TGR5 contributes to hepatic cystogenesis in rodents with polycystic liver diseases through cyclic adenosine monophosphate/Gαs signaling
Potential conflict of interest: Nothing to report.
Supported by the National Institutes of Health (DK24031), the Mayo Clinic, the Clinical Core and Optical Microscopy Core of the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30DK084567), the Mayo Translational PKD Center (NIDDK P30DK090728), the Mayo Translational PKD Center Pilot and Feasibility Award, and the Eileen Creamer O'Neill Award from the PKD Foundation.
Abstract
Hepatic cystogenesis in polycystic liver disease is associated with increased levels of cyclic adenosine monophosphate (cAMP) in cholangiocytes lining liver cysts. Takeda G protein receptor 5 (TGR5), a G protein–coupled bile acid receptor, is linked to cAMP and expressed in cholangiocytes. Therefore, we hypothesized that TGR5 might contribute to disease progression. We examined expression of TGR5 and Gα proteins in cultured cholangiocytes and in livers of animal models and humans with polycystic liver disease. In vitro, we assessed cholangiocyte proliferation, cAMP levels, and cyst growth in response to (1) TGR5 agonists (taurolithocholic acid, oleanolic acid [OA], and two synthetic compounds), (2) a novel TGR5 antagonist (m-tolyl 5-chloro-2-[ethylsulfonyl] pyrimidine-4-carboxylate [SBI-115]), and (3) a combination of SBI-115 and pasireotide, a somatostatin receptor analogue. In vivo, we examined hepatic cystogenesis in OA-treated polycystic kidney rats and after genetic elimination of TGR5 in double mutant TGR5−/−;Pkhd1del2/del2 mice. Compared to control, expression of TGR5 and Gαs (but not Gαi and Gαq) proteins was increased 2-fold to 3-fold in cystic cholangiocytes in vitro and in vivo. In vitro, TGR5 stimulation enhanced cAMP production, cell proliferation, and cyst growth by ∼40%; these effects were abolished after TGR5 reduction by short hairpin RNA. OA increased cystogenesis in polycystic kidney rats by 35%; in contrast, hepatic cystic areas were decreased by 45% in TGR5-deficient TGR5−/−;Pkhd1del2/del2 mice. TGR5 expression and its colocalization with Gαs were increased ∼2-fold upon OA treatment. Levels of cAMP, cell proliferation, and cyst growth in vitro were decreased by ∼30% in cystic cholangiocytes after treatment with SBI-115 alone and by ∼50% when SBI-115 was combined with pasireotide. Conclusion: TGR5 contributes to hepatic cystogenesis by increasing cAMP and enhancing cholangiocyte proliferation; our data suggest that a TGR5 antagonist alone or concurrently with somatostatin receptor agonists represents a potential therapeutic approach in polycystic liver disease. (Hepatology 2017;66:1197-1218).
Abbreviations
-
- ADPKD
-
- autosomal dominant PKD
-
- C1/C2
-
- compounds 1 and 2, respectively
-
- cAMP
-
- cyclic adenosine monophosphate
-
- ERK
-
- extracellular signal–regulated protein kinase
-
- GLP-1
-
- glucagon-like peptide 1
-
- OA
-
- oleanolic acid
-
- PCK
-
- polycystic kidney
-
- PCNA
-
- proliferating cell nuclear antigen
-
- PKD
-
- polycystic kidney disease
-
- PLD
-
- polycystic liver disease
-
- SBI-115
-
- m-tolyl 5-chloro-2-(ethylsulfonyl) pyrimidine-4-carboxylate
-
- shRNA
-
- short hairpin RNA
-
- SSTR
-
- somatostatin receptor
-
- TGR5
-
- Takeda G protein receptor 5
-
- TLCA
-
- taurolithocholic acid
-
- WT
-
- wild type
Polycystic liver disease (PLD) is a group of genetic disorders characterized by cholangiocyte-derived liver cysts. PLD coexists with autosomal recessive polycystic kidney disease (PKD) or autosomal dominant PKD (ADPKD). ADPKD results from mutations in the PKD1 and PKD2 genes, while mutations in the PKHD1 gene are responsible for renal and hepatic cystogenesis in autosomal recessive PKD. Isolated autosomal dominant PLD is a rare condition due to mutations in the SEC63, PRKCSH, or LRP5 genes.1-3 Hepatic cystogenesis in all the PLDs is sex-dependent; female patients often have a more severe disease and higher total liver volume.2
