30 minutes before sample collection. Experiments were performed according to the manufacturer's instructions.
QUANTITATIVE REAL-TIME PCR
The relative expression of specific genes was measured with the Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA) using the comparative threshold cycle method. Detailed procedures are described in the Supporting Information. All primers were synthesized by Sangon Biotech (Shanghai, China; Supporting Table S1).
FLOW CYTOMETRY
Single-cell suspensions were generated, and cells were stained with specific antibodies according to the manufacturer's instructions. Antibodies including human leukocyte antigen (HLA) A, HLA-B, HLA-C (W6/32), cluster of differentiation 68 (CD68; FA-11), CD45 (2D1), CD206 (19.2), CD163 (GHI/61), CD11b (ICRF44), and IL-1β (JK1B-1) were purchased from eBioscience (San Diego, CA) or Biolegend. Isotype controls were used for all staining. Cell sorting was performed using a FACSAria II cell sorter (BD Biosciences). Flow-cytometric analysis was performed on a BD FACS Canto-II Flow Cytometer (BD Biosciences).
MOUSE MODELS
Male BALB/c nude mice, 5-6 weeks old, were purchased from Shanghai Experimental Animal Center (Shanghai, China). Spontaneous HCC mice (Mst1–/–;Mst2–/flox;Alb-Cre) were a gift from Professor Bin Zhao (Life Science Institute Zhejiang University). Detailed procedures are shown in the Supporting Information.
HUMAN TISSUE ACQUIREMENT
Human tumor samples were obtained from patients with HCC who underwent curative resection between 2013 and 2017 at the Second Affiliated Hospital, Zhejiang University School of Medicine. Informed consent of patients was obtained. The protocol was approved by the Ethics Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine. The clinicopathological information of all samples used is shown in Supporting Tables S2-S6. HCC tissue microarrays were purchased from Shanghai Outdo Biotech (Shanghai, China).
STATISTICAL ANALYSIS
The data were analyzed using GraphPad Prism 6 (San Diego, CA) and are presented as means ± standard deviation or standard error of the mean (SEM). Continuous variables were analyzed using an unpaired Student t test or Mann-Whitney test as indicated to compare between two groups. Categorical variables were compared using a chi-squared test or Fisher exact test. Survival analysis was conducted using the Kaplan-Meier method with a log-rank test. P < 0.05 was considered statistically significant.
Results
MODERATE HYPOXIA FACILITATES IL-1β SECRETION BY MONOCYTES AND MACROPHAGES THROUGH HIF-1α
To investigate the impact of hypoxia on proinflammatory cytokine secretion of the monocyte–macrophage system, we first examined the levels of IL-1β and IL-6 secreted by THP-1, a widely used human monocyte cell line. Compared with unstimulated THP-1 cells cultured under normoxia, IL-1β secretion increased mildly but significantly with desferrioxamine treatment, a chemical mimicry of hypoxia (Fig. 1A). Similarly, moderate hypoxia mildly increased the level of IL-1β rather than IL-6 in both THP-1 cells and THP-1-derived macrophages (Fig. 1B). When activated by lipopolysaccharide (LPS), THP-1-derived macrophages and human MDMs were much more sensitive to hypoxia-enhanced IL-1β secretion (Fig. 1C). Similar effects were observed in mouse bone marrow–derived macrophages and peritoneal macrophages in the presence of LPS (Fig. 1D). Because LPS induces IL-1β synthesis by up-regulating HIF-1α,15 we used HIF-1α siRNA to confirm the role of HIF-1α in LPS-induced IL-1β production in THP-1-derived macrophages. As expected, inhibition of HIF-1α eliminated the LPS-induced pro-IL-1β expression in THP-1-derived macrophages (Fig. 1E). These results suggested that hypoxia in HCC could create a proinflammatory microenvironment.
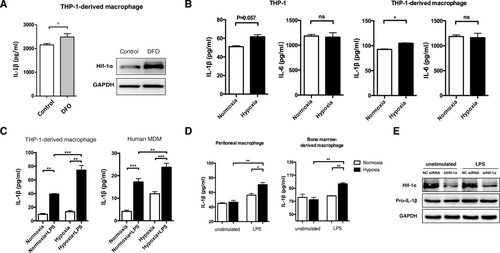
Hypoxia treatment promotes IL-1β expression through HIF-1α in monocytes and macrophages. (A) IL-1β levels (detected by ELISA) and HIF-1α expression (detected by western blotting) in THP-1-derived macrophages treated with or without desferrioxamine (100 μM, 24 hours). For IL-1β detection, 5 mM adenosine 5′-triphosphate was added to the medium 30 minutes prior to sample collection. (B) IL-1β and IL-6 levels in THP-1 cells or THP-1-derived macrophages increased under conditions of normoxia (21% O2) or hypoxia (1% O2). (C) IL-1β in THP-1-derived macrophages and human MDMs cultured in normoxia or hypoxia for 24 hours, followed by LPS (100 ng/mL, 24 hours) treatment. (D) Peritoneal macrophages (left panels) and bone marrow–derived macrophages (right panels) were cultured under normoxic or hypoxic conditions, followed by LPS (100 ng/mL, 3 hours) treatment. The IL-1β level was measured by ELISA. (E) THP-1-derived macrophages transfected with HIF-1α siRNA or negative control siRNA were stimulated with LPS (100 ng/mL, 12 hours), and the expression of HIF-1α and pro-IL-1β was measured. Results in (A)-(D) are expressed as the means ± SEM of a minimum of three independent experiments, analyzed with an unpaired t test. *P < 0.05, **P < 0.01, ***P < 0.001. Abbreviations: DFO, desferrioxamine; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NC, negative control; ns, not significant.
