Loss of L-selectin-guided CD8+, but not CD4+, cells protects against ischemia reperfusion injury in a steatotic liver
Potential conflict of interest: Nothing to report.
Supported by the National Institutes of Health (K08 grant DK091506, to N.A.G.).
Abstract
Steatotic liver responds with increased hepatocellular injury when exposed to an ischemic-reperfusion insult. Increasing evidence supports the role of immune cells as key mediators of this injury in a normal (lean) state, but data about their role in a steatotic liver are practically nonexistent. The objective of the current study was to delineate the contribution of specific phenotypes of T cells and adhesion molecules in exacerbated cell death in steatotic liver injury. RNA sequencing was performed on isolated steatotic primary hepatocytes, and T-cell markers were assessed in hepatic lymphocytes after ischemia reperfusion injury (IRI) in high-fat diet (HFD)–fed mice. Cluster of differentiation 8 knockout (CD8−/−) and CD4−/− mice along with CD8 and L-selectin antibody–treated mice were fed an HFD, and hepatocellular injury was assessed by histology, propidium iodide injection, and alanine aminotransferase after IRI. RNA sequencing demonstrated a strikingly differential gene profile in steatotic hepatocytes versus lean hepatocytes. After injury, the HFD liver showed increased necrosis, infiltrating CD8+ cells, alanine aminotransferase, and proinflammatory cytokines. Hepatic lymphocytes demonstrated increased CD8+/CD62L+(L-selectin) cells in HFD-fed mice after IRI. CD8−/− mice and CD8-depleted C57BL/6 mice demonstrated significant protection from injury, which was not seen in CD4−/− mice. L-selectin blockade also demonstrated significant hepatoprotection from IRI. L-selectin ligand MECA-79 was increased in HFD-fed mice undergoing IRI. Conclusion: Blockade of CD8 and L-selectin, but not CD4, ameliorated hepatocellular injury, confirming that CD8+ cells are critical drivers of injury in a steatotic liver; this represents a therapeutic target in steatotic liver injury, underlining the importance of development of therapies specific to a steatotic liver. (Hepatology 2017;66:1258-1274).
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- ANOVA
-
- analysis of variance
-
- CD
-
- cluster of differentiation
-
- H&E
-
- hematoxylin and eosin
-
- HFD
-
- high-fat diet
-
- IgG
-
- immunoglobulin G
-
- IL
-
- interleukin
-
- IRI
-
- ischemia reperfusion injury
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- PI
-
- propidium iodide
-
- WT
-
- wild type
The proportion of obese adults in the United States has greatly increased since the 1960s, from 13.4% to 35.7%; it has been estimated that more than two thirds of adults are overweight or obese.1-3 Nonalcoholic fatty liver disease (NAFLD) is a sequel of obesity with a range of clinical severity from benign steatosis to severe steatohepatitis and then potentially fatal end-stage liver disease that requires liver transplantation.4, 5 Ischemia reperfusion injury (IRI) in the liver occurs during shock, during myocardial infarction, following hepatectomy, and following liver transplantation. A fatty liver is increasingly susceptible to IRI and develops increased hepatocellular dysfunction and cell death compared with a nonfatty liver. When exposed to IRI, patients with NAFLD develop extensive liver injury, leading to long hospital stays and increased morbidity and mortality. NAFLD is expected to surpass hepatitis C as the most common indication for liver transplantation by 2030.6 With wait lists for liver transplant increasing, there has been a decrease in availability of healthy donor organs because significant proportions of donor livers are fatty and, hence, unsuitable for transplantation. Measures to reduce cell injury and death in fatty livers exposed to IRI could increase the number of organs available for transplantation. The mechanisms by which hepatocytes undergo cell death in the fatty liver after IRI have not been sufficiently explored. We have shown that a fatty liver, compared with a lean liver, has increased necrosis, apoptosis, and autophagy after IRI,7, 8 which is associated with progression to fibrosis, cirrhosis, and hepatocellular carcinoma. Several groups have shown that cluster of differentiation 4–positive (CD4+) cells are the main drivers of IRI in a lean state,9-11 but the role of lymphocytes in IRI of NAFLD is virtually unknown. Interestingly, there is an increase in lymphocyte trafficking across endothelial cells in NAFLD,12 suggesting that these cells may contribute to the exacerbated tissue response to injury after IRI.
