Cardiovascular predictors of death in patients with cirrhosis
Potential conflict of interest: Nothing to report.
Abstract
Cirrhotic cardiomyopathy is associated with poor outcomes in patients with cirrhosis. We investigated if subclinical cardiac morphologic and functional modifications can influence survival in patients with cirrhosis during follow-up. A series of patients with cirrhosis without cardiovascular or pulmonary disease underwent standard and tissue Doppler echocardiography to assess left ventricular geometry, systolic/diastolic function, and the main haemodynamic parameters. After baseline evaluation 115 patients with cirrhosis were followed up for at least 6 years. During follow-up 54 patients died (47%). On univariate analysis, age, body surface area (BSA), Model for End-Stage Liver Disease (MELD), mean arterial pressure, heart rate, cardiac index, systemic vascular resistance index, and the ratio of transmitral Doppler early filling velocity to tissue Doppler early diastolic mitral annular velocity (E/è) were associated with increased risk of death. In a Cox hazard regression analysis including these factors and other hypothesized important factors (but not MELD), increased age (P = 0.04) and left atrial dimension (P = 0.005) and lower BSA (P = 0.03) were the strongest predictors of death. When MELD was included in the analysis, the main predictors were MELD, age, and BSA. When multivariate analysis was performed incorporating only cardiovascular parameters, increased E/è (P = 0.003) and heart rate (P = 0.03) and reduced mean blood pressure (P = 0.01) were significantly associated with poor prognosis. Conclusion: In a large cohort of patients with cirrhosis and after a long follow-up, MELD, age, and BSA were the main predictors of death; among cardiovascular parameters, left atrium enlargement, increased heart rate and E/è, and reduced mean blood pressure were independent predictors of death. (Hepatology 2018).
Abbreviations
-
- ASE
-
- American Society of Echocardiography
-
- BSA
-
- body surface area
-
- CI
-
- cardiac index
-
- CO
-
- cardiac output
-
- DD
-
- diastolic dysfunction
-
- EACVI
-
- European Association of Cardiovascular Imaging
-
- EAE
-
- European Association of Echocardiography
-
- E/e′
-
- early diastolic transmitral and myocardial velocity on TDI ratio
-
- EF
-
- ejection fraction
-
- HRS
-
- hepatorenal syndrome
-
- LA
-
- left atrial
-
- LV
-
- left ventricular
-
- LVH
-
- LV hypertrophy
-
- LVM
-
- LV mass
-
- MAP
-
- mean arterial pressure
-
- MELD
-
- Model for End-Stage Liver Disease
-
- SV
-
- stroke volume
-
- SW
-
- stroke work
-
- TDI
-
- tissue Doppler imaging
Cardiovascular abnormalities play a marked role in the pathogenesis of major complications of cirrhosis such as ascites, hyponatremia, and hepatorenal syndrome (HRS). Cirrhotic cardiomyopathy is a very complex condition characterized by impaired myocardial contractility, left ventricular (LV) hypertrophy (LVH), diastolic dysfunction (DD), impaired chronotropic function, and electrophysiological abnormalities in the absence of other known causes of heart disease.1-3 In patients with cirrhosis, LV enlargement and a high prevalence of LVH despite low peripheral resistances and low to normal blood pressure have been reported and referred to the interaction between the high renin and aldosterone values and the work overload associated with the hyperdynamic circulatory state.3, 4 Regarding DD, its prevalence in patients with cirrhosis varies between 37% and 43% when assessed according to the classification proposed by the American Society of Echocardiography (ASE) and the European Association of Echocardiography (EAE) in 2009,5 being not associated with the stage of liver disease but strongly related to the increase in LV mass (LVM)3 but with no relevance in terms of prognosis and particularly with survival.6-8
Cirrhotic cardiomyopathy represents a condition of latent heart failure which manifests only under stress, resulting in a blunted increase in cardiac index (CI) and cardiac output (CO) during exercise or pharmacologic stimuli.2, 9 In fact, even if at rest no evidence of subclinical systolic dysfunction exists,3 along with the development and worsening of ascites, CO and CI do not further increase to compensate for the continuous lowering of peripheral vascular resistances and blood pressure; and therefore, their maintenance becomes critically dependent upon the heart rate. Moreover, a decreased CI has been identified as an independent predictor of development of HRS.10
In the literature, few studies have investigated the predictive value of cardiac parameters in patients with cirrhosis, with confounding results.6, 10-12
We performed a prospective study assessing cardiac dimensions, systolic and diastolic function, and hemodynamic parameters in a large cohort of patients with different stages of cirrhosis using a noninvasive state-of-the-art echocardiography technique, with the aim of investigating the predictive value of these parameters in the clinical course of these patients.
