Stratifying risk in the prevention of recurrent variceal hemorrhage: Results of an individual patient meta-analysis
Potential conflict of interest: Dr. Bosch consults, advises, and received grants from Conatus. He consults and advises Actelion and Exalenz. He consults and received grants from Gilead. He consults for Novo. Dr. Garcia-Pagan is on the speakers' bureau for Gore. He received grants from Novartis and Exalenz.
Supported by the Spanish Ministry of Health, Instituto de Salud Carlos III–Spain (Plan Estatal de I+D+I 2013-2016) (PI14/00876, PI051871, to A.A. and CIBEREHD; PI13/00896, to J.V. and CIBERESP), cofinanced by the European Development Regional Fund “A Way to Achieve Europe” (ERDF), and by the National Institutes of Health (P30 DK34989, to G.G.-T.).
Abstract
Endoscopic variceal ligation plus beta-blockers (EVL+BB) is currently recommended for variceal rebleeding prophylaxis, a recommendation that extends to all patients with cirrhosis with previous variceal bleeding irrespective of prognostic stage. Individualizing patient care is relevant, and in published studies on variceal rebleeding prophylaxis, there is a lack of information regarding response to therapy by prognostic stage. This study aimed at comparing EVL plus BB with monotherapy (EVL or BB) on all-source rebleeding and mortality in patients with cirrhosis and previous variceal bleeding stratified by cirrhosis severity (Child A versus B/C) by means of individual time-to-event patient data meta-analysis from randomized controlled trials. The study used individual data on 389 patients from three trials comparing EVL plus BB versus BB and 416 patients from four trials comparing EVL plus BB versus EVL. Compared with BB alone, EVL plus BB reduced overall rebleeding in Child A (incidence rate ratio 0.40; 95% confidence interval, 0.18-0.89; P = 0.025) but not in Child B/C, without differences in mortality. The effect of EVL on rebleeding was different according to Child (P for interaction <0.001). Conversely, compared with EVL, EVL plus BB reduced rebleeding in both Child A and B/C, with a significant reduction in mortality in Child B/C (incidence rate ratio 0.46; 95% confidence interval, 0.25-0.85; P = 0.013). Conclusion: Outcomes of therapies to prevent variceal rebleeding differ depending on cirrhosis severity: in patients with preserved liver function (Child A), combination therapy is recommended because it is more effective in preventing rebleeding, without modifying survival, while in patients with advanced liver failure (Child B/C), EVL alone carries an increased risk of rebleeding and death compared with combination therapy, underlining that BB is the key element of combination therapy. (Hepatology 2017;66:1219-1231).
Abbreviations
-
- BB
-
- beta-blocker
-
- CI
-
- confidence interval
-
- EVL
-
- endoscopic variceal ligation
-
- IPD
-
- individual patient data
-
- IRR
-
- incidence rate ratio
-
- ISMN
-
- isosorbide-5-mononitrate
-
- MIRR
-
- median IRR
Preventing recurrent variceal hemorrhage in patients with cirrhosis who have recovered from an episode of acute variceal hemorrhage is essential because otherwise the 1-year rebleeding and mortality rates are unacceptably high, at 60% and 30%, respectively.1 The current standard of care for the prevention of rebleeding is the combination of endoscopic variceal ligation (EVL) and nonselective beta-blockers (BB).2, 3 This recommendation is supported by several meta-analyses that have pooled trials comparing combination therapy versus monotherapy (BB or EVL alone).4-6 These meta-analyses uniformly concluded that combination therapy had greater efficacy in preventing recurrent variceal hemorrhage than either therapy alone, but this benefit did not translate into an improved survival.
However, because studies included in these meta-analyses did not report results by prognostic stage, this recommendation is extended to all patients with cirrhosis with a previous variceal bleeding, irrespective of cirrhosis stage. Cirrhosis is classified in two main prognostic stages: compensated and decompensated,7, 8 and the Child-Turcotte-Pugh (Child) classification is excellent at stratifying patients with cirrhosis,9 with Child A patients being mostly compensated and Child B/C patients being decompensated. Additionally, obtaining individual patient data (IPD) allows the performance of survival analysis as a time-dependent variable, which was not possible in published aggregate data meta-analyses on this topic.
Therefore, we performed a meta-analysis using IPD obtained from principal investigators of randomized controlled trials that compared combination therapy (EVL plus BB) versus monotherapy (EVL or BB) with the objective of comparing these strategies with regard to time-dependent rebleeding and mortality based on the stage of cirrhosis at the time of variceal hemorrhage using the Child-Turcotte-Pugh classification.
We hypothesized that the impact of each therapy, EVL or BB, on outcomes (rebleeding, mortality) would differ according to the severity of cirrhosis. This would be clinically relevant as risk stratification would allow tailoring therapy in accordance with patient characteristics. In the specific case of BB, it could provide information regarding their effect on outcomes in the two main stages of cirrhosis.