Proteins in the selectin family mediate leukocyte migration and recruitment.13, 14 L-selectin (also known as CD62L) is a lymphocyte-homing receptor, expressed by most leukocytes.15 It is an important mediator of inflammation, which is expressed on CD4+ and CD8+ cells, and is involved in the pathogenesis of several different disorders,16-19 including IRI in the lean liver.20-23
It has not been clear whether there is a role for L-selectin in the response of the fatty liver to IRI. We investigated the role of L-selectin-induced lymphocyte recruitment as an underlying mechanism of hepatocellular damage in fatty livers using a mouse model of IRI.
Materials and Methods
ANIMALS
The Institutional Animal Care and Use Committee of Emory University approved all procedures performed on animals. Mice were maintained on a 12-hour dark–light cycle and allowed free access to food and water under conditions of controlled temperature (25 ± 2°C).
DIETS
C57BL/6, CD8−/−, CD4−/− male mice were obtained from Jackson Research Laboratories at 4 weeks of age. Mice were divided into groups given a regular mouse chow or a high-fat diet (HFD; 60% fat; Research Diets Inc., NJ) ad libitum for 12 weeks before IRI. Body weights were monitored at regular intervals. Livers were collected, and steatosis was confirmed by oil red O staining on frozen sections.
TISSUE TRIGLYCERIDE QUANTIFICATION
Triglycerides were quantified in liver tissues by triglyceride assay according to the manufacturer's instructions and expressed as nanomoles per milligram of liver tissue.
IRI
Wild-type (WT) C57BL/6 mice fed an HFD were divided into four subgroups of 10 mice each: no IRI (control), IRI+, IRI with injection of a CD8-depleting antibody, or IRI with injection of antimouse CD62L (anti-L-selectin antibody). An equivalent number of mice fed normal chow were used as lean controls. Similar studies were also performed with CD8−/− and CD4−/− mice fed an HFD. Animals were anesthetized with pentobarbital (50 mg/kg), and IRI was induced as described.7 A clamp was placed on the portal vein and the hepatic artery, blocking blood flow to the left and medial lobes of the liver and inducing partial hepatic ischemia. The clamp was removed after 40 minutes to allow reperfusion. The mice were sacrificed after 24 hours of reperfusion, and liver tissue and blood samples were collected. Sham surgeries were also performed along with controls for all experiments.
PRIMARY HEPATOCYTE ISOLATION
At the time of sacrifice, livers were perfused with liberase enzyme (10 units) to separate parenchymal and nonparenchymal cells. Hepatocytes were purified using a Percoll gradient.
RNA SEQUENCING
Purified hepatocytes from lean and HFD-fed mice were analyzed for their genome-wide transcriptional profiles using a next-generation RNA-sequencing approach at Integrated Emory University Genomics Core. Genes were grouped into functionally related genes using the DAVID functional annotation tool, and heat maps were generated using R programming.
HISTOLOGY
Paraffin sections of the entire ischemic lobe of the liver of lean and HFD-fed mice undergoing IRI were stained with hematoxylin and eosin (H&E). Sections were scanned using a bright-field scanner (Hamamatsu Nano zoomer 2.0 H) in the Department of Pathology, Emory University. Aperio image scope software (Leica Biosystems Imaging Inc.) was used for assessment of total necrotic area and nuclear count per high-power field. Scanned, high-resolution images were used to outline the necrotic area (white line), and nuclear counting was performed using the algorithm provided in the software. Based on the recommendations of the Nomenclature Committee on Cell Death,24 we also calculated a manual, detailed morphologic score using selective histological criteria for necrosis, as shown in Table 1.