Patients and Methods
STUDY POPULATION
From 2009 to 2012 a large series of consecutive outpatients with cirrhosis underwent a detailed haemodynamic evaluation and standard transthoracic Doppler echocardiography in our Department. Exclusion criteria were arterial hypertension and history of cardiovascular disease, diabetes, or heart valve disease. Coronary heart disease was excluded in all participants on the basis of symptoms, negative family history, a normal standard 12-lead electrocardiogram, and normal wall motion on two-dimensional echocardiographic examination.
If used, β-blockers were stopped 48 hours before echocardiography. The diagnosis of liver cirrhosis was based on clinical, biochemical, imaging, and endoscopic findings in all patients. The presence of ascites was detected clinically and confirmed by an abdomen ultrasound examination. The diagnosis of refractory ascites was based on the criteria of the International Club of Ascites.13 In patients with refractory ascites, the hemodynamic evaluation was performed soon after the therapeutic paracentesis.
ECHOCARDIOGRAPHIC PARAMETER ANALYSIS
All echocardiographic examinations were performed by the same expert sonographer (M.C.) using a General Electric Vivid 7 ultrasound machine with a 2.5-MHz transducer. Echocardiograms were stored digitally and analyzed offline by a single experienced observer (M.C.). All measurements were made according to the guidelines of the ASE.14 LV end-diastolic and end-systolic diameters and wall thickness were assessed in M-mode. LV ejection fraction (EF) and fractional shortening were measured in biplane two-dimensional mode using the Simpson method.14 At a given contractile state, however, EF increases as LV geometry becomes more concentric.15 Thus, midwall fractional shortening was also calculated to assess underlying systolic dysfunction in the setting of concentric hypertrophy.16, 17 LVM was estimated using the anatomically validated formula of Devereux et al.14 LVM was normalized both by body surface area (BSA) and by height in meters to the power of 2.7. The latter has been demonstrated to maximize risk prediction.18 Criteria for LVH were LVM/height ≥48 g/m2.7 for men and ≥44 g/m2.7 for women according to the current recommendations.14
The following parameters were calculated: mean arterial pressure (MAP; systolic pressure + [2 × diastolic pressure]/3); stroke volume (SV) was computed as the difference between end-diastolic and end-systolic LV volume and used as a direct indicator of LV volume load; SV was also corrected by the BSA to obtain the SV index. CO was calculated as the product of SV and heart rate; CI was calculated as CO adjusted by the BSA; the systemic vascular resistance index was calculated as the product of MAP and 80/CI;19 the stroke volume-pulse pressure ratio was calculated as an index of arterial compliance; stroke work (SW), a measure of total cardiac workload, was calculated as the product of systolic blood pressure (pressure load) and SV (volume load) and converted into gram-meters per beat by multiplying by the conversion factor 0.0014.20
Pulsed Doppler recordings at the level of the mitral valve tips were obtained from apical four-chamber scans to measure early (E) and late (A) diastolic filling velocities, their ratio (E/A), and the early wave deceleration time (DT).5
The tissue Doppler imaging (TDI) program was set to pulse-wave Doppler mode. Filters were set to exclude high-frequency signals. Gains were minimized to allow a clear tissue signal with minimal background noise. The TDI of the diastolic velocities was obtained from the apical four-chamber view. The recorded wall was positioned in the center of the sector. A 1.5-mm sample volume was placed at the septal corner of the mitral valve annulus. The angle between the Doppler beam and the longitudinal motion of the septal mitral valve annulus was minimized as well. All Doppler parameters were recorded at a horizontal speed of 100 mm/second. The average values obtained from at least three consecutive cardiac cycles were taken into consideration. Early diastolic peak velocity of septal mitral annulus (è) was obtained, and the E/è ratio was derived, reflecting the LV filling pressure.21