SEVERE HYPOXIA-ASSOCIATED NECROTIC DEBRIS OF CANCER CELLS STIMULATES IL-1β PRODUCTION IN MACROPHAGES
Clinically, in some cases, hypoxia or even anoxia is so severe and persistent that HCC cells are forced to undergo necrosis. Macrophages are natural scavengers and are recruited to these regions to clear the necrotic debris.16 In patients with large HCCs exhibiting necrotic centers, we observed substantial CD68+ TAM infiltration in the tumor margin, tumor body, and necrotic center (Supporting Fig. S1). To mimic the local microenvironment in the necrotic center of HCC, we investigated whether Huh-7 conditioned medium or persistent hypoxia-associated necrotic debris of Huh-7 cells could stimulate IL-1β secretion in THP-1-derived macrophages in vitro. Persistent hypoxia-associated necrotic debris was confirmed by flow cytometry to ensure that the necrotic components were similar to those exhibited by human HCC samples (Supporting Fig. S2). Stimulation with Huh-7 necrotic debris significantly enhanced IL-1β production in macrophages (Fig. 2A). Moreover, expression of pro-IL-1β was enhanced in a time-dependent manner following the stimulation (Fig. 2B). Although the mRNA levels of proinflammatory cytokines, including IL-1β, IL-6, and TNF-α, were dramatically up-regulated following treatment with Huh-7 necrotic cell debris (Fig. 2C; Supporting Fig. S3), the secreted protein levels of IL-6 and TNF-α did not significantly increase (Fig. 2A).
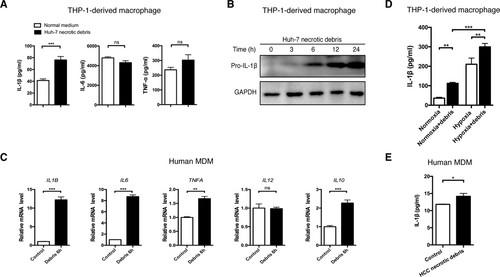
Necrotic debris of cancer cells stimulates IL-1β secretion in macrophages. (A) THP-1-derived macrophages were stimulated with necrotic debris of Huh-7 cells (debris from three Huh-7 cells per macrophage, 48 hours) and the levels of IL-1β, IL-6, and TNF-α were detected by ELISA. (B) Pro-IL-1β accumulation in THP-1-derived macrophages stimulated with Huh-7 debris from 0 to 24 hours. (C) Human MDMs were incubated with Huh-7 cell debris (6 hours), and mRNA levels of proinflammatory genes were measured by quantitative real-time PCR. (D) THP-1-derived macrophages were treated with Huh-7 necrotic debris in normoxia or hypoxia, and the IL-1β level was determined by ELISA. (E) The IL-1β level in human MDMs stimulated with necrotic debris isolated from HCC samples of the same patients. Data are expressed as the mean ± SEM of a minimum of three independent experiments, analyzed by an unpaired t test. *P < 0.05, **P < 0.01, ***P < 0.001. Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; ns, not significant.
To further confirm the effect of necrotic debris of cancer cells, we acquired MDMs from healthy donors and HCC patients. MDMs from healthy donors showed consistent alterations upon stimulation of Huh-7 necrotic debris with THP-1-derived macrophages (Fig. 2D). The proinflammatory effect of cancer cell necrotic debris was further enhanced in hypoxia (Fig. 2D). Necrotic debris isolated from HCC samples was then used to stimulate MDMs from the same patients, and IL-1β secretion by macrophages was significantly up-regulated (Fig. 2E). Altogether, we proved that HCC necrotic debris could prime macrophages, enhancing IL-1β production.
O-GLYCAN STRUCTURES CONTRIBUTE TO NECROTIC DEBRIS–INDUCED IL-1β PRODUCTION
We noted that Huh-7 conditioned medium failed to induce IL-1β secretion of macrophages (Supporting Fig. S4A), though it facilitated the differentiation of THP-1-derived macrophages (Supporting Fig. S4B). We further excluded any residual soluble components using debris eluent, and no IL-1β inductive effect was detected (Supporting Fig. S4C). Thus, direct cell-debris contact but not secretory cytokines or soluble damage-associated molecular patterns played a pivotal role in cell debris–induced macrophage activation. Furthermore, living cells and apoptotic cell debris were incapable of inducing IL-1β secretion in macrophages (Supporting Fig. S5). However, proteinase K and O-glycosidase, rather than peptide-N-glycosidase F, treatment could partially or completely inhibit the stimulatory effect of necrotic debris (Fig. 3A,B). For further validation, we used debris of benzyl-N-acetyl-D-galactosamine (an O-glycosylation inhibitor)–pretreated Huh-7 cells to stimulate human MDMs and observed no IL-1β inductive effect (Fig. 3C). More importantly, O-glycosidase treatment of Huh-7 necrotic debris simultaneously inhibited cytokine overexpression and reversed the expression of polarization-related genes including NOS2, CD163, ARG1, and MRC1 (Fig. 3D,E). We then investigated the protein change by gel electrophoresis (Supporting Fig. S6) and, using mass spectrometry, identified a group of membrane-located proteins that potentially contributed to IL-1β induction (Supporting Table S7). These results indicated that O-glycan structures in certain glycoproteins were required for the HCC necrotic debris–induced proinflammatory effect in macrophages.