Mutations in PLD-related genes initiate the formation of hepatic cysts, which continue to grow as a result of multiple downstream phenomena including cholangiocyte hyperproliferation, dysregulated cell cycle, enhanced fluid secretion, decreased intracellular calcium, and global changes in mRNA, microRNA, and protein expression.2, 3 Intracellular cyclic adenosine monophosphate (cAMP, levels of which are markedly increased in PLD)4 is a well-characterized regulator of cellular pathways associated with hepatic cystogenesis. For example, (1) cholangiocyte proliferation involves multiple cAMP downstream effectors, including exchange proteins activated by cAMP 1 and 2, protein kinase A, and extracellular signal–regulated protein kinase 1/2 (ERK1/2)5; (2) expression of the cell cycle proteins is controlled by components of cAMP signaling3, 6; (3) fluid secretion into the cystic lumen requires the water channel aquaporin 1 and the ion transporters cystic fibrosis transmembrane conductance regulator and anion exchanger 2, all influenced by cAMP7; and (4) cilia in cystic cells lack components of the cAMP machinery (e.g., adenylyl cyclase 5/6, A-kinase anchoring protein 150, protein kinase A, and phosphodiesterase 4C), subsequently interrupting crosstalk between cAMP and intracellular calcium; as a result, cholangiocyte proliferation is enhanced, contributing to cyst growth.3, 8 Taken together, these observations implicate cAMP as an important central component in the network of dysregulated signaling pathways in PLD and provide the rationale for cAMP-targeted strategies in disease treatment. Indeed, we have shown that suppression of cAMP in cystic cholangiocytes by octreotide and pasireotide, synthetic octapeptide agonists of somatostatin receptors (SSTRs), inhibited hepatic cystogenesis in animal models of PLD.4, 9 Moreover, octreotide decreased liver volume (by ∼5%) in patients with PLD and improved quality of life.10-12 While somatostatin analogues are considered to be the only available drug option, treatment is costly ($7,000-$11,000 per month), changes in liver volume are modest, up to 15% of patients do not respond to treatment, and after drug withdrawal the liver volume returns to pretreatment levels.11 Thus, a search for new therapeutic options remains of great importance.
Takeda G protein receptor 5 (TGR5), a G protein–coupled bile acid receptor linked to cAMP signaling, is expressed in a variety of tissues and is known to influence energy homeostasis, inflammation, immune responses, insulin secretion, and gallbladder relaxation.13-15 Recently, TGR5 has emerged as a promising therapeutic target.13, 14, 16, 17
In the liver, TGR5 is expressed in sinusoidal cells, Kupffer cells, gallbladder epithelia, and cholangiocytes but not in hepatocytes.13, 16, 18 TGR5 is stimulated by bile acids (e.g., lithocholic, chenodeoxycholic, deoxycholic, and cholic acids), xenobiotic ligands (i.e., oleanolic acid [OA]), and semisynthetic derivatives (e.g., INT-777 and obeticholic acid).13, 14 Importantly, we previously reported that the concentration of lithocholic, chenodeoxycholic, and cholic acids is significantly higher in livers of polycystic kidney (PCK) rats (an animal model of PLD) compared to wild-type (WT) counterparts.19 TGR5 agonists are known to affect downstream signaling events through coupling to Gαs, Gαi, and Gαq proteins.13, 18, 20 Moreover, stimulation of TGR5 in different cell types, including cholangiocytes, has been shown to increase cAMP and affect cell proliferation.18, 20-23
Thus, given that (1) hyperproliferation is one of the major mechanisms driving cyst expansion in PLD2, 3; (2) cAMP is increased in cholangiocytes of animal models and humans with PLD3, 4, 9; (3) levels of TGR5 agonists are increased in cystic livers; and (4) TGR5 is linked to cAMP signaling,13, 18 we investigated the effects of pharmacological activation and genetic and pharmacological inactivation of TGR5 on hepatic cystogenesis in PLD and the potential mechanisms involved.
We found that TGR5 and Gαs proteins are overexpressed in PLD. As anticipated, stimulation of TGR5 in cystic cholangiocytes increased cAMP levels, cell proliferation, and cyst growth in vitro; these effects were eliminated after TGR5 reduction by short hairpin RNA (shRNA). The TGR5 agonist OA worsened hepatic cystogenesis in vivo in PCK rats, while genetic depletion of TGR5 by generating TGR5−/−; Pkhd1del2/del2 double mutant mice reduced cyst growth. Finally, cAMP levels, cell proliferation, and cyst expansion in vitro were decreased in cystic cholangiocytes after treatment with a novel small molecule TGR5 antagonist, m-tolyl 5-chloro-2-(ethylsulfonyl) pyrimidine-4-carboxylate (SBI-115). Concurrent targeting of cAMP by SBI-115 and pasireotide, a synthetic agonist of SSTRs, suppressed cAMP, cell proliferation, and cyst expansion in vitro more effectively than each drug alone. Together these data suggest that TGR5 activation facilitates cyst growth, while its inhibition attenuates hepatic cystogenesis through modulation of cAMP signaling. Thus, TGR5 antagonists alone or concurrently with agonists of SSTRs represent a novel therapeutic approach for PLD.