Strain rate parameters were analyzed offline by the same operator (M.C.). Placing the region of interest on the medial corner of the mitral annulus, we assessed its peak systolic tissue velocity (septal). Placing the region of interest (6 × 4 mm) on the basal portion of the inferior interventricular septum, we assessed septal peak systolic strain, the systolic strain rate, and early and late diastolic strain rate parameters.22 Early and late diastolic strain rate parameters were derived, which is considered an index of segmental relaxation–segmental diastolic dysfunction.23
The diagnosis and grading of LV DD (Table 1) were based on both the ASE/EAE 2009 recommendations,5 the ASE and European Association of Cardiovascular Imaging (EACVI) 2016 recommendations,21 and the 2016 Thorax Center algorithm, which starts with assessment of the transmitral flow velocities (E/A ratio and DT) and subsequently the mitral annulus TDI parameters (E/e′) and the left atrial (LA) dimension (anteroposterior diameter).24 This algorithm was claimed to be much simpler, faster, and therefore more reproducible than the ASE/EAE algorithm.24 The high reproducibility of all echocardiographic parameters, in our laboratory, has also been reported.3
| Algorithm | Parameters Considered | Diagnosis of DD | Grading of DD |
|---|---|---|---|
| ASE/EAE 20095 |
LA volume Annular e′ velocity (septal and lateral e′) Average E/e′ E/A DT |
Septal e′ <8 cm/second or lateral e′ <10 cm/second and LA maximum volume index ≥34 mL/m2 |
E/A DT Average E/e′ |
| ASE/EACVI 201621 |
LA volume Annular e′ velocity (septal and lateral e′) Average E/e′ Peak velocity of tricuspid regurgitation |
DD is present if more than half of the available parameters meet these cutoff values: -Annular e′ velocity (septal e′ <7 cm/second, lateral e′ <10 cm/second) -Average E/e′ ratio >14 (if only the lateral e′ or septal e′ velocity is available, a lateral E/e′ ratio >13 or a septal E/e′ >15 is considered abnormal) -LA maximum volume index >34 mL/m2 -Peak velocity of tricuspid regurgitation >2.8 m/second |
|
| Thorax Center 201624 |
E/A DT E/e′ (using septal e′) LA diameter |
DT ≥ 220 or < 220 DT ≥160 or <160 E/e′ <11, 11-15, or >15 LA diameter >40 mm |
E/A DT E/e′ (using septal e′) LA diameter |
- Abbreviations: E/A, early and late diastolic velocity ratio; DT, early wave deceleration time.
STATISTICAL ANALYSIS
The data are expressed as mean ± SD (or median and range), as appropriate. All echocardiographic and hemodynamic parameters showed a normal distribution, which was formally verified by the Kolmogorov-Smirnov test. Survival free from death was estimated with the Kaplan-Meier method.
Potential predictors of mortality were analyzed with univariate Cox regression, and those statistically significant at the 10% level were introduced in a multivariate Cox regression model with backward selection of the variables. The significance level for the multivariate analysis was 5%.
Three multivariate models were considered: (1) with all predictors significant at the univariate analysis; (2) dropping Model for End-Stage Liver Disease (MELD); (3) dropping age, BSA, and MELD. Proportionality assumption was checked graphically. The results of the Cox regression were reported as P value, hazard ratio, and 95% confidence interval.
Results
We recruited 162 consecutive outpatients with cirrhosis. Patients were excluded if they had arterial hypertension (n = 11), history of cardiovascular disease (n = 5), diabetes mellitus (n = 16), heart valve disease (n = 7), or coronary heart disease (n = 5). Three patients were lost at follow-up. Therefore, for the prospective analysis we considered 115 patients with cirrhosis: 48 with refractory ascites, 31 with responsive ascites, 36 without ascites. The clinical, demographic, and biochemical data are reported in Table 2. Patients with cirrhosis were mostly male and in different stages of liver disease of different etiologies: 53 patients (46%) had alcoholic cirrhosis, 51 patients (44%) had postviral cirrhosis, 11 patients (10%) had other forms of cirrhosis.