O-glycan structures are responsible for the elevated IL-1β secretion in macrophages stimulated by cancer cell necrotic debris. (A) IL-1β levels in THP-1-derieved macrophages stimulated with Huh-7 necrotic debris with or without pretreatment with proteinase K (20 mg/mL, 24 hours). (B) THP-1-derived macrophages were stimulated with Huh-7 necrotic cell debris (debris from three Huh-7 cells per macrophage) pretreated with either peptide-N-glycosidase F (50 U/mL) or O-glycosidase (50 U/mL) for 24 hours. The IL-1β level was detected by ELISA. (C) Huh-7 cells were treated with 2 mM benzyl-N-acetyl-D-galactosamine for 72 hours, and necrotic debris was obtained to stimulate human MDMs. The IL-1β level was determined by ELISA. (D,E) The mRNA level of pro-inflammatory genes (D) and macrophage phenotype markers (E) in THP-1-derived macrophages stimulated with debris and the indicated treatment for 6 hours. Representative data from at least three independent experiments are shown. Error bars represent the SEM. *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant. Abbreviations: BADG, benzyl-N-acetyl-D-galactosamine; PNaseF, peptide-N-glycosidase F.
NECROTIC DEBRIS INDUCES PROINFLAMMATORY EFFECTS AND M2 POLARIZATION IN MACROPHAGES
HCC cells can “educate” TAMs to promote cancer progression by inducing TAM M2 polarization toward an anti-inflammatory phenotype.17 Unexpectedly, we detected M2 polarization of macrophages upon stimulation with cancer cell necrotic debris, which paradoxically induced proinflammatory effects. In the MDMs of HCC patients stimulated with the same patient-derived HCC necrotic debris, we detected increased CD206 expression and reduced inducible nitric oxide synthase (iNOS) expression (Fig. 4A,B). We also found an accumulation of CD206+ TAMs in both the tumor margin and necrotic center of HCC (Fig. 4C). Notably, the necrotic center had even more CD206+ TAMs than the tumor margin (Fig. 4D). Intriguingly, TAMs in the necrotic center of HCC also had an increased capacity of IL-1β production compared with those at the tumor margin (Fig. 4E,F). Using RNA sequencing, we found a general activation of proinflammatory response, and particularly, NF-κB signaling was activated (Supporting Fig. S7). In addition, Huh-7 necrotic debris and LPS exhibited the same metabolic changes (increased glycolysis and decreased oxidative phosphorylation), which are critical for M1 polarization of macrophages,18 in both human and mouse macrophages of distinct origins (Supporting Figs. S7 and S8). Therefore, our findings demonstrated the proinflammatory effects of M2 TAMs in response to stimulation with HCC necrotic debris.
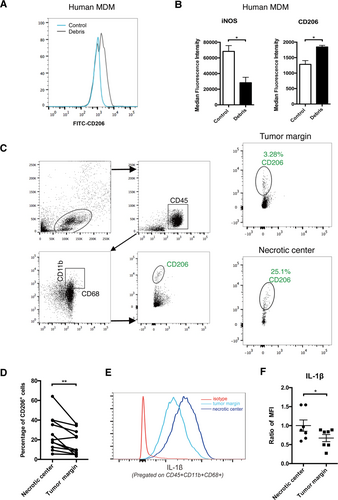
Necrotic debris induces M2 polarization in macrophages. (A) Human MDMs were treated with HCC necrotic debris (48 hours) derived from the same patients, and CD206 expression was measured. (B) Mean fluorescence intensity of iNOS and CD206 expression on human MDMs with or without treatment with HCC necrotic debris (48 hours) (n = 3). (C,D) CD206 expression of TAMs isolated from the necrotic center or tumor margin of human HCC samples. Gating strategies are shown in (C). Statistical analysis is shown in (D) (n = 10). (E,F) IL-1β production of TAMs isolated from the necrotic center or tumor margin of human HCC samples. A representative result is shown in (E). Statistical analysis is shown in (F) (n = 7). *P < 0.05, **P < 0.01, ***P < 0.001. Abbreviations: FITC, fluorescein isothiocyanate; MFI, mean fluorescence intensity.
TLR4/TRIF/NF-κB SIGNALING MEDIATES CELL NECROTIC DEBRIS–INDUCED IL-1β PRODUCTION
Next, we explored the potential mechanisms underlying the enhanced IL-1β production in necrotic debris–primed macrophages. We initially assumed that macrophages could nonselectively engulf necrotic debris and subsequently trigger a proinflammatory response; however, we did not observe a significant engulfment of necrotic debris by macrophages (Supporting Fig. S9A). Additionally, the enhanced secretion of IL-1β was not impaired when phagocytosis was suppressed by cytochalasin D (Supporting Fig. S9B), suggesting a phagocytosis-independent manner.
IL-1β secretion employs a two-step activation mechanism, and TLR signaling is crucial for up-regulating the transcription of IL1B. Because some glycoproteins harbor LPS-like domains and can activate TLR4 to induce a proinflammatory response,19 we assumed that O-linked glycoproteins activated TLR4 in a manner similar to LPS. Indeed, when we used the small molecular inhibitor CLI-095 or an antihuman TLR4 antibody to block TLR4 signaling, the IL-1β inductive role of necrotic debris was ablated (Fig. 5A). Consistently, the transcriptional activity of NF-κB, a downstream effector of TLR4 signaling, was induced by Huh-7 necrotic debris treatment and inhibited by CLI-095 and antihuman TLR4 antibody treatments (Fig. 5B; Supporting Fig. S10A,B). We further analyzed the expression of NF-κB and phosphorylated NF-κB (p-NF-κB) following Huh-7 necrotic debris stimulation. p-NF-κB was up-regulated in a time-dependent manner and reached its peak at 3 hours, while the total NF-κB remained stable (Fig. 5C). In addition, the HIF-1α level was elevated in parallel with p-NF-κB, while the expression of pro-IL-1β increased continuously. Using a TLR signaling pathway quantitative PCR array, we further confirmed the activation of NF-κB instead of the Jun N-terminal kinase/p38 mitogen-activated protein kinase, Janus kinase/signal transducer and activator of transcription, and interferon regulatory factor pathways (Supporting Fig. S11).