Materials and Methods
The current meta-analysis was designed to pool the data of individual patients participating in randomized controlled trials that compared the efficacy of combination therapy (EVL plus BB) versus either therapy alone (EVL or BB) in preventing recurrent variceal hemorrhage vis-à-vis the two main prognostic stages of cirrhosis. We searched and identified the trials according to previously defined outcomes, revised the data, and planned statistical analysis. To report the present review, we have adhered to the PRISMA-IPD guidelines for reporting meta-analysis of IPD.10
ELIGIBILITY CRITERIA
Studies that included patients with cirrhosis undergoing prevention of recurrent esophageal variceal hemorrhage were included in this analysis if they were published as full articles, if patients were randomly allocated to receive the combination of EVL plus BB versus either EVL or BB alone, if information regarding overall rebleeding and death was available, and if the original data sets of the trials were available.
IDENTIFYING STUDIES, INFORMATION SOURCES, AND SEARCH
We performed a literature search up to December 30, 2015, in Medline, Embase, the Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews, using the terms “rebleeding,” “recurrent hemorrhage,” “esophageal varices,” “variceal obliteration,” “beta-blockers,” “endoscopy,” “band ligation,” and “cirrhosis.” We also reviewed publications in personal reference lists and citation sections of the recovered articles; manually searched abstracts presented at meetings of the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the European Association for the Study of the Liver, the American Society of Gastrointestinal Endoscopy, and the British Society of Gastroenterology; and looked at authors of the abstracts to identify whether these abstracts had been published in full. Finally, we also searched clinical trials at www.controlled-trials.com and www.clinicaltrials.gov.
STUDY SELECTION AND DATA-COLLECTION PROCESSES
Two reviewers independently selected the studies by screening the titles and abstracts to identify those that fulfilled the inclusion criteria (J.M., A.A.). Principal investigators of the trials that met eligibility criteria were contacted to obtain individual patient data for all participants in their trials.
The original data sets were checked for completeness and internal consistency and amended through correspondence with the principal investigators. To build the final data set of IPD, we excluded studies with insufficient information and those that could not be obtained upon request.
DATA ITEMS
Patient-level data were analyzed by two authors (J.M. and D.A.). The following data were extracted from the data sets: patient characteristics (age, sex, etiology of cirrhosis, serum bilirubin, serum albumin, prothrombin time, Child-Turcotte-Pugh [Child] class),11 presence of ascites or encephalopathy, outcomes (all-source rebleeding, variceal rebleeding, and mortality), and follow-up (time-to-event for each outcome). Careful initial evaluation was performed to ensure completeness of data and to check consistency of the results of the primary analyses for each trial with published reports.
OUTCOMES
Primary endpoints were all-source rebleeding and mortality, and the secondary endpoint was variceal rebleeding. As described by the principal investigators of the included trials, all-source and variceal rebleeding were defined as the presence of melena or hematemesis with an upper gastrointestinal or an esophageal variceal source, respectively, identified by emergency endoscopy after randomization. All-source rebleeding was chosen as a primary outcome because EVL may reduce the risk for variceal hemorrhage but can also cause bleeding from esophageal ulcers, leaving the number of bleeding events per patient unchanged. Mortality was defined as death from any cause. The rate of adverse events was also explored.
STATISTICAL ANALYSIS
The main analysis was a one-stage IPD meta-analysis comparing time-to-event data. This uses the raw data from each study and allows for adjustments for confounders and tests for interactions. Because traditional meta-analysis models are not suited to analyze individual time-to-event patient data, a multilevel Poisson mixed-effects model with random intercepts was used to model time-to-event data that are clustered within studies. In the multilevel Poisson mixed-effects model, the effect of interventions is estimated as a hazard ratio approximated by incidence rate ratios (IRRs). This approach models survival with a fixed treatment effect and a different baseline hazard function for each study. So, the assumption of proportional hazards for all studies is relaxed, while still assuming proportional hazards between treatment groups within each study.12 The effect of intervention was stratified by the severity of cirrhosis, as assessed by Child class (A versus B/C). We specifically checked for the modifier effect of Child on the interventions by including interaction terms in the models. A significant interaction would indicate that the treatment effect was different between Child A and Child B/C patients.
Within each stratum, the model was further adjusted by including the following confounders: etiology (alcohol versus viral versus others), ascites (presence versus absence), encephalopathy (presence versus absence), and the continuous variables age, bilirubin, and albumin. Prothrombin time was not included because of the variability in expressing the result of this variable among the trials (prothrombin time in some trials, prothrombin activity in others). Inclusion of confounders allows fine-tuning of the model within each stratum compared with the coarse adjustment using Child as the sole category. A covariate was considered a confounder factor if the difference between adjusted and unadjusted model coefficients for the intervention variables varied >10%. In such a case, the IRR was shown along with the list of confounders used for adjustment. Otherwise, we reported the model without adjustment for confounders. Heterogeneity among trials was measured with the random effect parameters of the model, transformed into median IRRs (MIRRs). The MIRR shows the extent to which the probability of the event is determined by the trial in which the patients were recruited. The closer the MIRR is to 1, the lower the variability of the individual risk of an event among trials.13
We performed two meta-analyses: the first explored trials that compared combination therapy versus BB alone (control group), while the second explored trials that compared combination therapy versus EVL alone (control group).