| Variable |
Alive (n = 61) |
Dead (n = 54) |
P |
|---|---|---|---|
| Age (years) | 55 ± 11 | 62 ± 11 | 0.0004 |
| Gender M/F (%) | 75/25 | 68/32 | NS |
| Etiology of cirrhosis (%)
(alcohol/viral/other) |
(44/48/8) | (49/40/11) | NS |
| Weight (kg) | 78 ± 12 | 73 ± 14 | 0.02 |
| BSA (kg/m2) | 1.9 ± 0.2 | 1.8 ± 0.2 | 0.02 |
| MELD | 11 ± 5 | 16 ± 6 | 0.0001 |
| Child-Pugh score | 7 ± 2 | 9 ± 2 | 0.0001 |
| Obesity (%) | 10 | 12 | NS |
| MAP (mm Hg) | 95 ± 12 | 90 ± 11 | 0.01 |
| Heart rate (beats/minute) | 70 ± 11 | 77 ± 13 | 0.002 |
| Creatinine (μmol/L) | 82 (75-88) | 122 (95-149) | 0.008 |
| Cardiac dimensions | |||
| Left atrial size (cm) | 3.8 ± 0.4 | 4.0 ± 0.5 | 0.06 |
| LVEDD (mm) | 48 ± 4 | 48 ± 3 | NS |
| LVEDVol (mL) | 110 ± 23 | 107 ± 17 | NS |
| LVM/height (g/m2,7) | 46 ± 10 | 46 ± 10 | NS |
| LVH (%) | 39 | 41 | NS |
| LV systolic function | |||
| EF (%) | 70 ± 6 | 69 ± 5 | NS |
| MWFS | 17 ± 2 | 17 ± 2 | NS |
| Septal strain (%) | 23 ± 4 | 23 ± 3 | NS |
| Hemodynamic parameters | |||
| SV (mL/beat) | 76 ± 16 | 74 ± 12 | NS |
| SW (g/beat) | 14 ± 4 | 13 ± 3 | 0.04 |
| CI (L/minute/m2) | 2.73 ± 0.6 | 3.06 ± 0.58 | 0.008 |
| CO (L/minute) | 5.3 ± 1.3 | 5.6 ± 1.0 | NS |
| SVRI (dyn s/cm5 m2) | 2,946 (2,750-3,142) | 2,481 (2,285-2,677) | 0.0009 |
| LV diastolic function | |||
| E/A | 1.14 ± 0.38 | 1.11 ± 0.4 | NS |
| DT/HR | 3.48 ± 1.02 | 3.35 ± 1.17 | NS |
| Septal E′ (cm/second) | 8.8 ± 2.1 | 8.2 ± 2.2 | NS |
| E/e′ | 9.8 ± 2.9 | 11.3 ± 3 | 0.03 |
| SR e/a | 1.43 ± 0.65 | 1.50 ± 0.6 | NS |
- Data are reported as mean ± SD or median (and interquartile range), as appropriate.
- Abbreviations: DT, early wave deceleration time; E/A, early and late diastolic velocity ratio; HR, heart rate; LVEDD, LV end-diastolic diameter; LVEDVol, LV end-diastolic volume; MWFS, midwall fractional shortening; NS, nonsignificant; SR e/a, early and late diastolic TDI velocities ratio; SVRI, systemic vascular resistance index.
All patients with cirrhosis showed an increase in LV end-diastolic dimensions and volumes, in wall thickness, and in LVM, with an important increase in LVH (40%). This increase in LVM was less evident in patients with more advanced liver disease. Patients with alcoholic cirrhosis did not present the echocardiographic features of alcoholic cardiomyopathy (i.e., increased LV volumes, subclinical systolic dysfunction, or reduced EF) compared with other forms of cirrhosis.
In the overall population the prevalence of LV DD was 30% using ASE/EAE 2009, 17% using ASE/EACVI 2016, and 48% using the Thorax Center algorithm.
SURVIVAL
During a median follow-up of 5 years (range 20 days-6 years), 54 patients died (47%): 20 patients in the first 6 months and 27 patients in the first year. Main causes of death were complications of cirrhosis (gastrointestinal hemorrhage, HRS, liver failure, sepsis, hepatocellular carcinoma).
On univariate analysis the parameters associated with death at follow-up were age, BSA, MELD, MAP, heart rate, serum creatinine, SW, CI, systemic vascular resistance index, and E/e′ (Table 2).