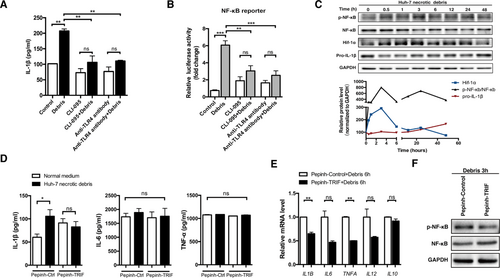
TLR4/TRIF/NF-κB signaling is responsible for necrotic debris–induced IL-1β secretion in macrophages. (A) The IL-1β level in Huh-7 necrotic debris–stimulated macrophages pretreated with or without CLI-095 (1 μg/mL, 6 hours) or anti-TLR4 antibodies (5 μg/mL, 1 hour). (B) NF-κB-luc THP-1-derived macrophages were stimulated with Huh-7 cell debris (debris from three Huh-7 cells per macrophage, 24 hours) with or without CLI-095 (1 μg/mL, 6 hours) or anti-TLR4 antibodies (5 μg/mL, 1 hour) pretreatment. NF-κB reporter luciferase activity was then detected. (C) Expression of pro-IL-1β, HIF-1α, NF-κB, and p-NF-κB in THP-1-derived macrophages was detected by western blotting following stimulation with Huh-7 cell debris for the indicated time interval. (D-F) Following Pepinh-TRIF or Pepinh-control (10 μM, 4 hours) pretreatment, THP-1-derived macrophages were stimulated with Huh-7 cell debris for 48, 6, and 3 hours, respectively. Expression of cytokines in the supernatants (D) and mRNA levels (E) as well as protein expression of NF-κB and p-NF-κB (F) was detected. Representative data from at least three independent experiments are shown. Error bars represent the SEM. *P < 0.05, **P < 0.01, ***P < 0.001. Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; ns, not significant.
Myd88 and TRIF are two TLR4 adapters and mediate overlapping but distinct signaling pathways in response to distinct TLR4 ligands.20 Inhibition of Myd88 using Pepinh-MYD did not affect the induced IL-1β production in both protein and mRNA levels following Huh-7 necrotic debris stimulation (Supporting Fig. S12). Interestingly, Pepinh-TRIF abrogated the enhanced IL-1β secretion induced by Huh-7 necrotic debris (Fig. 5D). Moreover, TRIF inhibition was associated with a consistent decrease of IL1B mRNA and reduced p-NF-κB expression and nuclear location (Fig. 5E; Supporting Fig. S13). The involvement of TLR4/TRIF/NF-κB signaling in IL-1β production and similar changes of M2 phenotypic markers were further verified using Huh-7 necrotic debris–stimulated human MDMs (Supporting Fig. S14). In addition, Huh-7 necrotic debris enhanced the recruitment of TLR4 into the cell membrane (Supporting Fig. S15), possibly due to the presence of saturated fatty acids in the cell membrane,21 further enhancing TLR4 signaling in macrophages. Collectively, these results indicated that TLR4/TRIF/NF-κB signaling mediated cancer cell necrotic debris–induced IL-1β production in macrophages.
The maturation and secretion of IL-1β require caspase-1, which itself needs to be activated.22 We found that Huh-7 necrotic debris induced caspase-1 activation (Supporting Fig. S16), indicating the possible existence of a second signal in our setting and the sufficiency of cancer cell necrotic debris to induce IL-1β production by macrophages.
MACROPHAGE-DERIVED IL-1β INDUCES EMT IN HCC CELLS
To confirm the inflammatory environment in HCC patients, we quantified the serum level of IL-1β in HCC patients, along with patients with cirrhosis and healthy donors. The IL-1β level in HCC patients was significantly elevated compared with that in the healthy controls (Fig. 6A), suggesting a possible association between inflammation and HCC. Notably, HCC patients with necrotic tumors had markedly higher IL-1β levels than patients without evidence of tumor necrosis (81.88 versus 30.48 pg/mL), indicating that tumor necrosis partially contributed to the increased level of IL-1β. To investigate the level of inflammation within HCC, we performed successive cross sections of human HCC tissues and found that the IL-1β level was closely related to the presence of CD68+ TAMs (Fig. 6B; Supporting Fig. S17). Using an HCC tissue microarray, we confirmed that a high level of IL-1β in the tumor margins was significantly associated with a poor prognosis in HCC patients (P = 0.0011; Supporting Table S8 and Fig. S18). Univariate and multivariate analyses showed that tumor–node–metastasis stage and elevated IL-1β expression in tumor margins were two independent factors for overall survival (P < 0.01; Supporting Table S9). These findings indicated that TAM-derived IL-1β may promote HCC progression.