We carried out a sensitivity analysis to verify the robustness of our results on all-source and variceal rebleeding. First, we considered death as a competing event for rebleeding, and we performed a competing risk analysis including study as a covariate. Second, we tested, using logistic multilevel regression, the impact of including all the patients into the analysis of rebleeding, even those patients excluded from the main analysis because they lacked information on time-to-event. Finally, we performed a post hoc exploratory analysis of the effect of the association of BB with isosorbide-5-mononitrate (ISMN) in those four trials that compared pharmacological therapy and EVL versus EVL alone, two of which used BB alone as pharmacological therapy, while the other two used BB with ISMN.
All analyses were done with Stata v13 (Stata Statistical Software, Release 13; StataCorp LP, College Station, TX) and the “xtmepoisson” command.
Results
STUDY SELECTION
Our search strategy identified 798 potential references. We excluded 792 references: 771 because they were clearly ineligible by reading the title and the abstract and 17 because of duplicity (Fig. 1). Four additional studies were also excluded: two of them because they were in abstract form only and data could not be obtained from the authors14, 15; another because its primary endpoint was the incidence and natural history of portal hypertensive gastropathy in patients with previous variceal bleeding treated with EVL or BB16; and the last one because it used the hepatic venous pressure gradient response to BB to allocate patients to combination therapy or BB alone, instead of randomly allocating consecutive patients.17 This left six studies for inclusion in the meta-analysis.
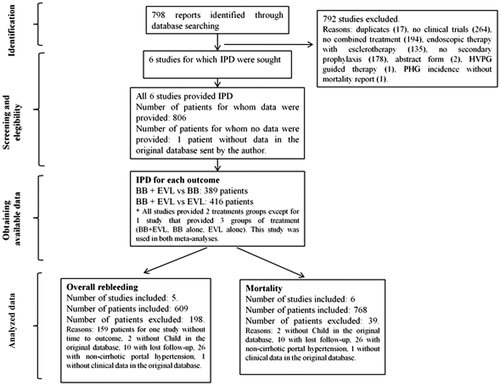
STUDY CHARACTERISTICS
Table 1 shows the general characteristics of the six studies that met inclusion criteria. All the trials, except the one by Ahmad et al.,18 had two study groups that compared the combination of EVL and BB versus BB (three trials) or versus EVL (four trials). The trial by Ahmad et al. compared four groups, BB plus ISMN combined with EVL, BB alone, BB plus ISMN, and EVL alone. The specific BB used was propranolol in two trials18, 19 and nadolol in four trials,20-23 at a dose adjusted to reduce resting heart rate by 25% or to 55 bpm18, 20-22 or to the maximum tolerated dose without reducing heart rate below 55 bpm.19, 23 In all studies, BB were started within 5 days of the index bleed. BB were associated with ISMN (20 mg twice daily) in the three trials that compared combination therapies with BB alone18, 22, 23 and in two of the four studies that compared combination therapy with EVL alone.18, 19 In one of the other two trials, BB were administered together with sucralfate.22 EVL sessions were performed with multiband devices in all the trials and repeated at intervals that varied between 10 days and 4 weeks. The mean follow-up of the trials varied from 14 to 23 months.
| Reference | No. of Patients | Additional Inclusion Criteria | Exclusion Criteriaa | Trial Design | Included Patients | Treatment Groups | Definition of Rebleeding | Mean Sessions to Variceal Obliteration | Time to Start BB (Days) | Drug Therapy Mean Dose (Range or SD) | Follow-up. Median or Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EVL and BB versus BB | |||||||||||
| Ahmad et al.18 | 160 | First episode of bleeding |
Acute-on-chronic liver failure |
Single center, no blinding |
111 |
EVL+BB: 37 BB: 74 |
Hematemesis/melena with >2 blood units transfusion or drop in hemoglobin >2 g/dL | 3.0 | 5 |
EVL+BB: Propranolol 50 ± 21 mg/day ISMN 33 ± 10 mg/day BB: Propranolol 53 ± 21 mg/day ISMN 35 ± 9 mg/day |
24 months EVL+BB: 24 BB: 24 |
| Lo et al.22 | 120 | No |
Bilirubin >10 mg/dL, hepatic encephalopathy >grade 2 |
Single center, endoscopy- blinded |
120 |
EVL+BB: 60 BB: 60 |
Hematemesis/melena with >2 blood units transfusion or drop in hemoglobin >2 g/dL | 3.8 | 2 |
Both groups: nadolol 40 mg/day (20-120) ISMN 20 mg/day (0-40) |
23 months EVL+BB: 23 BB: 22.7 |
| García-Pagán et al.23 | 158 | No |
Multicenter, no blinding |
158 |
EVL+BB: 80 BB: 78 |
Hematemesis/melena with >2 blood units transfusion | 2 | 5 |