| Score | 0 | 1 | 2 | 4 | |
|---|---|---|---|---|---|
| Zone 1 | |||||
| Nucleus | Normal | >50 cells | <50 cells | — | |
| Membrane | Normal | >50 cells | <50 cells | — | |
| Discernible necrotic tissue | Absent | Present | |||
| Zone 2 | Nucleus | Normal | >50 cells | <50 cells | — |
| Membrane | Normal | >50 cells | <50 cells | — | |
| Discernible necrotic tissue | Absent | Present | |||
| Zone 3 | Nucleus | Normal | >50 cells | <50 cells | — |
| Membrane | Normal | >50 cells | <50 cells | — | |
| Discernible necrotic tissue | Absent | Present | |||
| Bridging necrosis | Absent | <5 | >5 | Present | |
| Hemorrhagic necrotic tissue | Absent | Present | |||
| Total score | 32 |
HEPATOCELLULAR INJURY
Hepatocellular injury was assessed in vivo by measuring serum levels of alanine aminotransferase (ALT) in the Division of Animal Resources, Emory University. To assess cell death due to cell membrane permeability, mice were given intravenous injections of propidium iodide (PI; Sigma-Aldrich) 10 minutes before sacrifice. Frozen sections were cut, and red fluorescent intensity was captured by fluorescent microscopy (Olympus) and quantified using Fiji software.
LUMINEX ASSAY
Levels of cytokines in serum samples of lean and HFD-fed mice were determined using the mouse Luminex Bead Cytokine 20-plex Kit (Invitrogen) according to the manufacturer's protocol.
HEPATIC LYMPHOCYTE ISOLATION
Liver tissues were minced and placed in Roswell Park Memorial Institute medium with 10% fetal bovine serum. Cells were centrifuged at 300g for 5 minutes to discard hepatocytes. Then the pellet was resuspended in 40% Percoll, topped over 60% Percoll solution, and centrifuged at 800g × 20 minutes. Hepatocytes were in the surface layer, and lymphocytes were collected from the interface. The lymphocyte fraction was resuspended in Roswell Park Memorial Institute medium and centrifuged at 500g for 5 minutes. The single-cell suspension was checked for viability and stained for CD3, CD4, and CD8, and CD62L (L-selectin).
IMMUNOFLUORESCENCE
Frozen liver tissue sections from all groups were fixed, blocked, and incubated with primary antibody CD8, CD34, CD62L (Abcam, MA), and MECA-79 (Biolegend, CA). Then the tissues were probed with secondary antibodies and counterstained with nuclear stain 4′,6-diamidino-2-phenylindole. Images were taken using an Olympus FV1000 confocal laser-scanning microscope (Olympus America Inc.), and the generated data sets were analyzed using Imaris 8.4 (Bitplane) for three-dimensional colocalization and visual overlap at Emory Integrated Cellular imaging Core.
IMMUNOHISTOCHEMISTRY
Liver tissue sections were deparaffinized, and antigens were retrieved. They were exposed to H2O2 to quench endogenous peroxidase. Tissues were blocked and incubated with an anti-CD3 antibody, antirabbit secondary antibody, and then diaminobenzidine substrate. After counterstaining with hematoxylin, tissue sections were visualized under a light microscope.
FLOW CYTOMETRY
One million hepatic lymphocytes were washed and incubated with an Fc receptor blocker and then with antibodies against CD3, CD4, or CD8 at 4°C. A live/dead cell kit was used (Biolegend). Cells were compensated with ArC-reactive and ArC-negative beads (Molecular Probes) and analyzed on a Becton-Dickenson flow cytometer (LSR II) at the Emory Children's Pediatric Flow Cytometry Core.