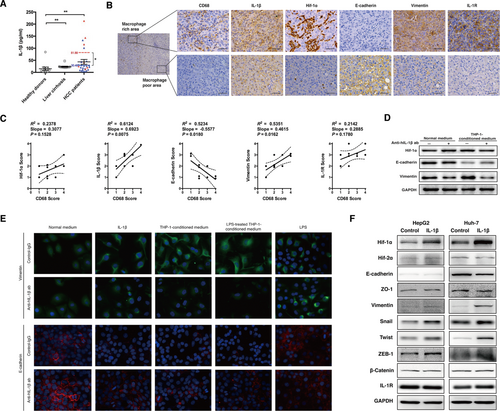
Macrophage-induced IL-1β promotes EMT in HCC cells. (A) Serum concentrations of IL-1β were measured by ELISA. Samples were obtained from the peripheral blood of healthy donors (n = 11), patients with cirrhosis (n = 12), and HCC patients (n = 27). Red triangles indicate samples from HCC patients whose tumors were found to have significant necrosis by imaging or postoperative pathology (n = 7), and blue triangles indicate samples from HCC patients with no evidence of tumor necrosis (n = 20). Red and blue dashes with numbers beside them indicate the means of the two groups, respectively. Data are presented as mean ± SEM. The Mann-Whitney test was used. *P < 0.05, **P < 0.01. (B,C) Cross sections of human HCC tissues (n = 10) were performed and stained with antibodies as indicated. Representative images (magnification, ×50) from areas of distinct CD68 expression are shown in (B). Right panels represent expression of relative proteins in magnified views (× 400) indicated by boxes in the left panel. Scale bar, 100 μm. Immunohistochemical staining and scoring of indicated markers were performed in HCC tissue sections (n = 10) and are shown in (C). Correlations between each of the indicated markers with intratumoral CD68+ macrophage density were interpolated using linear regression. (D) Huh-7 cells were cultured with normal medium or THP-1 conditioned medium, together with control immunoglobulin G or anti-hIL-1β antibodies (1:100, 48 hours). Expression of HIF-1α, E-cadherin, and vimentin was detected by western blotting. (E) Huh-7 cells were incubated with the indicated medium, together with the control immunoglobulin G or anti-hIL-1β antibodies (20 μg/mL, 48 hours). Immunofluorescence staining of the images of E-cadherin (red) and vimentin (green) under specific treatments are shown (magnification, ×400). (F) Expression of EMT markers was measured following IL-1β treatment (250 pg/mL, 48 hours). Abbreviations: ab, antibody; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IgG, immunoglobulin G; IL-1R, IL-1 receptor; ZEB1, zinc finger E-box binding homeobox 1; ZO-1, zonula occludens-1.
We observed that in human HCC tissues the cancer cells in the areas with abundant TAMs exhibited a higher expression of IL-1 receptor and HIF-1α, as well as more significant mesenchymal characteristics (up-regulation of vimentin and down-regulation of E-cadherin) compared with those located in areas with poor TAM infiltration (Fig. 6B,C; Supporting Fig. S17). Given that HIF-1α mediates hypoxia-induced EMT in HCC cells,6 we hypothesized that IL-1β derived from TAMs promoted EMT in HCC cells by inducing a local proinflammatory microenvironment. To test this hypothesis, the conditioned medium from THP-1-derived macrophages was used to culture Huh-7 cells. The conditioned medium elevated vimentin expression and reduced E-cadherin expression in Huh-7 cells; however, this effect was reversed by the IL-1β neutralizing antibody (Fig. 6D), suggesting that IL-1β mediated macrophage-induced EMT in HCC cells. In addition, immunofluorescence assays revealed that there were morphological changes associated with the increased vimentin and decreased E-cadherin expression in Huh-7 cells treated with IL-1β or THP-1-conditioned medium but not LPS stimulation (Fig. 6E). Transwell assays further confirmed that the THP-1-conditioned medium significantly enhanced the migratory and invasive capacities of both Huh-7 and HepG2 cells (Supporting Fig. S19). This effect was retarded by IL-1β neutralizing antibodies, indicating an IL-1β-dependent effect (Supporting Fig. S19A,B). Furthermore, we detected enhanced expression of vimentin, Snail, Twist, and zinc finger E-box binding homeobox 1, as well as a decreased level of E-cadherin in HCC cells treated with IL-1β (Fig. 6F). In summary, these data indicated that macrophage-derived IL-1β facilitated EMT in HCC cell lines.
HIF-1α IS UP-REGULATED BY IL-1β AND MEDIATES IL-1β-INDUCED EMT IN HCC CELLS
Previous studies have demonstrated that IL-1β regulates HIF-1α expression in a cell type–dependent manner. For instance, IL-1β led to HIF-1α overexpression in lung cancer cells but promoted HIF-1α degradation in glioblastoma cells.23, 24 We showed that in both Huh-7 and HepG2 cells HIF-1α rather than HIF-2α was up-regulated by IL-1β at a dose as low as 250 pg/mL in normoxia (Fig. 7A). Consistently, HIF-1α mRNA was selectively up-regulated only in HepG2 and Huh-7 cells (both are epithelial phenotype) but not in SNU-449 cells (mesenchymal phenotype; Fig. 7B). In line with this, the transcriptional activity of HIF-1α was dramatically increased in Huh-7 cells but not in SNU-449 cells (Supporting Fig. S20A). Moreover, IL-1β was unable to affect the expression of EMT-related proteins, including E-cadherin, zonula occludens-1, vimentin, and Snail, in SNU449 cells (Supporting Fig. S20B).