Materials and Methods
CELL CULTURES, REAGENTS, AND ANIMALS
Control and PCK rat cystic cholangiocytes (derived from WT and PCK rats, respectively); and control and ADPKD human cystic cholangiocytes (derived from healthy humans and ADPKD patients, respectively) were used and maintained as described.9 TGR5 was activated by taurolithocholic acid (TLCA), OA (both from Sigma-Aldrich, St. Louis, MO), and two synthetic compounds—compound “1” (C1), (2-(ethylamino)-6-(3-(4-(trifluoromethoxy)phenyl)-propanoyl)-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine-4carbox-amide), and compound “2” (C2), 3-(2-chlorophenyl)-N-(4-chlorophenyl)-N,5-dimethyl-isoxazole-4-carboxamide. The small molecule TGR5 antagonist (SBI-115) was identified by throughput screening of a 50,000-compound chemical library in TGR5-expressing CHO-K1 cells. C1, C2, and SBI-115 were synthesized in the Sanford Burnham Prebys Medical Discovery Institute at >95% purity by high-performance liquid chromatography following the outlined procedure.24, 25 Cystic cholangiocytes were stably transfected with TGR5 or control shRNAs (Santa Cruz Biotechnology, Santa Cruz, CA). Rodents were housed in a 12-hour light–dark facility on a standard diet and water ad libitum. The number of animals used is indicated for each experiment. The protocol was approved by the Mayo Institutional Animal Care and Use Committee.
RNA preparation, sequencing, and data analysis of rat and human control and cystic cholangiocytes (n = 3 for each cell line) are described in Supporting Information.
WESTERN BLOT
Proteins were separated by 4%-15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA), and incubated first with primary anti-TGR5 (Santa Cruz H-90, which was found to work best in our experimental systems), anti-p-Erk1/2 (Santa Cruz), anti-total Erk1/2 (Cell Signaling, Danvers, MA), anti-Gα (Abcam, Cambridge, MA), and anti-β-actin antibodies and then with corresponding secondary horseradish peroxidase–conjugated (Invitrogen, Carlsbad, CA) or IRdye 680 or 800 (Odyssey) antibodies. Bands were visualized with ECL Plus Western Blotting Detection kit (BD Biosciences, San Jose, CA) or Odyssey Li-Cor Scanner (Li-Cor, Lincoln, NE). Integrated density of protein bands was assessed by ImageJ (National Institutes of Health, Bethesda, MD), and data are presented as arbitrary units compared to β-actin.
IMMUNOFLUORESCENCE CONFOCAL MICROSCOPY
Livers from WT (Harlan Sprague Dawley) and PCK (our colony) rats; WT, Pkd2WS25/–, Pkhd1del2/del2, and TGR5−/− mice (all of C57BL/6 background and our colonies) and healthy humans and patients with ADPKD and autosomal dominant PLD were used. Archive human liver tissues were provided by the Mayo Clinical Core after IRB approval. Livers were incubated with primary antibodies to TGR5, Gα, and proliferating cell nuclear antigen (PCNA) (Santa Cruz) and then with respective secondary fluorescent antibodies (Molecular Probes, Eugene, OR). Nuclei were stained with 4,6-diamidino-2-phenylindole (Invitrogen). Livers were analyzed by Zeiss LSM-510 microscope (Carl Zeiss, Thornwood, NY). Fluorescence intensity (arbitrary units) of protein staining was assessed by Adobe Photoshop Elements 10 and expressed as percent change in cystic cholangiocytes compared to respective controls in which fluorescence intensity was considered to be 100%. Quantitation of TGR5 colocalization with Gαs was performed using Image J.
IMMUNOGOLD TRANSMISSION ELECTRON MICROSCOPY
Livers from WT and PCK rats were incubated with anti-TGR5 antibody (1:20) and then with a gold-conjugated secondary antibody (1:100; Electron Microscopy Sciences), postfixed with 2.5% glutaraldehyde, enhanced with silver mixture (R-Gent SE-EM), and incubated again with 1% osmium tetroxide. Specimens were observed using a Jeol 12 electron microscope (Jeol USA, Peabody, MA). The number of immunogold particles was assessed by ImageJ.
cAMP DETECTION
cAMP was detected by the Bridge-It cAMP designer cAMP assay (Mediomics, St. Louis, MO). Cholangiocytes (10,000/well) were incubated with TLCA, OA, C1 and C2 (all, 25 μM), SBI-115 (100 and 200 μM), and pasireotide (20 μM; MedChemexpress, Monmouth Junction, NJ) for 15-30 minutes. Doses of drugs were chosen based on published data9, 18 or dose ranging (SBI-115).
