Using the Icelandic genealogical database to define the familial risk of primary biliary cholangitis
Potential conflict of interest: Nothing to report.
Unmarked set by kristjanornolfsson.
Abstract
Hereditary factors in primary biliary cholangitis (PBC) have been well defined in genome-wide association studies, but there are few direct data available that define the relative risk (RR) for family members with an affected proband. An increased risk in first-degree relatives has been demonstrated in a variety of studies, but data have been lacking on further detailed associations for subsequent generations. The objective of this study was to use the unique Icelandic genealogical database to study the familiality of PBC. All patients with positive antimitochondrial antibody measurements in Iceland during the period 1991-2015 who fulfilled diagnostic criteria for PBC were included. The Icelandic genealogical database was used to assess familial relations. For each case of PBC, 10,000 control subjects matched for age, sex, and number of known relatives were randomly chosen from this database to calculate the familial RR of PBC. The average kinship coefficient (KC) of the patients was calculated and compared with the average KC of controls. Overall, 222 PBC patients were identified (182 females, 40 males; median age, 62 years). First-, second- and third-degree relatives of the PBC patients had a high RR of the disease: 9.13 (P < 0.0001), 3.61 (P = 0.014) and 2.59 (P = 0.008), respectively. In fourth- and fifth-degree relatives, the RR was also increased to 1.66 (P = 0.08) and 1.42 (P = 0.08), respectively. The average KC of the patients was also higher than that of the control subjects, with 21.34 × 10−5 versus 9.56 × 10−5 (P < 0.0001). Conclusion: Relatives of PBC patients had markedly higher risk for development of the disease compared with controls and importantly our data demonstrate that the risk was significantly increased even in second- and third-degree relatives. (Hepatology 2018;68:166-171).
Abbreviations
-
- AMA
-
- antimitochondrial antibodies
-
- KC
-
- kinship coefficient
-
- PBC
-
- primary biliary cholangitis
-
- RR
-
- relative risk
The etiology of primary biliary cholangitis (PBC) remains enigmatic, and the relative contributions of genetic and environmental factors to disease susceptibility have yet to be fully elucidated. Familial, twin, and genetic studies indicate that genetic factors play a significant role in the pathogenesis of the disease.1 Several studies have demonstrated an increased risk of developing PBC among first-degree relatives of PBC patients compared with the general population.2-8 Moreover, an increased prevalence of disease-specific antimitochondrial antibodies (AMAs) has been demonstrated in first-degree relatives of patients with PBC.9 A study of eight monozygotic and eight dizygotic twin pairs in which at least one member of the pair had PBC found a concordance rate of 63%, which is one of the highest twin concordance rates reported for any autoimmune disease.10 An increased risk of other autoimmune conditions among PBC patients and their relatives has been observed, indicating an overlap between genetic factors involved in the pathogenesis of PBC and other autoimmune diseases.11-13 This finding has been further corroborated by the results of genetic studies that have found associations with variations in genes involved in immune function, some of which have been associated with other autoimmune conditions as well.14-16 Genome-wide association studies have identified common variants of several loci associated with PBC, many of which are in or adjacent to genes of the immune system.17-20 Most studies on the familiality of PBC have assessed the prevalence of the disease in first-degree relatives of PBC cases.3, 5, 21 The lack of information on disease in more distantly related relatives has made it difficult to differentiate between the contributions of shared genetic and environmental factors to the familial aggregation of the disease.
The objective of the current study was to use a population-based database on PBC patients and an extensive genealogy database containing genealogic information on all living Icelanders and most of their ancestors since the settlement of Iceland, to throw further light on the etiology of the disease.