In a Cox hazard regression analysis incorporating these parameters (but not MELD) and all ecochardiographic and hemodynamic parameters (model 2), the most significant predictors of death were age, BSA, and LA dimension (Table 3 and Fig. 1). When MELD was included in the Cox analysis (model 1), MELD (P < 0.0001), age (P = 0.008), and BSA (P = 0.03) were the best predictors and the prognostic value of LA dimension was blunted.
| Predictors |
Hazard Ratio (95% Confidence Interval) |
P |
|---|---|---|
| MELD | 1.15 (1.08-1.23) | 0.0001 |
| BSA (kg/m2) | 0.09 (0.01-0.81) | 0.03 |
| Age (years) | 1.06 (1.02-1.10) | 0.008 |
| Dropping MELD | ||
| LA dimension (cm) | 3.42 (1.45-8.06) | 0.005 |
| BSA (kg/m2) | 0.11 (0.01-0.81) | 0.03 |
| Age (years) | 1.04 (1.00-1.07) | 0.04 |
| Dropping age, BSA, and MELD | ||
| E/e′ | 1.22 (1.07-1.38) | 0.003 |
| MAP (mm Hg) | 0.96 (0.93-0.99) | 0.011 |
| HR (beats/minute) | 1.04 (1.00-1.08) | 0.03 |
- Abbreviation: BSA: body surface area; E/e' early diastolic transmitral and myocardial velocity on TDI ratio; MAP, mean arterial pressure; HR, heart rate.
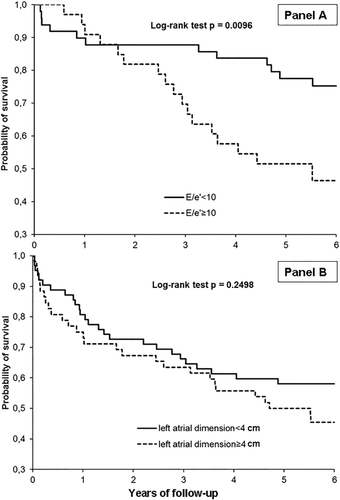
When multivariate analysis was performed incorporating only cardiovascular parameters (model 3), increased E/e′ and heart rate and reduced MAP were significantly associated with the occurrence of death (Table 3 and Fig. 1).
Regarding LV DD, if defined according to the ASE/EAE 2009 recommendations, the prevalence in the alive and dead groups was 28% and 31%, respectively (nonsignificant). If defined according to the ASE/EACVI 2016 recommendations, the prevalence in the alive and dead groups was 15% and 21%, respectively (nonsignificant). At variance, when assessed by the newly proposed Thorax Center algorithm, the prevalence in the alive and dead groups was 42% and 63%, respectively (nonsignificant).
No association with prognosis emerged regarding LV DD when assessed with both the ASE/EAE 2009 and ASE/EACVI 2016 recommendations or with the Thorax Center algorithm.
PATIENTS WITH ALCOHOL-RELATED CIRRHOSIS
When we performed the univariate analysis only in the 53 patients with alcohol-related cirrhosis, the parameters associated with death at follow-up were increased MELD (P = 0.0003), reduced systolic blood pressure (P = 0.04) and MAP (P = 0.05), and lower LV SW (P = 0.03). At multivariate Cox regression analysis the main predictors of poor outcome were increased MELD (P < 0.0001) and reduced LV SW (P = 0.003); if MELD was excluded, the best independent predictor remained low LV SW (P = 0.03).
TYPE 1 HRS DEVELOPMENT DURING FOLLOW-UP
During the observation period 12 patients developed type 1 HRS. The median time between the initial evaluation and the development of the complication was 3 months (range 20 days-3.5 years). We compared the 48 patients with refractory ascites who did and did not develop type 1 HRS. Only patients with refractory ascites were compared because no patients without ascites and only 1 patient with responsive ascites developed the syndrome. The 11 patients with refractory ascites who developed type 1 HRS showed significantly lower body mass index, higher MELD and Child-Pugh scores, and increased systolic blood pressure, pulse pressure, LV SW, and CI (Table 4). A multivariate analysis identified increased MELD (P = 0.006) and CI (P = 0.01) as the main predictors of type 1 HRS.