REAL-TIME PCR
Total RNA was extracted from livers of lean mice and HFD-fed mice, with and without IRI. Liver tissues were homogenized and RNA was extracted using an RNAeasy Minikit (Qiagen). Complementary DNA was made using the high-capacity complementary DNA reverse transcription kit (Applied Biosystems). The sequences of the primers used for real-time PCR for P-selectin, E-selectin, and L-selectin are listed in Table 2.
| Primer Name | Sequence |
|---|---|
| L-selectin forward | TGTGGAGCATCTGGAAACTG |
| L-selectin reverse | AGGAATGAAGAGGGGGTTGT |
| P-selectin forward | TGCTGTCCATTGTCCTGAAG |
| P-selectin reverse | CTTTGGTCCGAACACCACTT |
| E-selectin forward | AGCTACCCATGGAACACGAC |
| E-selectin reverse | CGTTATCCCAGATGCCAGAT |
BLOCKADE OF L-SELECTIN
A functional grade purified antibody for antimouse CD62L (anti-L-selectin) was obtained from eBiosciences (clone MEL-14). Two doses of 100 μg each were given intravenously to lean and HFD-fed mice on day –1 and day 0 (day of IRI). Rat immunoglobulin G2a (IgG2a) isotype control was used in similar doses (eBiosciences).
CD8+ DEPLETION
Before IRI, mice fed the HFD were given three intraperitoneal injections (400 μg each) of CD8 antibody (clone 53-6.72, BioXcell) on day –2, day –1, and the day of IRI. A rat monoclonal antibody against IgG2a was used as a negative control.
STATISTICAL ANALYSES
Groups were compared using Student t test for two groups, one-way analysis of variance (ANOVA) for three or more groups, and two-way ANOVA for four or more groups (GraphPad Prism 6). A probability value of P < 0.05 was considered significant.
Results
HFD-FED MICE HAVE INCREASED HEPATOCELLULAR INJURY AND NUMBER OF INTRAHEPATIC CD3+ T CELLS AFTER IRI
Mice were fed an HFD diet for 12 weeks, and their weight gain measurements, liver fat accumulation, and liver triglyceride content are shown in Supporting Fig. S1A-D. Additionally, differential gene expression analysis by RNA sequencing of isolated primary lean and steatotic hepatocytes is shown in Supporting Fig. S1E. To further explore the immune response, we assessed the frequency of CD3+ cells in the liver after IRI by immunohistochemistry. Livers of lean and HFD mice showed no difference in numbers of CD3+ cells (Fig. 1A, top panel) at baseline. After IRI, CD3+ cells were significantly increased in livers of mice with steatosis (Fig. 1A, bottom panel). Quantification for CD3+ cells is shown in Fig. 1B (P < 0.0004).

To confirm our immunohistochemical findings, we did flow-cytometric analysis of total-liver lymphocytes. At baseline, lean and HFD-fed mice showed no difference in CD3+ cells (30.2 ± 1.1% versus 32.5 ± 1.5%; Fig. 1C, top panel, D). On the other hand, mice fed the HFD subjected to IRI had significantly more CD3+ cells in the liver (44.8 ± 2.4%) compared to lean mice (32.4 ± 0.1%, P < 0.0001; Fig. 1C, bottom panel, D), confirming immunohistochemical findings. Thus, IRI in fatty liver not only increases necrosis as shown in our earlier studies7, 8 but also leads to increased infiltration of CD3+ cells compared to the lean liver undergoing IRI.