EVL+BB: nadolol 102 ± 52 mg/day ISMN 36 ± 9 mg/day BB: nadolol 90 ± 48 mg/day ISMN 36 ± 10 mg/day |
15 months EVL+BB: 14 BB: 15.3 | |
| EVL and BB versus EVL | |||||||||||
| Ahmad et al.18 | 160 | First episode of bleeding |
Acute-on-chronic liver failure |
Single center, no blinding |
76 |
EVL+BB: 37 EVL: 39 |
Hematemesis/melena with >2 blood units transfusion or drop in hemoglobin >2 g/dL |
EVL+BB: 3.0 EVL: 3.5 |
5 |
Propranolol 53 ± 21 mg/day ISMN 35 ± 9 mg/day |
23 months EVL+BB: 24 EVL: 21 |
| Kumar et al.19 | 177 | NCPH, wide interval from bleed to inclusion |
Single center, no blinding |
177 |
EVL+BB: 88 EVL: 89 |
Hematemesis/melena with >2 blood units transfusion |
EVL+BB: 4.6 EVL: 4.6 |
5 | Propranolol 120 mg/day ISMN 40 mg/day |
EVL+BB: 15 EVL: 15 |
|
| De la Peña et al.20 | 80 | No | Refractory ascites |
Multicenter, no blinding |
80 |
EVL+BB: 43 EVL: 37 |
Hematemesis/melena with fall in hemoglobin |
EVL+BB: 3 EVL:3 |
5 | Nadolol 58 mg/day |
16.4 months EVL+BB: 17 EVL: 15 |
| Lo et al.22 | 122 | No |
Hepatic encephalopathy >grade 2 |
Single center, no blinding | 122 |
EVL+BB: 60 EVL: 62 |
Hematemesis/melena with >2 blood units transfusion or drop in hemoglobin >2 g/dL | EVL+BB: 3.3 EVL: 3.6 | 2 |
Nadolol (60 mg/day) + sucralfate |
21.5 months EVL+BB: 22 EVL: 21 |
- a Exclusion criteria common to all studies were previous therapy with endoscopy or BB, bleeding from gastric varices, presence of hepatocellular carcinoma, portal hypertensive gastropathy bleeding, debilitating disease, transjugular intrahepatic portosystemic shunt, pregnancy.
- Abbreviations: NCPH, noncirrhotic portal hypertension; SD, standard deviation.
The Child score was used to assess the severity of cirrhosis in all trials, and the publication by Pugh et al.11 was only referenced in the trial by Lo et al.21 In all trials, rebleeding was considered as such if significant (Table 1). Significant rebleeding was defined in all studies, except the one by De la Peña et al.,20 by the need to transfuse more than two blood units or by a drop in hemoglobin >2 g/dL. De la Peña et al. required a drop in hemoglobin to consider rebleeding, but they did not describe the amount.20
Supporting Table S1 shows the methodological quality indices of the included trials.24 All trials had an adequate generation of allocation sequence and adequate allocation concealment. The baseline characteristics of treatment and control groups were balanced in all trials. No trial had blinded investigators, and only the trial by Kumar et al. had a blinded endoscopist.19 The reasons for withdrawal were clear in all trials.
IPD OBTAINED
The final IPD data set was built after excluding patients with insufficient information in the original data sets and those for whom data could not be obtained upon request to the principal investigators. Supporting Table S2 shows the reasons for and the number of patients excluded from the original data sets: 2 lacked Child class,21 1 lacked any clinical information,20 26 had noncirrhotic etiology of portal hypertension,19 and 10 were lost to follow-up immediately after randomization and discharge.19 In addition, 150 patients from the study of Ahmad et al.18 and 9 patients from the study of Lo et al.21 were excluded from the main analysis because they lacked information on time-to-event but were included in the analysis of rebleeding using logistic multilevel regression models. The data set from Lo et al.'s study lacked information regarding the source of rebleeding; thus, 120 patients from this study were not included in the analysis of variceal rebleeding.22 All patients included in the final data set had information regarding death. Because the studies lacked a uniform standard definition of adverse events and the information reported in the databases was insufficient, we could not perform a safety analysis.
PATIENT CHARACTERISTICS
Characteristics of patients allocated to each treatment group in the six included trials are shown in Supporting Table S3. The final IPD base was built with 768 patients: 350 patients allocated to combination of EVL and BB, 206 to EVL alone, and 212 to BB alone. Patient characteristics were similar between the combination therapy arms of both sets of trials and between the combination and the monotherapy arms of each set of trials (Table 2).