Discussion
In a previous study we performed a cross-sectional evaluation of our cohort to investigate for the occurrence of LV systolic and/or diastolic dysfunction. We observed that patients with cirrhosis showed a reduction in peripheral vascular resistance; a compensatory hyperdynamic syndrome; and significant increases in CI, CO, and cardiac work, with consequent increases in the prevalence of LVH and associated LV DD. The latter was strongly related to increased age and LVM but not related to the etiology or the severity of the liver disease. Systolic dysfunction was not evident at rest, becoming evident as a lack of an appropriate LV contractile response in the advanced stage of the liver disease, when CO became critically dependent upon the heart rate (thus suggesting a possible detrimental effect of β-blockers in these patients). To better define the importance of these cardiovascular parameters as predictors of poor outcomes, we performed a prospective study with a long follow-up. To our knowledge, we studied the largest cohort of patients with cirrhosis (115 patients) for the longest follow-up (at least 6 years). The long follow-up justifies the high incidence of mortality (47% at the end of follow-up). In smaller cohorts and with shorter follow-up (1-2 years) a similar incidence of mortality was reported compared with our findings (27% in the first year in our study, 21% in the study of Ruiz-del-Arbol et al.,12 27% in the study of Merli et al.11).
Previous studies which evaluated the prognostic role of cardiac parameters in patients with cirrhosis have shown conflicting results (Table 5). Merli et al. investigated the prognostic value of cardiovascular parameters in 90 patients with cirrhosis followed for 24 months. An increased LA dimension and a decreased LVM, but not E/e′ ratio, correlated with survival.11 Ruiz-del-Arbol in 80 patients with cirrhosis followed up for 12 months identified the E/e′ ratio as an independent predictive factor of mortality.12 Survival was significantly greater in the E/e′ <10 group compared to the E/e′ >10 group. Of interest, basal LVM was significantly increased in patients who presented type 1 HRS and in patients dead at 12 months compared with alive patients, in contrast with the results of Merli et al.
| Variable |
With Type 1 HRS (n = 11) |
Without Type 1 HRS (n = 37) |
P |
|---|---|---|---|
| Age (years) | 65 ± 9 | 60 ± 11 | NS |
| Weight (kg) | 68 ± 6 | 78 ± 14 | NS |
| BMI (kg/m2) | 23 ± 2 | 26 ± 4 | 0.03 |
| MELD | 21 ± 5 | 16 ± 6 | 0.03 |
| Child-Pugh score | 11 ± 2 | 10 ± 2 | <0.05 |
| SBP (mm Hg) | 126 ± 13 | 116 ± 12 | 0.03 |
| PP (mm Hg) | 55 ± 13 | 44 ± 10 | 0.003 |
| Cardiac dimensions | |||
| LA size (cm) | 4.2 ± 0.4 | 3.9 ± 0.5 | NS |
| LVEDVol (mL) | 112 ± 21 | 107 ± 19 | NS |
| LVM/height (g/m2.7) | 44 ± 5 | 44 ± 9 | NS |
| Hemodynamic parameters | |||
| SW (g/beat) | 15 ± 4 | 12 ± 2 | 0.005 |
| CI (L/minute/m2) | 3.40 ± 0.50 | 2.83 ± 0.59 | 0.03 |
| CO (L/min) | 6.2 ± 1.3 | 5.5 ± 1.0 | 0.08 |
| SVRI (dyn s/cm5 m2) | 2,122 (1,803-2,440) | 2,547 (2,262-2,831) | NS |
| Biochemical parameters | |||
| Creatinine (μmol/L) | 122 (91-154) | 118 (90-146) | NS |
| Plasma renin activity (ng/mL/hour) | 32 (26-37) | 29 (6-52) | NS |
| Plasma aldosterone (pmol/L) | 2,686 (933-5,903) | 2,308 (406-7,066) | NS |
- Data are reported as mean ± SD or median (and interquartile range), as appropriate.