CELL PROLIFERATION
Cell proliferation was determined by CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI) and by cell counting using the Cellometer Auto4 (Nexcelom Bioscience) cell counter. Cholangiocytes (2,500 cells/well) were grown for 24-48 hours and then treated with TGR5 agonists (all, 25 μM), SBI-115 (100 and 200 μM), and pasireotide (20 μM) for additional 24 hours. Alterations in cell proliferation after treatment were expressed as percent change compared to untreated cholangiocytes in which cell proliferation was considered to be 100%.
THREE-DIMENSIONAL CULTURES
Microdissected from PCK rat livers cystic bile ducts or cultured cholangiocytes were grown in three-dimensional matrices as described4, 5, 9 and treated with TLCA and OA (both, 25 μM) daily. Images were taken at days 1 (24 hours after seeding) and 3. The circumference of cystic structures was measured by ImageJ as described.4
THREE-DIMENSIONAL CHOLANGIOCYTE SPHEROIDS
Cholangiocyte spheroids were generated by a hanging drop approach26, 27 using a Perfect 96-well hanging drop plate (3-D Biomatrix, Ann Arbor, MI). Each hanging drop (40 μL of medium) contains 10,000 cholangiocytes in suspension. Spheroids were formed within 24 hours and grown for an additional 96 hours before treatment. Microphotographs were taking before (day 1) and after (day 3) treatment. Circumferences of spheroids were assessed by Image J.
TREATMENT PROTOCOL
PCK rats (4-6 weeks old, n = 5 female, n = 5 male) were injected intraperitoneally with OA (25 mg/kg body weight) daily for 6 weeks. Dose of OA and route of administration were chosen based on published studies.28 Drug doses were adjusted to animal weight weekly. Control PCK rats (4-6 weeks old, n = 4 female, n = 4 male) received equal doses of dimethyl sulfoxide. Serum biochemistry, gross anatomy, and cystic and fibrotic areas were analyzed as described.9 Both male and female rats were used in this study because of gender differences in human PLD patients.2
STATISTICAL ANALYSIS
The data are expressed as the mean ± standard deviation. Statistical analysis was performed by Student t test; results were considered statistically significant at P < 0.05.
Details on other experimental procedures are described in the Supporting Information.
Results
TGR5 IS OVEREXPRESSED IN RAT AND HUMAN CYSTIC CHOLANGIOCYTES
Copy numbers of TGR5 transcript were increased ∼20-fold (Fig. 1A), and expression of TGR5 protein was higher by 40%-45% (Fig. 1B) in cultured cystic cholangiocytes compared to control. Increased immunoreactivity of TGR5 (up to 2-fold) was seen in vivo in cholangiocytes of patients and animal models with PLD (Fig. 1C). Immunogold transmission electron microscopy demonstrated that the number of TGR5-positive immunogold particles was ∼4.5-fold greater in cholangiocytes of PCK rats compared to WT, confirming overexpression of TGR5 in PLD (Fig. 1D).
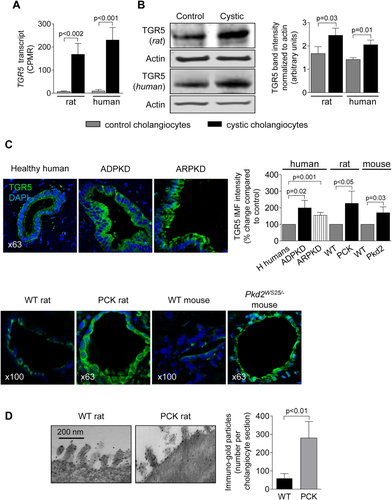
TGR5 AGONISTS INCREASE cAMP LEVELS IN CULTURED CYSTIC CHOLANGIOCYTES
Levels of cAMP upon TLCA, OA, C1, and C2 treatment were increased by 39%-50% in PCK cholangiocytes and by 41%-46% in ADPKD cholangiocytes (Fig. 2A). TGR5 agonists also increased cAMP (by 27%-33%) in cholangiocytes derived from WT rats and healthy humans (Supporting Fig. S1).