| EVL and BB versus BB | EVL and BB versus EVL | |||
|---|---|---|---|---|
|
EVL+BB (n = 177) |
BB (n = 212) |
EVL+BB (n = 210) |
EVL (n = 206) |
|
| Age (years) | 53.6 ± 0.9 | 54.1 ± 0.7 | 51.1 ± 0.8 | 50.7 ± 0.9 |
| Sex (male/female) (%) | 66/34 | 68/32 | 72/28 | 79/21 |
| Etiology (%) | ||||
| Alcohol | 45 | 41 | 49 | 44 |
| Virus | 47 | 53 | 39 | 40 |
| Others | 8 | 6 | 12 | 16 |
| Bilirubin (md/dL) | 2.3 ± 0.2 | 2.2 ± 0.1 | 2.4 ± 0.1 | 2.5 ± 0.2 |
| Albumin (g/dL) | 3.0 ± 0.2 | 3.1 ± 0.1 | 3.0 ± 0.2 | 3.0 ± 0.4 |
| Prothrombin ratio (%) | 69 ± 14 | 68 ± 14 | 69 ± 15 | 67 ± 19 |
| Child-Pugh (%) | ||||
| A | 21 | 20 | 25 | 24 |
| B/C | 79 | 80 | 74 | 76 |
- Data are shown as mean ± standard deviation or as percentage.
PRIMARY OUTCOMES IN TRIALS COMPARING COMBINATION THERAPY (EVL PLUS BB) VERSUS BB ALONE
All-Source Rebleeding
We used 278 patients from two trials to assess overall rebleeding (Fig. 2; Supporting Table S2).22, 23 Fifty-four out of 140 (39%) patients rebled in the combination therapy group, and 58 out of 138 (42%) patients did in the BB group. The IRR (adjusted for etiology, bilirubin, and encephalopathy) for overall rebleeding was 1.00 (95% confidence interval [CI], 0.68-1.47; P = 0.996); that is, there were no differences in rebleeding between treatment groups in the overall group.
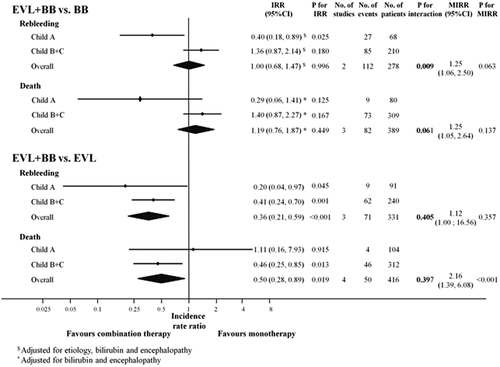
Sixty-eight of the 278 patients were Child A, and 27 of them rebled (40%), 9 on combination therapy and 18 on BB. Eighty-five out of 210 Child B/C patients rebled (40%), 45 on combination therapy and 40 on BB. The combination of EVL plus BB was more effective than BB alone in preventing overall rebleeding in Child A patients (IRR, 0.40; 95% CI, 0.18-0.89; P = 0.025) but not in Child B/C patients (IRR, 1.36; 95% CI, 0.87-2.14; P = 0.180) (adjusted for etiology, bilirubin, and encephalopathy) (Fig. 2). This interaction (i.e., difference in response to therapy by Child strata) was statistically significant (P = 0.009). There was no statistically significant heterogeneity among trials (MIRR, 1.25; 95% CI, 1.06-2.50; P = 0.063).
Death
A total of 389 patients from three trials were used to assess mortality (Fig. 2; Supporting Table S2).18, 22, 23 Overall, 39 out of 177 (22%) patients on combination therapy died compared to 43 out of 212 (20%) patients on BB. Mortality was similar in both study groups (IRR [adjusted for bilirubin and encephalopathy], 1.19; 95% CI, 0.76-1.87; P = 0.449).
Nine of the 80 Child A patients (11%) died, 2 on combination therapy and 7 on BB, whereas 73 of 309 Child B/C patients died (24%), 37 on combination therapy and 36 on BB. The combination of EVL and BB was associated with a lower mortality compared to BB alone in Child A patients, although this effect was not statistically significant (IRR, 0.29; 95% CI, 0.06-1.41; P = 0.125). Mortality was greater in Child B/C patients on combination therapy than in those on BB, but again the difference was not statistically significant (IRR, 1.40; 95% CI, 0.87-2.27; P = 0.167) (Fig. 2). Interaction (i.e., difference in response to therapy by Child class) was not statistically significant (P = 0.061). There was no statistically significant heterogeneity between trials (MIRR, 1.25; 95% CI, 1.05-2.64; P = 0.137).
PRIMARY OUTCOMES IN TRIALS COMPARING COMBINATION THERAPY (EVL PLUS BB) VERSUS EVL ALONE
All-Source Rebleeding
A total of 331 patients from three trials were used to assess overall rebleeding (that is, rebleeding from any source) (Fig. 2 and Supporting Table S2).19-21 Twenty-one of 169 (12%) patients rebled in the combination therapy group compared to 50 of 162 (31%) patients in the EVL group. The IRR for overall rebleeding was significantly lower in patients in the combination therapy group (IRR, 0.36; 95% CI, 0.21-0.59, P < 0.001).