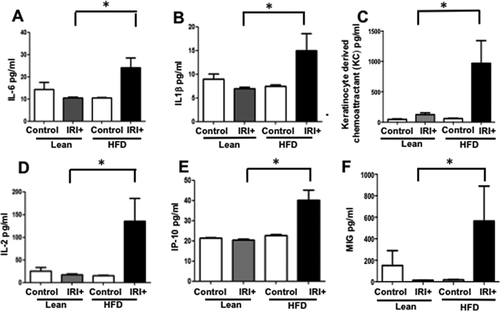
INCREASED SERUM LEVELS OF PROINFLAMMATORY CYTOKINES IN HFD-FED MICE AFTER IRI
We analyzed levels of proinflammatory cytokines in serum samples from lean and HFD-fed mice, with and without IRI. Serum from mice fed the HFD had significantly higher levels of proinflammatory cytokines compared to lean mice after IRI or mice on the HFD without IRI (Fig. 2). Levels of interleukin 6 (IL-6) were 10.5 ± 0.5 pg/mL in lean mice undergoing IRI and 24.1 ± 4.4 pg/mL in mice on the HFD undergoing IRI (P < 0.0004): IL-1β, lean IRI+ 7 ± 0.2 versus HFD IRI+ 15.0 ± 3.6 pg/mL (P < 0.005); keratinocyte-derived chemoattractant, lean IRI+ 123 ± 30 versus HFD IRI+ 970 ± 372 pg/mL (P < 0.0001); IL-2, lean IRI+ 18 ± 1.6 versus HFD/ IRI+ 135 ± 64 (P < 0.0001); interferon-gamma-induced protein 10, lean IRI+ 20 ± 0.5 versus HFD IRI+ 40 ± 4.9 pg/mL (P < 0.0009); monokine induced by interferon-gamma, lean IRI+ 14 ± 1.2 versus HFD IRI+ 564 ± 324.6 pg/mL (P < 0.0001). These findings indicate that imposing IRI on steatotic liver increases systemic production of proinflammatory cytokines.
LOSS OF CD8+, BUT NOT CD4+, CELLS PROTECTS AGAINST IRI IN HFD-FED MICE
Because the livers of HFD-fed mice contain more CD3+ cells than lean livers after IRI, we investigated the role of CD3+ subsets as potential mediators of increased liver injury. We compared liver injury after HFD and IRI in C57BL/6, CD4−/−, and CD8−/− mice and CD8 antibody–depleted mice.
Compared to WT mice, CD8−/− mice on HFD exposed to IRI exhibited significantly less necrosis as outlined by white lines in H&E images (Fig. 3A). Necrosis was further quantified by Aperio software as described in Materials and Methods. CD8−/− mice showed more intact nuclei compared to WT (136 versus 38 ± 9/field; P < 0.0001) along with a substantially decreased necrotic area (4.2 × 106 versus 3.5 × 107; P < 0.002). Calculated necrosis score was also significantly lower (11.6 versus 25.1 ± 3.9; P < 0.01). Serum ALT levels were significantly lower in CD8−/− mice (326 versus 986 IU/L; P < 0.04) corroborating the histological findings. There was no statistical difference in necrotic changes between WT and CD4−/− either histologically or biochemically. The body weight changes (Supporting Fig. S2) between CD4−/− and CD8−/− mice fed an HFD were similar. These results suggest that CD8+ cells, but not CD4+ cells, play a critical role in IRI-mediated liver cell injury in steatotic liver. To further corroborate the data from knockout mice, we depleted CD8+ cells using anti-CD8-specific antibody and determined whether such depletion conferred protection from IRI in steatotic liver. C57BL/6 WT mice were given either the anti-CD8-depleting antibody (under conditions that depleted >90% of CD8+ cells; Supporting Fig. S3) or the control IgG. Compared to mice receiving control IgG, mice receiving a CD8-depleting antibody exhibited decreased necrosis histologically (Fig. 3D), significantly more intact nuclei (83 ± 6 versus 41 ± 5; P < 0.0001), lesser necrotic area (2.8 × 106 versus 4.1 × 107; P < 0.001), lower calculated necrosis score (5.7 ± 1.4 versus 19.2 ± 3.1; P < 0.0002), and lower serum ALT (657 ± 103.8 versus 1,536 ± 294 IU/L; P < 0.05), corroborating the findings in CD8−/− mice.