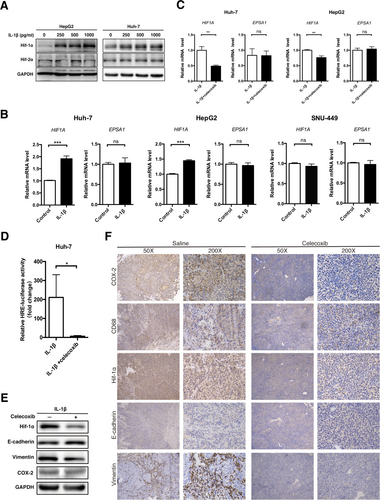
IL-1β enhances EMT in HCC cell lines through the IL-1β/HIF-1α/COX-2 axis. (A) HepG2 and Huh-7 cells were incubated with distinct concentrations of recombinant human IL-1β (48 hours), and expression of HIF-1α and HIF-2α was detected. (B) mRNA levels of HIF1A and EPSA1 (normalized to ACTB) in Huh-7, HepG2, and SNU-449 cell lines treated with recombinant human IL-1β (250 pg/mL, 48 hours). (C-E) Huh-7 or HepG2 cells were treated with IL-1β (250 pg/mL, 48 hours) with or without celecoxib (25 μM, 12 hours) pretreatment. (C) mRNA levels of HIF1A and EPSA1, (D) hypoxia-response element luciferase activity, and (E) expression of the indicated proteins were detected. (F) Spontaneous HCC mice (Mst–/–;Mst–/flox;Alb-Cre) were intragastrically administered saline or celecoxib (200 mg/kg/day for 1 month). Immunohistochemical staining of mouse liver tissues was performed, and representative images are shown. Scale bar, 100 μm. Data from at least three independent experiments are shown. Error bars represent the SEM. *P < 0.05, **P < 0.01, ***P < 0.001. Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HRE, hypoxia-response element; ns, not significant.
It has been demonstrated that IL-1β activates HIF-1α through the NF-κB/cyclooxygenase-2 (COX-2) pathway.25 To test this theory in HCC cells, a COX-2 selective inhibitor, celecoxib, was added prior to IL-1β treatment. Interestingly, the inhibition of COX-2 activity by celecoxib abrogated IL-1β-enhanced HIF-1α expression and transcriptional activity (Fig. 7C-E), as well as the IL-1β-induced EMT process in HCC cells (Fig. 7E; Supporting Fig. S21). Additionally, we treated a spontaneous HCC mouse model with celecoxib to confirm the role of COX-2 in vivo. As expected, celecoxib significantly reduced the expression of HIF-1α and vimentin (Fig. 7F). We then analyzed the necrotic center of surgically resected HCC from patients who received preoperative transarterial chemoembolization. The necrotic center also revealed increased expression of HIF-1α, IL-1β, and vimentin as well as decreased expression of E-cadherin (Supporting Fig. S22). Altogether, these results indicated that COX-2/HIF-1α mediated IL-1β-induced EMT in HCC cells.
THE HIF-1α/IL-1β LOOP ENHANCES EMT AND METASTASIS OF HCC CELLS IN VIVO
To confirm IL-1β-induced HCC EMT through the up-regulation of HIF-1α in vivo, we first used an intratumoral injection of LPS to enhance the IL-1β secretion of TAMs in a Huh-7 xenograft mouse model. Xenografts in the LPS group showed clearly increased TAM accumulation and overexpression of HIF-1α (Supporting Fig. S23). The up-regulation of vimentin and down-regulation of E-cadherin were also quite apparent in mice locally treated with LPS. In xenografts of mice with decreased TAMs depleted by clodronate,26 expression of IL-1β, HIF-1α, and vimentin was decreased, while E-cadherin expression was recovered (Supporting Fig. S24). Moreover, by neutralizing TAM-secreted IL-1β in the mouse model, we observed reduced Hif-1α expression accompanied by up-regulated E-cadherin and down-regulated vimentin in HCC cells (Supporting Fig. S25). We next established a metastatic mouse model of a red fluorescence protein (RFP)-LM3 xenograft to further verify the role of IL-1β in vivo. We injected recombinant human IL-1β into the xenograft weekly for a total of 5 weeks to create an inflammatory microenvironment within the tumor (Fig. 8A). Two weeks of IL-1β treatment already significantly increased the number of RFP+ cells in the circulation of mice compared to those treated with phosphate-buffered saline (Fig. 8B). HLA staining confirmed the increased number of circulating tumor cells in the peripheral blood of mice that received an IL-1β injection (Fig. 8C), indicating the potential role of IL-1β in facilitating HCC cell detachment and metastasis through the bloodstream. Three months later, we observed lung macrometastatic nodules in two of six mice treated with IL-1β and zero of eight in the control group. Hematoxylin and eosin staining confirmed a higher incidence and an increased number of micrometastases in IL-1β-treated mice (P < 0.05; Fig. 8D; Supporting Table S10). We also observed increased recruitment of myeloid-derived suppressor cells in the lungs of IL-1β-treated mice (Supporting Fig. S26A), indicative of an immunosuppressive effect. Immunohistochemistry of the original xenografts revealed overexpression of HIF-1α and COX-2 in mice treated with IL-1β, in conjunction with elevated vimentin expression and reduced E-cadherin expression (Fig. 8E); however, the tumor burden of the xenografts was not significantly affected by the IL-1β injection at the time of analysis (Supporting Fig. S26B,C). Because IL-1β was injected intratumorally and the serum levels of IL-1β, IL-6, and TNF-α were comparable between the experimental and control groups (Supporting Fig. S26D), we believed that metastases formation was primarily mediated by IL-1β-induced HCC EMT within the xenografts.