- Abbreviations: BMI, body mass index; LVEDVol, LV end-diastolic volume; NS, nonsignificant; PP, pulse pressure; SBP, systolic blood pressure; SVRI, systemic vascular resistance index.
| Reference | Number of Patients |
Follow-Up (Years) |
Cardiovascular Prognostic Parameters |
|---|---|---|---|
| Krag et al.10 | 24 | 1 |
↓ CI (<1.5 L/minute/m2) |
| Merli et al.11 | 90 | 2 |
↑ LA dimension ↓ LV mass |
| Ruiz-Del Arbol et al.12 | 80 | 1 |
↑ E/e′ LVDD |
| Sampaio et al.6 | 98 | 0.5 | ↓ MAP |
| Cesari et al.(present study) | 115 | 6 |
↑ LA dimension ↑ E/e′ ↑ heart rate ↓ MAP |
Sampaio et al. evaluated the prognostic impact of cardiac systolic and diastolic dysfunction (assessed by tissue Doppler and speckle tracking analysis) in 98 patients with cirrhosis followed up for 6 months.6 None of the echocardiographic parameters were associated with the occurrence of death. The only independent predictors of mortality were a Child score >10 points and a MAP below the median (Table 5). Krag et al. reported that a low CI (below 1.5 L/minute/m2) assessed by gated myocardial perfusion imaging was associated with the development of HRS type 1 within 3 months in 24 patients with advanced cirrhosis and ascites, more important than MELD score to predict prognosis (Table 5).10
As expected, in our study MELD, age, and BSA were the main predictors of poor outcome. Of interest, when MELD was excluded by the Cox analysis, the increased LA dimension entered in the model and was an independent predictor of death (Table 3 and Fig. 1).
In our models, LV volume, mass, and prevalence of hypertrophy did not predict death, as well as the parameters of LV systolic function (midwall fractional shortening, EF, and septal strain).
When multivariate analysis was performed incorporating only cardiovascular parameters, increased E/e′ and heart rate and reduced MAP were significantly associated with the occurrence of death (Table 3 and Fig. 1).
In line with other studies (Table 5), we observed a predictive value of increased LA dimension, increased E/e′ (Fig. 1), and low MAP, whereas no effect of LVM was evident. Because increased LA dimension and E/e′ are strongly associated with increased LV filling pressure,25 we suggest that in patients with cirrhosis with normal systolic function the reduction of MAP and the increase in LV filling pressure are the main cardiovascular predictors of death.
At variance with previous results, the systolic function and SV being equal, in patients with a poor outcome we observed an increase in heart rate and a reduction of BSA and MAP, therefore inducing a consequent concomitant reduction of SW and systemic vascular resistance index and an increase of CI (all parameters which are related to heart rate, BSA, and MAP). Also, Ruiz-del-Arbol reported a reduction (not significant) of MAP and SW in 14 patients with cirrhosis with moderate ascites who presented type 1 HRS during 12 months of follow-up.12
The prevalence of LV DD showed wide variation depending on the algorithm considered, being lower using the ASE/EACVI 2016 recommendations and higher using the newly proposed Thorax Center algorithm. However, no association with prognosis emerged regarding LV DD when assessed with all of the above algorithms.
Regarding the development of type 1 HRS, we identified increased MELD (P = 0.006) and CI (P = 0.01) as independent predictors of development of this complication. This is in contrast with the results of Krag et al.,10 who reported a higher incidence of type 1 HRS in patients with low CI (below 1.5 L/minute/m2), and with the results of Ruiz-del-Arbol et al.,12 who observed an association between plasma renin activity and E/e′ ratio, with the development of this complication in 14 patients with cirrhosis and moderate ascites who presented type 1 HRS during 12 months of follow-up.
In conclusion, among echocardiographic and hemodynamic parameters, an increased LA dimension and E/e′ (as indexes of increased LV filling pressure) and low MAP with related compensatory increased heart rate seem to be useful in identifying patients with cirrhosis at increased risk of dying.
From the clinical standpoint, these results suggest performing an accurate echocardiographic evaluation in all patients with cirrhosis, paying specific attention to the assessment of LA dimensions and E/e′ ratio, to better identify patients with a worse prognosis which requires a lower threshold to initiate workup for liver transplantation. Further research is needed to collect follow-up outcome data in these patients to support this contention.
It might be argued that we should have used LA volume, which might have a stronger prognostic value for cardiovascular events, instead of LA diameter. However, the latter is more readily measurable and was available in all our patients, including those in the older studies, and provides an accurate estimate of LA size in 94% of patients.24
Strengths of this study to be underscored include the long follow-up and the large sample size of phenotypically well-characterized patients with cirrhosis, the use of state-of-the art echo-Doppler techniques entailing TDI for LV assessment, recording of echocardiograms, as well as centralized readings by a single experienced cardiologist.
REFERENCES
Author names in bold designate shared co-first authorship.