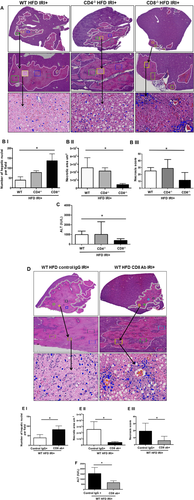
These findings clearly support the suggestion that CD8+ cells are important mediators of IRI-induced injury in a steatotic liver.
CD8+ CELLS MEDIATE IRI IN HFD-FED MICE THROUGH L-SELECTIN
If CD8+ cells mediate the damage in steatotic liver IRI, their trafficking to the liver is likely mediated by adhesion molecules, the genetic signature for which was up-regulated in steatotic hepatocytes (Fig. 1). Hence, we further investigated if the selectin family of adhesion molecules modulates this process. Analysis of selectin expression in the liver shows that levels of L-selectin mRNA were significantly increased in the liver of HFD-fed mice with IRI compared to lean mice with IRI (2.0 ± 0.2 in HFD mice with IRI versus 0.83 ± 0.1-fold increase in lean mice with IRI; P < 0.003) and compared to HFD liver without IRI (0.25 ± 0.1; P < 0.0003). In contrast, levels of P-selectin and E-selectin mRNAs were not significantly increased after IRI in HFD-fed mice (Fig. 4A). Sham-treated mice did not show any difference from controls.
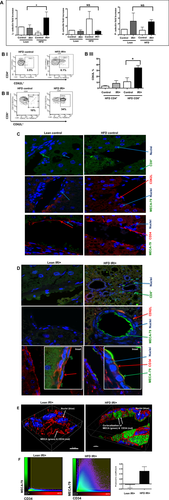
Flow-cytometric analysis of lymphocytes from livers of mice fed an HFD indicated that CD4+/CD62L+ cells (CD3 gated) were slightly increased with IRI (not significant), whereas CD8+/CD62L+ cells were significantly increased following IRI (Fig. 4B, P < 0.0002).
MECA-79 is a carbohydrate epitope found on a family of sialomucins known as “peripheral node addressins” that serve as ligands for L-selectin. Immunofluorescence analyses showed that livers of HFD-fed mice subjected to IRI had increased expression of MECA-79, which colocalized with endothelial cells (marker CD34). MECA-79 was not detected in livers of lean mice undergoing IRI or HFD-fed mice without IRI (Fig. 4C,D). Thus, HFD IRI mice showed endothelial cell transformation by expressing MECA-79. This was further confirmed by three-dimensional image analysis and quantification (Fig. 4E,F). These observations strongly suggest that the L-selectin adhesion molecule, with its ligand MECA-79, leads to the trafficking of CD8+ cells into the liver, which contributes to the exacerbated liver injury seen in steatotic mice undergoing hepatic IRI.
In order to further evaluate this observation, we blocked L-selectin and assessed if this would independently lead to amelioration of hepatocellular injury in HFD-fed mice after IRI.
BLOCKING L-SELECTIN REDUCES HEPATOCELLULAR INJURY IN HFD-FED MICE AFTER IRI
Mice were given L-selectin blocking antibody, and blockade was confirmed by flow cytometry (Supporting Fig. S4). Livers from HFD-fed mice given the L-selectin blocking antibody demonstrated less necrosis after IRI compared with HFD-fed mice given the control antibody, as outlined by white lines in H&E images (Fig. 5A). L-selectin antibody–treated mice showed more intact nuclei compared to control IgG (99 ± 7 versus 37 ± 5/field; P < 0.0001) along with a substantially decreased necrotic area (2.5 × 106 versus 2.7 × 107; P < 0.005). The calculated necrosis score was also significantly lower (5.2 ± 2.0 versus 19.2 ± 3.1, P < 0.002). Serum ALT levels were significantly lower in L-selectin antibody–treated mice (289.9 ± 79.8 versus 986.5 ± 87.4 IU/L; P < 0.004), corroborating the histological findings. The decreased liver injury was also confirmed by determining the number of PI-positive cells in the liver, with a remarkable reduction in PI-positive cells in HFD-fed mice treated with L-selectin antibody compared to IgG-treated HFD control mice (20.2 ± 0.2 versus 48.6 ± 4.6 pixels, P < 0.003; Fig. 5D).