TGR5 AGONISTS INCREASE PROLIFERATION OF CYSTIC CHOLANGIOCYTES
All TGR5 agonists increased proliferation of PCK cholangiocytes by 37%-51% and ADPKD cholangiocytes by 39-49% as assessed by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium absorbance (Fig. 2B) and cell counting (Fig. 2C). TGR5 agonists also increased (by 23%-30%) proliferation of cholangiocytes derived from WT rats and healthy humans (Supporting Fig. S2).
TGR5 AGONISTS ACCELERATE GROWTH OF ISOLATED PCK BILE DUCTS IN THREE-DIMENSIONAL CULTURE
PCK bile ducts progressively grew under basal conditions (Fig. 2D). At day 3, they were ∼60% larger compared to day 1. TLCA and OA accelerated cyst growth, increasing circumferences of bile ducts, respectively, by 88% and 92%.
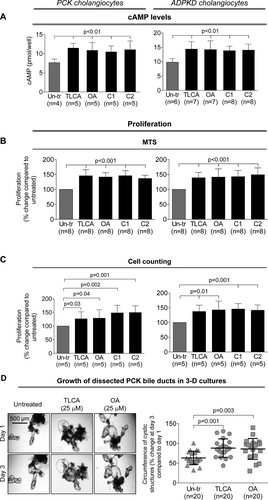
OA INCREASES HEPATIC AND RENAL CYSTOGENESIS IN PCK RATS
OA was well tolerated, without mortality or toxicity (i.e., no hair or weight loss). Serum biochemistries were comparable in untreated and OA-treated PCK rats (Supporting Table S1). OA worsened hepatorenal cystogenesis as evidenced by increased (1) liver weights (∼25%), (2) kidney weights (∼22%), (3) hepatic cystic areas (∼32%), (4) hepatic fibrotic areas (∼19%), (5) renal cystic areas (∼26%), and (6) renal fibrotic areas (∼22%; Supporting Table S2; Fig. 3A,B). After OA treatment, the numbers of PCNA-positive nuclei were higher by 2.5-fold in cystic cholangiocytes and 2.8-fold in cystic renal epithelia compared to respective controls (Fig. 3C).
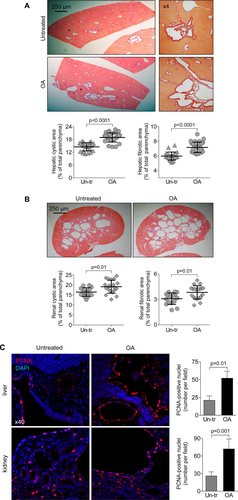
TGR5 REDUCTION IN CYSTIC CHOLANGIOCYTES ABOLISHES EFFECTS OF TGR5 AGONISTS ON cAMP LEVELS, Erk1/2 PHOSPHORYLATION, CELL PROLIFERATION, AND CYST GROWTH IN VITRO
Western blots demonstrate that TGR5 levels were decreased by ∼80% in TGR5 shRNA-transfected cholangiocytes compared to cholangiocytes transfected with control shRNA (Fig. 4A). Compared to untransfected cholangiocytes, control shRNA-transfected cholangiocytes have comparable levels of cAMP, cell proliferation, and rate of cyst growth after treatment with TGR5 agonists (Supporting Fig. S3). Control shRNA-transfected cholangiocytes respond to TGR5 ligands by increasing cAMP levels, Erk1/2 phosphorylation, and cell proliferation; these effects were eliminated in cholangiocytes with reduced levels of TGR5 (Fig. 4B-D). Similar to freshly isolated PCK bile ducts, growth of cystic structures formed by cultured control shRNA-transfected ADPKD cholangiocytes was enhanced by 47% and 36% in the presence of TLCA and OA, respectively (Fig. 4E). No changes in circumference of cystic structures were noticed in TGR5 shRNA-transfected cholangiocytes (Fig. 4F).