Materials and Methods
STUDY POPULATION
The study population included all patients who were diagnosed with PBC in Iceland from January 1, 1991, to December 31, 2015. Data on patients diagnosed from 1991 to 2010 were compiled earlier for a previous study on the epidemiology of PBC in Iceland.22 We also collected data for patients diagnosed from 2011 to 2015 using the same case-finding methods to increase the power of the study. The case-finding strategies have been described in detail previously.22 In short, patients were found by identifying all positive AMA measurements at the immunology laboratory of the National University Hospital of Iceland, which is the only laboratory in Iceland that conducts AMA measurements. We could therefore be assured that any positive AMA measurements in Iceland during the study period were not missed. We also conducted a search for positive diagnostic codes for PBC in the computerized database of the Departments of Pathology in Iceland to identify AMA-negative patients.
Patients were included in the study if they fulfilled at least two of the following three criteria as recommended by the American Association for the Study of Liver Disease guidelines23: 1) positive AMA (a titer of at least 1/40); 2) elevation of alkaline phosphatase above the upper limit of normal; and 3) liver histology compatible with PBC.
GENEALOGY DATABASE
The Icelandic genealogical database maintained by deCODE Genetics, a computerized database of the genealogy of Icelanders, contains genealogic data on more than 760,000 individuals. The records include almost all individuals born in Iceland in the last two centuries, and for that period around 95% of parental connections are known.24 For each case of PBC, 10,000 control subjects matched for age, sex, and number of known relatives were randomly chosen from this database to obtain the familial relative risk (RR) of the disease and compare the average kinship coefficient (KC) of the patients to that of the general population.
STATISTICAL ANALYSES
RR calculations
The RR for relatives of PBC patients was defined as the risk of disease in relatives divided by the risk in the general population. This ratio is related to the ability to find susceptibility genes.25 Obtaining an accurate assessment of the RR is not always straightforward, however, as a variety of factors leading to bias can result in skewed estimates.26, 27 The use of a population-based cohort minimizes the potential for a sampling bias. Furthermore, the use of a complete and extensive genealogy database allowed us to assess the RR well beyond the nuclear family, thus enabling us to make a clearer distinction between the impact of shared genetic and environmental factors on disease risk. Empirical P values were calculated using the RRs of each control group; thus, a P value of 0.05 would indicate that 5% of the control groups had an RR value equal to or higher than that for the PBC patients. Approximately 95% confidence intervals were also constructed based on the distribution of the RR values for the control groups.27
Estimated prevalence of PBC among relatives of patients by degree of relatedness was also calculated based on the prevalence of PBC in Iceland and the respective RR values. In addition, to assess for genetic anticipation (i.e., to estimate whether those with affected relatives were more likely to be diagnosed at an early age than controls), the average age at diagnosis of those patients with affected first- and second-degree relatives was compared with the average age at diagnosis of those patients without such affected relatives.
Kinship coefficient
The average KC of the PBC patients was calculated and compared with the average KC of control subjects. The KC is defined as the probability that two randomly selected autosomal alleles from each member of a pair, was inherited from a common ancestor. We determined the average KC of the PBC patients and control subjects by averaging the KCs of each possible pairwise combination of the PBC patients and control subjects, respectively. To examine the effect of close relations on the KC values, we also calculated the KCs after the exclusion of relatives in a stepwise manner from within one to six meiotic distances, because close relatives are more likely to share a common environment and may have a large impact on the results.28 Meiotic distance was defined as the number of meiotic events between two individuals, thus parent–offspring relationships were first removed from the calculations (one meiotic event), followed by siblings, grandparents, grandchildren (two meiotic events), and so forth. For both the RRs and KCs, a P value of 0.05 was considered significant.
Results
A total of 222 patients, whose median age at diagnosis was 62 years (range, 13-92), were diagnosed with PBC in Iceland during the study period, of whom 82% were women (Table 1). The prevalence of PBC at the end of the study period was 41 per 100,000 (Table 1). RR estimates for disease along with prevalence estimates calculated from the RR values in first- through fifth-degree relatives of PBC patients are presented in Table 2. First-degree relatives of PBC patients were found to be 9.13-fold more likely to have been diagnosed with the disease than the general population, and the estimated prevalence of disease among them was 37.3 per 10,000 (Table 2). The RR remained significantly higher for both second- and third-degree relatives, with an RR of 3.61 and 2.59, respectively (Table 2). The RRs for fourth- and fifth-degree relatives were also increased as shown in Table 2, but the increased risk did not reach statistical significance. The RRs decreased incrementally by degree of relatedness consistent with a decreasing proportion of alleles shared by descent. The RR was similar in females and males (data not shown).