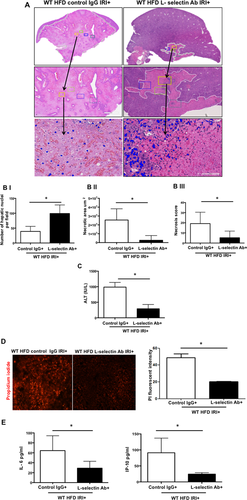
Levels of proinflammatory cytokines in serum were also decreased after L-selectin blockade. Mice fed the HFD and given L-selectin antibody with IRI had decreased levels of IL-6 (29.0 ± 6.2 pg/mL) compared to mice fed the HFD but given control IgG (64.4 ± 13.3 pg/mL; P < 0.04). Similarly, serum levels of interferon-gamma-induced protein 10 in mice on the HFD given L-selectin antibody with IRI were low (24.4 ± 2.2 pg/mL) compared to mice given a control IgG (91.0 ± 2.4 pg/mL; P < 0.03; Fig. 5E). Thus, blockade of the L-selectin adhesion molecule led to decreased hepatocellular injury and lower levels of proinflammatory cytokines in HFD-fed mice undergoing IRI.
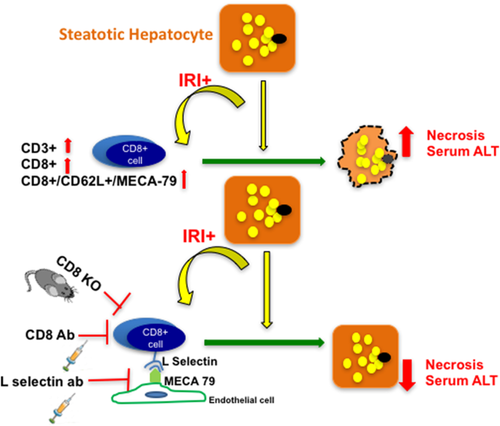
Figure 6 is a schematic representation of the possible mechanism by which CD8+ cells, guided by L-selectin/MECA-79-mediated trafficking, lead to hepatocellular injury in HFD-fed mice undergoing IRI.
Discussion
With increasing incidence of NAFLD, it is now being recognized that a steatotic liver when exposed to injury has a worse clinical outcome.25-34 Dynamics of microcirculation35-38 and ischemic injury39 differ in steatotic liver compared to lean liver, implying that our current understanding of host response to injury in a “lean or normal” state does not necessarily apply fully to a steatotic liver. In this study, we show that CD8+ cells are essential for the increased liver damage that occurs in steatotic liver during IRI compared with lean liver. We observed that CD8+ cells are recruited to the liver by L-selectin, a receptor on the T cells that binds to the ligand MECA-79, seen on endothelial cells of the fatty liver but not in lean liver after IRI. Depletion of CD8+ cells or knockout of CD8 in mice protects the liver from the combined damage of steatosis and IRI, reducing necrosis and serum levels of ALT. We also observed similar protective effects by treating the mice with L-selectin blocking antibody. These novel findings demonstrate the role of CD8+ cells as critical regulators of liver damage in a steatotic liver undergoing IRI versus the current understanding of CD4+ cells as being the main drivers of injury based on data from lean settings.