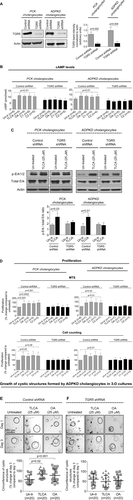
GENETIC ELIMINATION OF TGR5 IN MICE WITH PLD DECREASES HEPATIC CYSTOGENESIS
To further examine the involvement of TGR5 in PLD, we generated double-mutant mice yielding TGR5−/−;Pkhd1del2/del2 phenotype. Consistent with a previous report, TGR5−/− mice were healthy and fertile.29 Serum biochemistries (Supporting Table S3), gross anatomy (Supporting Table S4), and morphology of liver, cholangiocyte, and primary cilium (Supporting Fig. S4) were comparable in WT and TGR5−/− mice. Pkhd1del2/del2 rodents have multiple hepatic cysts.30 Consistent with our observations in other animal models of PLD, TGR5 was overexpressed in Pkhd1del2/del2 mice compared to WT but was not detected in TGR5−/− and TGR5−/−;Pkhd1del2/del2 mice (Supporting Fig. S5). In contrast to Pkhd1del2/del2 littermates, in double mutant TGR5−/−;Pkhd1del2/del2 mice (both male and female), we observed reductions in (1) liver weight by 30%, (2) hepatic cystic areas by 31%, and (3) hepatic fibrotic areas by 33% (Fig. 5A; Supporting Table S4). Serum biochemistries were similar in all groups of mice (Supporting Table S3). Attenuated hepatic cystogenesis in double mutant TGR5−/−; Pkhd1del2/del2 mice was associated with a 3-fold decreased quantity of PCNA-positive nuclei (Fig. 5B).
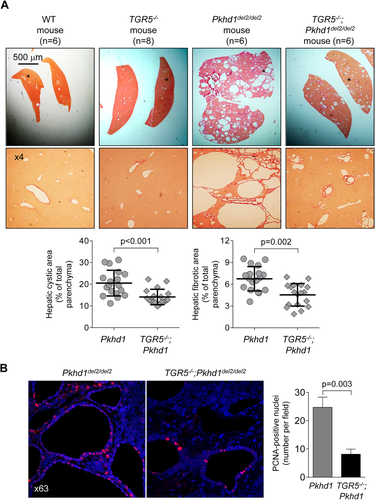
EXPRESSION OF Gαs PROTEINS IS INCREASED IN PLD
We investigated the expression of Gα proteins known to be coupled with TGR5.20 Levels of Gαi and Gαq were identical in control and cystic cholangiocytes (Fig. 6A,B). In contrast, immunoreactivity of Gαs in cholangiocytes of PCK rats was increased by ∼67% compared to WT rats (Fig. 6A). Similarly, in cultured cystic cholangiocytes expression of Gαs was ∼50% higher compared to control (i.e., cholangiocytes derived from WT rats; Fig. 6C,E). OA treatment increased levels of Gαs by ∼30% in control shRNA-transfected cholangiocytes, but no changes were observed in TGR5-depleted cholangiocytes (Fig. 6C,E). Up-regulation of TGR5 (by ∼50%) was also seen in response to OA (Fig. 6D,E). Consistent with an in vitro observation, 59% and 48% greater immunoreactivity of Gαs and TGR5, respectively, was observed in cholangiocytes of OA-treated PCK rats (Fig. 6F). Finally, we found by confocal microscopy that colocalization of TGR5 with Gαs proteins in cystic cholangiocytes was increased ∼2-fold after TGR5 stimulation (Costes' test, P < 0.001; Fig. 6E,F).
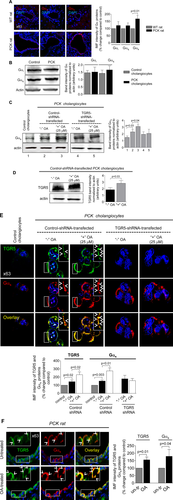
A TGR5 ANTAGONIST, SBI-115, DECREASES CELL PROLIFERATION, CHOLANGIOCYTE SPHEROID GROWTH, AND cAMP LEVELS IN CYSTIC CHOLANGIOCYTES
SBI-115 alone had no effects on cell proliferation, spheroid growth, and cAMP levels in shRNA-transfected ADPKD cholangiocytes. In contract, stimulation of cholangiocytes with TLCA followed by SBI-115 treatment resulted in dose-dependent (by 32%-48%) inhibition of cell proliferation (Fig. 7A). Under similar conditions, SBI-115 reduced growth of cholangiocyte spheroids (Fig. 7B) and levels of cAMP (Fig. 7C) by ∼30%. These processes were not affected in TGR5-depleted ADPKD cholangiocytes pretreated with TLCA (Fig. 7A-C). Moreover, to provide additional evidence that TGR5 antagonism decreases cAMP in a TGR5-dependent fashion, we increased cAMP levels in cystic cholangiocytes by forskolin (i.e., independently of TGR5) and found that SBI-115 had no effect on forskolin-induced cAMP production (Supporting Fig. S6).