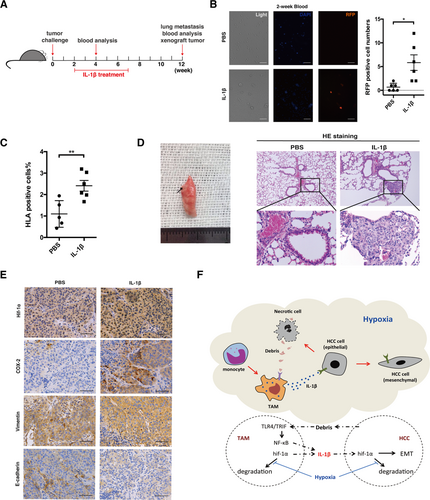
The HIF-1α/IL-1β loop enhances EMT and metastasis of cancer cells in vivo. (A) Recombinant human IL-1β (0.5 μg/mouse, intratumoral injection per week) or phosphate-buffered saline treatment of RFP-LM3 tumor-bearing mice. (B) Following IL-1β treatment for 2 weeks, single-cell suspensions were isolated from the peripheral blood (100 μL) of tumor-bearing mice, and the fluorescence intensity was detected. The statistical analysis of RFP+ cell detection is shown. (C) At 12 weeks post–tumor challenge, the percentages of HLA-A-positive, HLA-B-positive, and HLA-C-positive cells circulating in the peripheral blood were analyzed by flow cytometry. (D) Gross examination and hematoxylin and eosin staining of the lungs from RFP-LM3 tumor-bearing mice. Arrow indicates lung metastases observed after IL-1β treatment. Representative images of lung metastatic foci (magnification ×100) are shown. Lower panel represents magnified views (×400) indicated by boxes in the top panel. (E) Immunohistochemical staining of HIF-1α, COX-2, E-cadherin, and vimentin was performed on the xenografts of the mice. Representative images are shown. Scale bar, 100 μm. Error bars represent the SEM. *P < 0.05, **P < 0.01, ***P < 0.001. (F) A schematic model depicting the HIF-1α/IL-1β signaling loop mediates the crosstalk between HCC cells and TAMs in a hypoxic-inflammatory microenvironment. When exposed to moderate hypoxia, HIF-1α accumulation up-regulates IL-1β secretion in macrophages. Moreover, necrotic cancer cell debris caused by severe hypoxia can “educate” TAMs and induce proinflammatory effects (i.e., IL-1β secretion) through the TLR4/TRIF/NF-κB signaling pathway. Accumulation of macrophage-derived IL-1β deepens the inflammatory signaling in the tumor microenvironment and enhances EMT in cancer cells through the IL-1β/HIF-1α/COX-2 axis, which promotes invasive and metastatic capacities of HCC cells in a hypoxic microenvironment. Abbreviations: HE, hematoxylin and eosin; PBS, phosphate-buffered saline.
Discussion
Hypoxia and inflammation exist in all HCCs and have been frequently studied; however, their relationship remains poorly understood. In fact, these two factors can aggravate each other. For instance, hypoxia amplifies the proinflammatory activity of innate immune cells (e.g., neutrophils and macrophages),25 while innate immune cells exaggerate hypoxia by increasing oxygen consumption due to an outburst of cytokine release.27 Additionally, HIF-1α and NF-κB, the key transcriptional factors of hypoxia and inflammation, respectively, can activate each other.28 Here, we demonstrated an interaction between hypoxia and inflammation using a cellular and molecular perspective. We found that TAMs induced HCC cells to undergo EMT by secreting IL-1β and that necrotic HCC cell debris enhanced IL-1β release by activating TAMs through the TLR4/TRIF/NF-κB signaling pathway. It is important to note that these processes are molecularly linked by HIF-1α and IL-1β (HIF-1α induces overexpression of IL-1β in TAMs, and IL-1β in turn increases HIF-1α synthesis in HCC cells), which form a positive loop between the TAMs and HCC cells, ultimately inducing EMT and facilitating HCC metastasis.
We also found that hypoxia can promote EMT of HCC cells in at least two aspects. Firstly, hypoxia limits the function of prolyl hydroxylase domain-containing protein 2,29 thus impairing HIF-1α degradation and leading to HIF-1α accumulation in both TAMs and HCC cells. Secondly, IL-1β induced by necrotic debris of cancer cells exposed to severe and persistent hypoxia enhances HIF-1α synthesis in HCC cells. Our previous and current work demonstrated that either way sufficiently induces HCC cell EMT.6 Because the two mechanisms of inducing HIF-1α overexpression are relatively independent, in HCC tissue, where both hypoxia and IL-1β exist, they likely exhibit synergistic effects. Therefore, this combination can result in a stronger induction of EMT in HCC cells.
Although nearly all HCC cells suffer from mild or moderate hypoxia depending on their distance to the vessels, necrosis primarily occurs in large HCC lesions due to severe and persistent hypoxia in the cores. Hypoxia-induced necrosis differs from apoptosis, another common type of cancer cell death, in several ways. In particular, necrosis induces proinflammatory effects due to release of intracellular damage-associated molecular patterns.30 Here we report a group of membrane-located, O-linked glycoproteins as potential damage-associated molecular patterns, which trigger IL-1β secretion in TAMs. Unfortunately, the precise structures of these glycoproteins remain undefined; however, we speculate that some LPS-like, serine-related domains might be involved in the activation of necrotic debris–induced TLR4 signaling and consequent sterile inflammation. This is because serine is closely related to both O-linked glycosylation and enzymolysis of proteinase K.31, 32 Future analysis using TLR4 affinity chromatography and mass spectroscopy is required to clarify the detailed structures in certain glycoproteins. Additionally, we found that HCC necrotic debris could recruit TLR4 to the cell membrane of macrophages. Because saturated fatty acids lead to TLR4 recruitment while unsaturated fatty acids inhibit this process, we infer that the proportion of saturated fatty acids in necrotic debris increases due to up-regulated intracellular reactive oxygen species following exposure to hypoxia and reduced antioxidant generation following cell death.33 Thus, necrotic debris may function as an inflammatory inducer through both O-linked glycoproteins and saturated fatty acids, a pattern conceptually similar to the composition of the O-antigen and lipid A in LPS.