Ninety-one of the 331 patients were Child A, and 9 of them rebled (10%), 2 on combination therapy and 7 on EVL. Sixty-two of 240 Child B/C patients rebled (26%), 19 on combination therapy and 43 on EVL. The combination of EVL plus BB was more effective than EVL alone in preventing overall rebleeding both in Child A patients (IRR, 0.20; 95% CI, 0.04-0.97; P = 0.045) and in Child B/C patients (IRR, 0.41; 95% CI, 0.24-0.70; P = 0.001) (Fig. 2). The effect of treatment was similar in both strata (P value for the interaction of 0.405). There was no significant heterogeneity (MIRR, 1.12; 95% CI, 1.00-16.56; P = 0.357).
Death
A total of 416 patients from four trials contributed to the analysis of mortality (Fig. 2; Supporting Table S2).18-21 Seventeen out of the 210 (8%) patients on combination therapy died compared to 33 out of the 206 (16%) patients on EVL, the IRR for mortality being 0.50 (95% CI, 0.28-0.89; P = 0.019). Heterogeneity among trials was present for this analysis (MIRR, 2.16; P < 0.001).
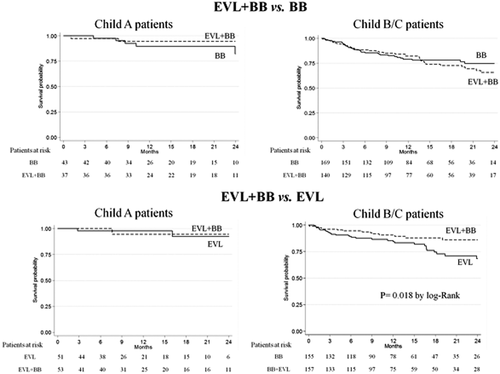
Four of the 104 Child A patients (4%) died, 2 on combination therapy and 2 on EVL, whereas 46 out of 312 Child B/C patients died (15%), 15 on combination therapy and 31 on EVL. Therefore, the combination of BB and EVL did not significantly influence mortality in the low-risk Child A patients (IRR, 1.11; 95% CI, 0.16-7.93; P = 0.915). However, in Child B/C patients combination therapy did result in a significantly lower mortality compared to treatment with EVL alone (IRR, 0.46; 95% CI, 0.25-0.85; P = 0.013). Figure 3 shows that the cumulative probability of survival was significantly (P < 0.01) higher in Child B/C patients on combination therapy but not in Child A patients.
SECONDARY OUTCOME
Rebleeding from a variceal source was assessed in 278 patients from the trials that compared combination therapy versus BB alone.22, 23 Forty-one of 140 patients (29%) rebled from varices in the combination therapy group versus 51 of 138 patients in the BB group (37%). The overall IRR (adjusted for bilirubin and encephalopathy) for variceal rebleeding was 0.81 (95% CI, 0.53-1.23; P = 0.320). When stratified by Child, 68 of the 278 patients were Child A, and 25 of them rebled (37%), 7 on combination therapy and 18 on BB therapy. Sixty-seven of 210 Child B/C patients rebled (32%), 34 on combination therapy and 33 on BB. Combination therapy (BB plus EVL) was more effective than BB alone in preventing variceal rebleeding in Child A patients (IRR, 0.26; 95% CI, 0.11-0.61; P = 0.002) but not in Child B/C patients (IRR, 1.24; 95% CI, 0.75-2.05; P = 0.410), showing a significant interaction (P = 0.002). No heterogeneity was detected among trials (MIRR, 1.23; 95% CI, 1.05-2.70; P = 0.109).
Variceal rebleeding was assessed in 220 patients from the trials that compared combination therapy versus EVL alone.19, 20 Twelve of 115 (10%) patients rebled from varices in the combination therapy group versus 18 of 105 (17%) patients in the EVL group. The overall IRR for variceal rebleeding was 0.52 (95% CI, 0.25-1.11; P = 0.091). When stratified by Child, 71 of the 220 patients were Child A, and 4 of them rebled (6%), 1 on combination therapy (25%) and 3 on EVL therapy (75%). Twenty-six of 149 Child B/C patients rebled (17%), 11 on combination therapy and 15 on EVL. Therefore, the combination of BB and EVL was not more effective than EVL in preventing variceal rebleeding either in Child A patients (IRR, 0.22; 95% CI, 0.02-2.13; P = 0.191) or in Child B/C patients (IRR, 0.63; 95% CI, 0.28-1.41; P = 0.264). There was no significant heterogeneity in this analysis (P = 0.497).
SENSITIVITY ANALYSIS
We performed two sensitivity analyses for overall and variceal rebleeding, one considering death as a competing event and the other including all the patients in the analysis, even those without information on time-to-event.