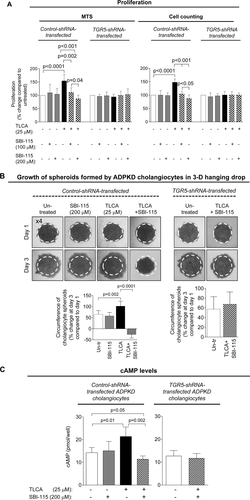
CONCURRENTLY, SBI-115 AND PASIREOTIDE DECREASE CELL PROLIFERATION, CHOLANGIOCYTE SPHEROID GROWTH, AND cAMP LEVELS IN VITRO MORE EFFECTIVELY THAN EACH DRUG ALONE
Finally, we tested how the simultaneous targeting of elevated cAMP in cystic cholangiocytes by SBI-115 and pasireotide will affect the cellular processes known to play an important role in hepatic cystogenesis. We observed that cell proliferation, spheroid growth, and cAMP levels in control shRNA-transfected ADPKD cholangiocytes were decreased after treatment with SBI-115 by 20%-33% and by 35%-40% in response to pasireotide. However, a greater reduction (by 45%-55%) in cell proliferation, spheroid growth, and cAMP was detected in response to drug combination (Fig. 8A-C). In addition, cAMP levels, cell proliferation, and spheroid growth were decreased by ∼30%-35% in TGR5 shRNA-depleted cystic cholangiocytes in response to pasireotide alone (Supporting Fig. S7), suggesting that pasireotide affects these processes independently of TGR5.

Discussion
The key findings of our study are (1) TGR5 is overexpressed in PLD, and its agonists accelerate disease progression; (2) pharmacologic inhibition of TGR5 in cystic cholangiocytes in vitro by a novel small molecule antagonist or in vivo by genetic elimination of TGR5 in an animal model of PLD attenuates disease progression; (3) TGR5 contributes to hepatic cystogenesis by enhancing cAMP production and Gαs expression; and (4) concurrent targeting of cAMP machinery in cystic cholangiocytes by TGR5 antagonists and SSTR agonists inhibits cell proliferation and cyst growth in vitro more substantially than each drug alone. Together, these data suggest that TGR5 contributes to PLD and its inhibition represents a potential therapeutic approach. Furthermore, targeting of elevated cAMP in cystic cholangiocytes through two different pathways (i.e., TGR5 antagonists and SSTR agonists [currently the only available pharmacologic option for PLD patients]) may be an approach worth evaluating.
Our data show that the hyperproliferative effects of TGR5 agonists in PLD are TGR5-dependent. All tested TGR5 agonists modestly but significantly accelerated proliferation (i.e., one of the major mechanisms involved in cyst growth) of cultured rat and human cystic cholangiocytes and increased the number of PCNA-positive cholangiocytes in PCK rats treated with OA. In TGR5-depleted cholangiocytes in vitro or in cholangiocytes of double mutant TGR5−/−; Pkhd1del2/del2 mice with genetically eliminated TGR5, the effects of TGR5 agonists on cell proliferation were absent. In line with our observations, cholangiocyte hyperproliferation in response to TGR5 agonists has been reported.21, 31 For example, TLCA feeding increased the number of PCNA-positive cholangiocytes in rats, while cholic acid triggered cholangiocyte proliferation in WT, but not in TGR5−/−, mice.21, 31 Moreover, TGR5 agonists have been shown to enhance growth of human cells derived from endometrial cancer, gastric adenocarcinoma, and cholangiocarcinoma.21, 22, 32
Using different approaches, we demonstrated that TGR5 is up-regulated in PLD at the message and protein levels and that its activation worsens hepatic cystogenesis. Accumulating data suggest that TGR5 levels are increased in a number of different pathological conditions and might influence disease outcome. Indeed, overexpression of TGR5 in gastric adenocarcinoma was associated with decreased rate of patient survival,22 and a high copy number of TGR5 gene was found to be an indicator of worse prognosis in gastric and breast cancer patients.33 Up-regulation of TGR5 was also reported in patients with cholangiocarcinoma and pancreatic cancers.21, 33 Thus, there appears to be a growing number of diseases17, 21, 23, 32 in addition to PLD in which an effective antagonist to TGR5 might be of value.
Why expression of TGR5 is increased in PLD (or in other pathological conditions) is unclear. Our preliminary data suggest that overexpression of TGR5 in cystic cholangiocytes is associated with decreased levels of microRNAs 204 and 708, which target TGR5 mRNA. This potential mechanism of TGR5 up-regulation in PLD is now under investigation in our laboratory.