| Characteristics | Values |
|---|---|
| Age, years, median (range) | 62 (13-92) |
| Sex, n (%) | |
| Female | 182 (82) |
| Male | 40 (18) |
| AMA status, n (%) | |
| Positive | 216 (97) |
| Negative | 6 (3) |
| Cirrhosis at diagnosis, n (%) | |
| Yes | 19 (9) |
| No | 203 (91)a |
| Liver transplantation, n (%) | 11 (5) |
- a Overall, 119 (54%) patients underwent a liver biopsy at diagnosis.
| Degree of Relatedness | RR (95% CI) | P | Number of Affected Relatives of Patients | Number of Relatives of Patients | Mean Number of Affected Relatives of Control Groups | Prevalence of PBC (per 10,000)b |
|---|---|---|---|---|---|---|
| 1° | 9.13 (4.17-16.76) | <0.0001 | 14 | 1990 | 1.52 | 37.3 |
| 2° | 3.61 (1.48-8.92) | 0.014 | 10 | 6948 | 2.84 | 14.8 |
| 3° | 2.59 (1.35-4.67) | 0.008 | 20 | 17,431 | 7.45 | 10.6 |
| 4° | 1.66 (1.00-3.02) | 0.07 | 26 | 39,118 | 16.31 | 6.8 |
| 5° | 1.42 (0.99-2.20) | 0.08 | 50 | 91,536 | 35.52 | 5.8 |
- a The RR values for each degree of relatedness were obtained by comparing the number of affected relatives of the PBC patients with the average number of affected relatives of 10,000 matched control groups.
- b Prevalence values by degree of relatedness were obtained by multiplying the respective RR values by the prevalence of PBC in Iceland.
The relatedness of the PBC patients was compared with the relatedness of the general population by comparing their KC with that of the control subjects. As is shown in Table 3 and Figure 1, the PBC patients were significantly more interrelated than the control subjects, as is evident by their higher KC value.
| Meiotic Distances Excluded | KC of PBC Patients | Average KC of Control Groups | P |
|---|---|---|---|
| None | 21.34 | 9.6 | <0.0001 |
| ≤1 meiotic event | 19.30 | 9.27 | <0.0001 |
| ≤2 meiotic events | 13.69 | 8.69 | <0.0001 |
| ≤3 meiotic events | 11.15 | 7.98 | 0.0007 |
| ≤4 meiotic events | 9.11 | 7.00 | 0.003 |
| ≤5 meiotic events | 7.45 | 5.93 | 0.005 |
| ≤6 meiotic events | 5.64 | 4.72 | 0.02 |
- All KCs are multiplied by 100,000.
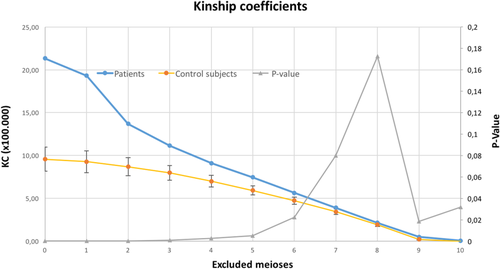
The KCs of the PBC patients remained significantly increased even after the exclusion of relatives within six or fewer meiotic events from the calculations (Table 3 and Fig. 1).
Discussion
The results of this population-based study using an extensive computerized genealogical database demonstrated a significant familial clustering of PBC in Iceland, which extended beyond the nuclear family.
First-degree relatives of Icelandic PBC patients had a markedly higher risk of development of the disease compared with subjects from the general population, with an RR greater than 9. A population-based study by Jones et al.5 found an RR of 10.5 for siblings. Similarly, studies from the United States and France reported values of 10.7 and 9.2, respectively.6, 7 Thus, the results of the current study are in accordance with the results of previous studies on the risk in first-degree relatives.5-7 Furthermore, the results presented here demonstrate a significantly increased risk of disease in second- and third-degree relatives.