Competing risk regression confirmed the estimates of the main analysis (Table 3).
|
Competing Risk Regression Analysis |
Multilevel Logistic Regression Analysis | ||||
|---|---|---|---|---|---|
| SHR | Pa | OR | Pa | ||
|
EVL and BB versus BB |
|||||
|
All-source rebleeding |
Child A | 0.46 (0.21-1.04) | 0.024 | 0.38 (0.14-1.00) | 0.067 |
| Child B/C | 1.35 (0.86-2.12) | 1.05 (0.64-1.72) | |||
| Variceal rebleeding | Child A | 0.32 (0.14-0.76) | 0.009 | 0.27 (0.09-0.76) | 0.043 |
| Child B/C | 1.23 (0.73-2.06) | 0.88 (0.53-1.47) | |||
|
EVL and BB versus EVL |
|||||
|
All-source rebleeding |
Child A | 0.21 (0.04; 0.97) | 0.444 | 0.21 (0.04; 1.03) | 0.206 |
| Child B/C | 0.39 (0.23; 0.68) | 0.62 (0.36; 1.07) | |||
| Variceal rebleeding | Child A | 0.23 (0.02; 2.17) | 0.429 | 0.43 (0.04; 4.98) | 0.579 |
| Child B/C | 0.59 (0.26; 1.33) | 0.89 (0.41; 1.89) | |||
- a P value for the interaction between treatment effect and Child-Pugh groups.
- Abbreviations: OR, odds ratio for the effect of combination EVL and BB versus monotherapy (BB or EVL) (as reference); SHR, subhazard ratio for the effect of combination of EVL and BB versus monotherapy (BB or EVL) (as reference).
In the second sensitivity analysis, we tested whether exclusion of 159 patients from the studies by Ahmad et al.18 and Lo et al.21 who lacked time-to-event information could have biased the results. Logistic multilevel regression analyses of all patients with information on Child class and treatment outcome independently of time-to-event also confirmed the estimates of the model using time-to-event data. The only different result was the P value for interaction in the response to EVL by Child strata, which in contrast to the main analysis was marginally significant in this analysis (P = 0.067) (Table 3).
Additionally, the post hoc exploratory analysis in the trials that compared combination therapy versus EVL alone showed that compared to EVL alone trials that used BB without ISMN20, 21 showed a statistically significantly (P = 0.007) larger impact on reducing all-source rebleeding than those trials associating ISMN to BB,18, 19 whereas the effect on mortality was not significant (Supporting Table S4).
Discussion
Currently, combination therapy consisting of BB plus EVL is recommended in the prevention of recurrent variceal hemorrhage in all patients with cirrhosis who have recovered from variceal hemorrhage.2, 3 This recommendation is based on meta-analyses that have analyzed all patients with cirrhosis, independent of the severity of liver disease. Per recent Baveno VI consensus conference and the 2016 Practice Guidance by the American Association for the Study of Liver Diseases on Portal Hypertensive Bleeding in Cirrhosis, risk stratification is essential in the treatment of portal hypertension.3, 25 Therefore, the objective of our IPD meta-analysis was to determine whether risk stratification would identify subgroups of patients who would benefit the most from combination therapy.
The current IPD meta-analysis, in addition to increasing the statistical power of previous aggregate data meta-analyses, allowed us to examine the effect of combination therapy versus monotherapy depending on Child class (A versus B/C) and to analyze survival as a time-to-event variable. We elected to stratify patients by Child A versus B/C because this would stratify patients in the two main prognostic stages of cirrhosis, compensated and decompensated, and most Child A patients are compensated whereas the majority of Child B/C patients are decompensated.9 Because trials of combination therapy had either BB or EVL as control therapy, we could address the relative contribution of BB versus EVL to combination therapy by performing separate meta-analysis.
Our meta-analysis included IPD from 389 patients of three trials comparing combination therapy versus BB and 416 patients of four trials comparing combination therapy versus EVL.
The overall results of our meta-analysis confirm results from aggregate published meta-analyses,5, 6 that is, that combination therapy is associated with lower rebleeding rates when compared to EVL alone but not when compared to BB alone, suggesting that BB therapy could be sufficient. It also supports results from aggregate meta-analyses demonstrating a lack of effect on mortality of combination therapy versus monotherapy. Notably, there was a decrease in mortality with combination therapy in trials where it was compared to EVL alone, but there was significant heterogeneity among trials; therefore, this conclusion is not strong.
Importantly, our meta-analysis showed a different effect of combination therapy in the two main stages of cirrhosis.
In compensated (Child A) patients with cirrhosis, combination therapy (BB plus EVL) was clearly associated with lower all-source rebleeding whether it was compared to BB alone or to EVL alone but without an effect on mortality. Lower variceal rebleeding rates were observed when combination therapy was compared to EVL alone but not when compared to BB alone, suggesting that BB therapy could be sufficient. However, as recently defined by consensus, the important outcome in a patient who has bled from varices is all-source rebleeding, rather than variceal rebleeding.2
In decompensated (Child B/C) patients with cirrhosis, combination therapy was associated with lower all-source rebleeding rates only in trials where it was compared to EVL alone, but no significant differences in all-source rebleeding were shown when combination therapy was compared to BB alone, suggesting that BB alone could be sufficient to prevent all-source rebleeding in this setting. More importantly, mortality was significantly lower only in trials in which combination therapy was compared to EVL alone, with a tendency for a higher mortality with combination therapy in trials where it was compared to BB alone, suggesting a deleterious effect of EVL on mortality. This deleterious effect was confirmed in time-to-event analysis that, in Child B/C patients, showed a significantly higher probability of survival with combination therapy when compared to EVL alone. This confirms that, in the setting of decompensated cirrhosis, combination therapy actually improves survival and that BB use is the key element of combination therapy. The different behavior of EVL according to Child class is further supported by the statistically significant interaction between both variables on rebleeding. Such a finding is in line with the studies observing an inverse relationship between the severity of liver disease and the efficacy of endoscopic therapy on rebleeding prevention.26
It is likely that BB improve survival in cirrhosis by lowering the rates of rebleeding and of other complications of cirrhosis through hemodynamic (reduction in splanchnic blood flow, gastroesophageal collateral blood flow, and portal pressure) and nonhemodynamic (reduction in gut bacterial translocation) mechanisms. Because portal hypertension and bacterial translocation worsen as cirrhosis progresses to the decompensated stage, the benefit of BB on rebleeding and mortality extends through the whole spectrum of the disease.