Unexpectedly, we found that HCC necrotic debris activated the TLR4 signaling pathway through TRIF rather than Myd88, which mediates LPS-triggered inflammatory cytokine release.20 Using multiple approaches, including PCR array and RNA sequencing, we confirmed that the TLR4/TRIF/NF-κB signaling pathway was activated during necrotic debris–induced inflammatory response. This noncanonical pathway requires signal rewiring within macrophages. The absence of increased transcription of TBK1, IRF3, and TNFA suggested a relatively specific activation of the NF-κB signaling pathway. Moreover, while the down-regulation of CASP8 and FADD implied inactivation of the classic TRIF-associated apoptosis pathway,34 a 2-fold increase of IFNB1 in the PCR array is consistent with TRIF activation. We assume that the noncanonical activation of NF-κB through TLR4 and TRIF is due to the distinct structures and high complexity of the active components in the HCC necrotic debris.
Unlike the classic M1 polarization induced by LPS, HCC necrotic debris appears to induce an M2 phenotype in TAMs or may preserve some M2 phenotypic characteristics. M2 polarized macrophages are conventionally considered to be anti-inflammatory.35 In rare conditions (e.g., rheumatoid arthritis), M2 polarized macrophages can also exhibit proinflammatory functionality by releasing IL-1β, IL-6, and TNF-α.36 Vogelpoel et al. verified this M2 proinflammatory phenotype in healthy subjects,36 and we have also observed that this phenomenon exists in pancreatic cancer (data not shown). This evidence suggests that relatively conservative functions and mechanisms underlie this phenomenon. In the present study, we showed that necrotic debris from both Huh-7 cells and patients' HCC lesions simultaneously induced a proinflammatory response and M2 polarization in THP-1-derived macrophages and human MDMs. The mechanisms of the proinflammatory effects exhibited by M2 polarized macrophages are largely unknown. Evidence indicates that the coactivation of additional signaling pathways (e.g., Fc gamma receptor signaling) may be required to prime M2 macrophages.36 Cancer cell necrotic debris may contain some types of additional stimulators required to induce the proinflammatory effects of M2 polarized TAMs. Mechanically, M2 polarized macrophages express proinflammatory cytokines, including IL-1β, in a manner that differs from M1 polarized macrophages, in which an Myd88-dependent early response and a TRIF-dependent later response of NF-κB activation are both involved to maintain a stable secretion of IL-1β.37 However, Myd88-dependent activity was not found in this study and has not been reported in any other condition involving M2 polarized macrophage-associated inflammation.36 The increased expression of IL10 (a classic M2 polarized macrophage-expressed gene) found by quantitative real-time PCR and the decreased expression of IRG1 (a highly expressed gene in classic M1 polarized macrophages) found by RNA sequencing also suggest an alternatively activated phenotype of the macrophages stimulated by HCC necrotic debris.38 Furthermore, M1 polarized macrophages disturb mitochondrial function through iNOS-mediated nitric oxide production, which prevents M1 polarized macrophages from being repolarized into an M2 phenotype.39 Under HCC conditions, although we observed similar metabolic changes upon stimulation with LPS and HCC necrotic debris, mitochondrial function under conditions of necrotic debris was more likely to be temporarily attenuated but not permanently damaged. This is because the level of iNOS mRNA was down-regulated following treatment with the necrotic debris. The failure of iNOS induction in macrophages was also observed when the necrotic debris of pancreatic cancer cells was used (data not shown). Therefore, although they remain elusive, several unknown mechanisms could be involved in M2 polarized macrophage-associated inflammation.
Given the enhanced invasive and metastatic capacities of HCC cells induced by IL-1β both in vitro and in vivo, we believe that anti-inflammatory effects, at least locally if not systematically, is beneficial for disease control in HCC patients. Indeed, anti-inflammatory agents (e.g., aspirin and celecoxib) have been proven to inhibit HCC development and progression in human and mouse models.40, 41 The anti-HCC role of estrogen is also partially attributed to its anti-inflammatory effects42; however, currently there is no consensus regarding the use of anti-inflammatory strategies for HCC treatment. Anti-inflammation is now typically applied only when pathogen-triggered inflammation is demonstrated by laboratory tests or relative symptoms are found in HCC patients. Our present work showed that blocking the positive loop between hypoxia and inflammation within the HCC microenvironment is critical to inhibit inflammation-induced metastasis. Thus, an anti-inflammatory response could be more valuable for patients receiving transarterial chemoembolization or sorafenib treatment or for those exhibiting large HCC lesions with imaging-indicated necrosis.
In conclusion, we demonstrate that the HIF-1α/IL-1β loop mediates crosstalk between HCC cells and TAMs in a hypoxic-inflammatory microenvironment that exists in each HCC patient and provides evidence for IL-1β-induced EMT in HCC metastasis (Fig. 8F). These findings may deepen our understanding regarding the role of inflammation in HCC progression and indicate the potential benefits of an anti-inflammatory strategy during HCC treatment.
Acknowledgment
We thank Prof. Bin Zhao from Life Science Institute Zhejiang University and Prof. Qingqing Wang from School of Medicine Zhejiang University for providing spontaneous HCC mice and plasmids, respectively. We appreciate Dr. Liyuan Chen from the Critical Care Medicine Department, Clinical Center, National Institutes of Health, for critical discussion. We thank Dr. Yang Yuan, Danfeng Qian, Liqiang Hu, Yi Wang, and Hao Qin for their technical support. We also appreciate Dr. Jie Feng from Beijing Neurosurgical Institute, Capital Medical University, for her help with RNA-sequencing analysis.
REFERENCES
Author names in bold designate shared co-first authorship.