Lymphocytes in general and T lymphocytes in particular have been implicated in IRI of several organs. It is well known that lymphocytes play more than a bystander role. Thus far, CD4+ cells have been considered to be the key mediators of IRI. Several studies have shown that in IRI of the lung,40 brain,41 myocardium,42 intestine,43 and kidney,44 deficiency of CD4+ cells leads to protection from injury. CD4+ cells have also been similarly implicated in hepatic IRI.9, 45 Zwacka et al. demonstrated the pathogenicity of CD4+ cells by subjecting athymic (nu/nu) mice to hepatic IRI,10 and Khandoga et al. showed improvement in hepatic perfusion in CD4-deficient mice after IRI.46 It is important to note that both these studies were conducted in normal or “lean” mice and not in HFD-fed mice as in our study, which is a different functional and activation state. This once again underlines the importance of differential cell types and mechanisms which are operational in a steatotic liver IRI.
The role of CD8+ cells has not been as well studied in IRI as that of CD4+ cells. Many studies have been done in Rag1−/− or T cell–deficient mice,41, 47 which have combined T-cell deficiencies, making the exact contribution of the CD4 and CD8 compartments difficult to assess. In a study by Yang et al., CD4−/− mice had a significantly decreased infarct size compared to control mice, implicating CD4+ cells in the injury. Interestingly, they also showed that CD8 deficiency was not protective and did not change the infarct size.42 Similarly, Burne et al. also showed an insignificant role of CD8+ cells in renal IRI.44 Hence, our study proposes CD8+ cells as new players in IRI of the steatotic liver, though thus far they have not been considered key mediators of IRI.
With the implication of CD8+ cells in steatotic liver IRI, we wanted to investigate the underlying mechanism of recruitment of CD8+ cells into the liver. Adhesion molecules, particularly the selectin family, are involved in homing and recruitment of lymphocytes in IRI.13, 48 They have also been implicated as mediators of injury in several disease states.12, 40, 44, 46 Studies have shown that blockade of L-selectin attenuates injury after IRI in the kidney21 and the heart.23 Martinez-Mier et al. showed that anti-L-selectin treatment improved liver function and decreased chemokine response.22 This study, however, alluded to the role of L-selectin in recruiting neutrophils and not CD8+ cells as in our study. Importantly, this study was conducted in lean mice and not in an HFD-fed steatotic mouse model.
The findings of our study provide insight into the mechanisms by which steatosis increases liver damage from IRI. These observations are important in light of the increasing incidence of fatty liver disease, which is making a greater proportion of livers unavailable for transplantation. We show in mice that fatty liver is more highly damaged by ischemia reperfusion than lean liver, and we identify CD8+ cells as an important cell extrinsic component mediating this damage. Blocking of CD8+ cells, whether by preventing trafficking or by depleting, protects against liver injury. These findings might be applied to make steatotic livers from patients who suffer from NAFLD available for transplantation, which would be an important advance due to the increasing shortage of lean donor livers. Decreasing the effects of IRI in a steatotic donor liver not only might make it usable49 but also could improve outcomes of patients who receive marginal-steatotic livers.33 Furthermore, a large proportion of patients undergoing hepatectomies, myocardial infarction, and shock have fatty livers, which respond poorly with delayed recovery.25, 28, 30, 32 Our findings show that these patients are likely to have more severe liver damage than patients with lean livers and provide strategies for reducing this damage. Though these results need to be verified in different strains of mice and nonalcoholic steatohepatitis models, we propose that strategies to block L-selectin-mediated homing of CD8+ cells to the fatty liver might reduce the damage caused by ischemia and reperfusion.
Another exciting area of opportunity is the demonstration of acquisition of MECA-79-positive endothelial cells of the liver sinusoidal cells. This needs to be further investigated because these cells might have a role in immune-mediated liver disease and provide new strategies for therapeutic intervention.
Acknowledgment
We thank Dr. Neil Anthony for his help with three-dimensional image analysis, Dr. Suresh Venkateswaran for generation of heat maps, and Dr. Ezio Laconi and Dr. Sarma Dittakavi for their guidance in primary hepatocyte isolation.
REFERENCES
Author names in bold designate shared co-first authorship.