Compared with familial RR values reported for other diseases, the RR values for relatives of PBC patients was higher than the RR values for relatives of patients with Crohn's disease, ulcerative colitis, atrial fibrillation (RR for first-degree relatives: 5.9, 5.4, and 1.77, respectively) and type 2 diabetes (RR for siblings and offspring: 2.7 and 2.0, respectively) but lower than the RR values for relatives of patients with ankylosing spondylitis and psoriatic arthritis (RR for first-degree relatives: 75.5 and 39.2, respectively).29-33 The average KC of Icelandic PBC patients was higher than the average KC of Icelandic patients with rheumatoid arthritis and atrial fibrillation (17.0 and 13.9, respectively) and lower than the average KC of Icelandic patients with psoriatic arthritis (50).30, 31, 34
When the average age at diagnosis of those PBC patients with affected first- and second-degree relatives was compared with the average age at diagnosis of those patients without such relatives, no significant difference was found (data not shown). Thus, there was no indication of genetic anticipation or bias after an initial close relative had been diagnosed.
It would have been of interest to analyze whether certain families had early onset disease or a more aggressive phenotype. Unfortunately, due to the limited number of hard endpoints, we were unable to analyze whether family history affected clinical outcomes.
The current study has several advantages compared with previous studies on the familiality of the disease. First, the current study employed more adequate methods to identify both patients and their relatives. Previous studies have all identified affected relatives by questioning patients, which introduces a substantial bias. In addition, they have mostly been hospital-based, which introduces a further bias. By using a population-based cohort of all PBC patients diagnosed in Iceland during the study period, the sampling bias inherent in the design of previous studies was largely avoided. Furthermore, the use of an extensive genealogical database enabled us to evaluate familial relationships of the patients more accurately and identify more distant relations than previous studies have. Thus, the current study enabled us to make a better distinction between the role of genetic and environmental factors in increasing the risk of PBC in relatives. Second, the present study used more reliable methods to estimate the risk of disease among relatives of patients compared with the general population. Previous studies have only presented prevalence values of PBC among relatives of patients or relied on estimates of the population prevalence to calculate their RR. The current study, however, used 10,000 matched control subjects from the genealogical database for each PBC patient in the study, which gave the study much greater statistical power than previous studies.
Contrary to many other liver diseases, strong associations between PBC and specific environmental factors have not been identified, although smoking, urinary tract infections, and hormonal factors have been reported to affect risk.35 Epigenetic changes have also been implicated in the pathogenesis of the disease.36-39 It therefore seems likely that an interplay between genetic and environmental components is necessary for the development of the disease.
First-degree relatives tend to share a more similar environment with each other than with more distantly related relatives. We cannot completely exclude the impact of similar environmental factors that might explain the results, although this is unlikely. As the RR in first-degree relatives of PBC patients in the current study is similar to the results of other studies5-7 despite rather different environments, it argues against a major contribution of environment, explaining the current results.
The increased risk of disease in second- and third-degree relatives shown here provides a stronger indication for the role of genetic factors in the etiology of PBC than previous reports of increased risk in first-degree relatives. Furthermore, we observed a graded decline in RR with increasing degree of relatedness, which is consistent with a decreasing number of alleles shared between relatives and strongly suggests genetic influences on disease risk.40
In conclusion, our results extend previous observations on the familiality of PBC substantially by showing that an increased risk of disease extends significantly to both second- and third-degree relatives. This finding supports a strong role of genetics in the pathogenesis of PBC and provides a further rationale for using genetic research methods to uncover novel genetic factors associated with development of the disease. More knowledge on the genetics of PBC may enable us to gain better insight into the pathophysiological mechanisms underlying the disease, which could lead to the development of new treatments.
Acknowledgements
The study was partly supported by an unrestricted grant from Intercept.