The absence of impact of BB on mortality in Child A in our study is probably related to the very low number of events (deaths) in this population. Alternatively, it can be suggested that effective prevention of rebleeding has a limited contribution in prolonging survival in initial stages of cirrhosis and that BB are only able to reduce mortality when cirrhosis progresses and the patient is at risk of complications other than bleeding. In fact, much of the observed differences in the survival curves between patients on combination therapy and those on EVL occurred beyond 18 months, which suggests an improvement in the natural history of cirrhosis by BB in the long term. These results are in agreement with those of Lo et al. who also observed a survival benefit of BB independently of rebleeding prevention.27
The availability and analysis of a large number of IPD is the main strength of the present study. Indeed, obtaining IPD data is a worthy effort. The final database was built after a thorough revision of the original data sets to detect inconsistencies and errors, which provided a robust database for analysis on a topic in which new trials with current therapies are unlikely. As is the case in our study, it is commonly acknowledged that IPD meta-analysis provides further insights to the estimation of treatment effects compared to the more frequently performed aggregate data meta-analysis.28 Besides, using aggregate data limits the possibility of assessing the impact of covariates to trial-level ones. Using IPD overcomes this limitation, allowing evaluation of the impact of patient-level covariates on the estimation of efficacy of interventions.29 Specifically, our IPD meta-analysis was able to analyze the modifier effect of individual patients' clinical characteristics as the severity of the disease (i.e., Child) on the efficacy of combined therapy. This is of importance as the severity of liver failure is the main prognostic determinant in patients with cirrhosis surviving an episode of variceal bleeding. We obtained deeper insights on the differential effect of the combined therapy depending on individual patients' Child score (A versus B/C). IPD allowed us to perform a multivariate adjustment of the effect of combined therapy by other confounders, when needed. So, we can assure that the significant decreases of mortality and rebleeding are not affected by any of the covariates included either by their distribution among the studies. Furthermore, the results of our main analysis on all-source and variceal rebleeding were reinforced by two sensitivity analyses, a competing risk analysis considering death as a competing event, and a logistic multilevel regression analysis, including all the patients regardless of time-to-event information.
The association of ISMN to BB in some but not all trials deserves a comment. ISMN was associated with BB in all three trials comparing combination therapy (BB and EVL) versus BB alone, a fact that should be taken into account when considering therapy in this context. The effect of pharmacological therapy using BB and ISMN (versus BB) could be explored in trials comparing combination therapy versus EVL alone. Interestingly, combination pharmacological therapy (BB and ISMN) had a lower effect in reducing all-source rebleeding compared to BB alone. These results indicate that BB exert their beneficial effect combined with EVL, an effect that may be hampered by the concomitant use of ISMN.
Our study, however, has several limitations. (1) As in every meta-analysis, the overall quality depends on that of the individual data. We built the final data set of IPD after checking for completeness and internal consistency and excluding those IPD with insufficient information or that could not be obtained upon request. For these reasons and in order to build a robust database, we excluded from the variceal rebleeding analysis those patients from trials lacking an individual source of bleeding information.18, 21 (2) The absence of uniform standard definitions and the insufficient information on adverse events precluded performing safety analysis. (3) The accuracy of conclusions in Child A is limited by the rather low, but otherwise expected, number of events in this population.
Altogether, our results, while confirming results from aggregate meta-analyses, point out differences in response to secondary prophylaxis therapies in patients with compensated versus decompensated cirrhosis. In those with compensated cirrhosis, combination therapy is indicated to prevent all-source rebleeding (although without an effect on mortality), while in those with decompensated cirrhosis in whom the key outcome is mortality, combination therapy, but mostly the BB component of combination therapy, is associated with a reduction in mortality. The study demonstrates that EVL should not be used as monotherapy as this is associated with lower overall efficacy in preventing rebleeding and with increased mortality in Child B/C patients. Current evidence does not support a harmful effect of BB in patients with decompensated cirrhosis but rather a beneficial effect. Therefore, careful thought should be given before discontinuing BB (or before considering not initiating them when indicated) in patients with cirrhosis, particularly in those at a decompensated stage.