To further reveal the contribution of TGR5 to PLD, we genetically eliminated this bile acid receptor in cystic cholangiocytes by crossing TGR5−/− and Pkhd1del2/del2 mice. It has been reported that TGR5−/− mice, which we used for cross-breeding, have body weight and bile acid pool size comparable to WT littermates.29 We also observed no differences in morphology of the liver (e.g., no cysts), of cholangiocytes, and of cholangiocyte primary cilia in TGR5−/− mice compared to WT. In contrast, Pkhd1del2/del2 mice, a well-characterized model of PLD, develop multiple liver cysts by the age of 7-9 months.30 Consistent with our observations in other animal models of PLD (i.e., PCK rat and Pkd2WS25/– mice), TGR5 was significantly overexpressed in cholangiocytes lining liver cysts in Pkhd1del2/del2 rodents. In contrast, no TGR5 expression was observed in TGR5−/−; Pkhd1del2/del2 double-mutant mice, and depletion of TGR5 significantly reduced cystic and fibrotic areas in TGR5−/−; Pkhd1del2/del2 mice, providing perhaps the best evidence that TGR5 plays an important role in hepatic cystogenesis.
Several reports suggest that TGR5 could be coupled to Gαs, Gαi, and Gαq proteins.18, 20, 34 In order to understand the mechanisms by which stimulation of TGR5 enhances hepatic cystogenesis, we studied the presence of Gα proteins in PLD. Expression of Gαi and Gαq was similar between control and diseased cholangiocytes, but levels of Gαs (the release of which accelerates cAMP production) were up-regulated in PCK and ADPKD cystic cholangiocytes. In line with this, all four tested TGR5 agonists increased cAMP levels in cystic cholangiocytes to a higher extent (by ∼12%-17%) than in control cholangiocytes. Effects of TGR5 stimulation were abolished after TGR5 reduction by shRNA. We noticed that levels of cAMP were comparable in control shRNA-transfected and TGR5 shRNA-transfected cholangiocytes in the absence of treatment. TGR5 enhances cAMP production only in response to its stimulation. In cultured cholangiocytes under basal conditions, TGR5 is silent due to the absence of TGR5 agonists in culture media. However, control shRNA-transfected cholangiocytes (but not TGR5-depleted cholangiocytes) respond to TGR5 stimulation by increased cAMP levels.
We also demonstrated that expression of both Gαs and TGR5 proteins and their colocalization was increased in response to OA in vitro and in vivo. These data suggest that exposure of cystic cholangiocytes to TGR5 agonists might account for overexpression of both TGR5 and Gαs in PLD, leading to cAMP elevation. An increased expression of TGR5 upon exposure to its ligands has also been observed in esophageal epithelium, gastric myenteric plexus, and macrophages.35-37
To our knowledge, no reports describe the identification, synthesis, or pharmacologic activity of TGR5 antagonists. SBI-115 (further details of which will be published elsewhere), a novel small molecule, was identified from a high-throughput screen in CHO-K1 cells expressing TGR5. We confirmed and validated the effects of SBI-115 in PLD and found that this TGR5 antagonist reduced cell proliferation and growth of cholangiocyte spheroids in vitro by decreasing cAMP levels in cystic cholangiocytes in a TGR5-dependent manner. Moreover, concurrent targeting of cAMP machinery by SBI-115 and an SSTR analogue, pasireotide, inhibited these processes more effectively compared to each drug alone. While in vivo preclinical studies are needed to further demonstrate the potential beneficial effects of TGR5 targeting in PLD, our extensive experience using different activators and inhibitors of hepatic cystogenesis4-7, 9, 38, 39 suggests that results of two in vitro experimental systems (i.e., cell proliferation and cyst growth) reliably predict outcomes of in vivo studies in different animal models of PLD.
Finally, while our in vitro and in vivo data strongly suggest that TGR5 agonists/antagonists affect hepatic cystogenesis in PLD by direct activation/inhibition of cholangiocyte TGR5, we acknowledge that other mechanisms might also be contributing factors. One such indirect mechanism might involve modulation of cholangiocyte proliferation by the hormone glucagon-like peptide 1 (GLP-1), which is released by intestinal L cells in response to TGR5 stimulation.40 Although experimental evidence suggests that cholangiocytes express the receptor for GLP-1 and that its activation increases cell proliferation,41 further studies are needed to assess the role of GLP-1 in TGR5-mediated cyst growth. However, we found that levels of GLP-1 receptor are decreased in human and rat cystic cholangiocytes, respectively, 16-fold and 37-fold (Supporting Fig. S8), suggesting that a potential contribution of GLP-1 to hepatic cystogenesis might not be significant.
In conclusion, we demonstrated that TGR5 agonists contribute to hepatic cystogenesis by increasing cAMP production and expression of Gαs proteins. We also showed that TGR5 antagonist alone or in combination with other drugs that lower cAMP in cystic cholangiocytes is a promising therapeutic target in PLD.
REFERENCES
Author names in bold designate shared co-first authorship.




