How healthy are the “Healthy volunteers”? Penetrance of NAFLD in the biomedical research volunteer pool
Potential conflict of interest: Nothing to report.
Supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Abstract
Healthy volunteers are crucial for biomedical research. Inadvertent inclusion of subjects with nonalcoholic fatty liver disease (NAFLD) as controls can compromise study validity and subject safety. Given the rising prevalence of NAFLD in the general population, we sought to identify its prevalence and potential impact in volunteers for clinical trials. We conducted a cross-sectional study of subjects who were classified as healthy volunteers between 2011 and 2015 and had no known liver disease. Subjects were classified as presumed NAFLD (pNF; alanine aminotransferase [ALT] level ≥ 20 for women or ≥ 31 for men and body mass index [BMI] > 25 kg/m2), healthy non-NAFLD controls (normal ALT and BMI), or indeterminate. A total of 3160 subjects participated as healthy volunteers in 149 clinical trials (1-29 trials per subject); 1732 of these subjects (55%) had a BMI > 25 kg/m2 and 1382 (44%) had abnormal ALT. pNF was present in 881 subjects (27.9%), and these subjects were older than healthy control subjects and had higher triglycerides, low-density lipoprotein cholesterol, and HbA1c and lower high-density lipoprotein cholesterol (P < 0.001 for all). The 149 trials included 101 non-interventional, 33 interventional, and 15 vaccine trials. The impact on study validity of recruiting NAFLD subjects as controls was estimated as likely, probable, and unlikely in 10, 41, and 98 trials, respectively. The proportion of pNF subjects (28%-29%) did not differ by impact. Only 14% of trials used both BMI and ALT for screening. ALT cutoffs for screening were based on local reference values. Grade 3-4 ALT elevations during the study period were rare but more common in pNF subjects than in healthy control subjects (4 versus 1). Conclusion: NAFLD is common and often overlooked in volunteers for clinical trials, despite its potential impact on subject safety and validity of study findings. Increased awareness of NAFLD prevalence and stricter ALT cutoffs may ameliorate this problem. (Hepatology 2017;66:825–833)
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- BMI
-
- body mass index
-
- CTCAE
-
- Common Terminology Criteria for Adverse Events
-
- HDL-C
-
- high-density lipoprotein cholesterol
-
- IQR
-
- interquartile range
-
- LDL-C
-
- low-density lipoprotein cholesterol
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NIH
-
- National Institutes of Health
-
- pNF
-
- presumed NAFLD
-
- ULN
-
- upper limit of normal
Nonalcoholic fatty liver disease (NAFLD) is rapidly becoming the most common chronic liver disorder in the United States,1 with a reported prevalence of 10%-46% in studies using different screening tests including laboratory results, hepatic imaging, and histopathology.2 The National Health and Nutrition Examination Survey III data suggests that as much as 23% of the population have unexplained liver enzyme elevations, which, in the absence of other identifiable causes, can be presumed to be NAFLD.3 The rise in NAFLD prevalence mirrors the rise of obesity and metabolic syndrome.4 This is supported clinically, because patients with NAFLD tend to be overweight or obese5 and often exhibit additional components of the metabolic syndrome, including insulin resistance, dyslipidemia, and hypertension.6, 7
The rising prevalence of NAFLD has been described in population studies and in cohorts followed by hepatologists, such as in liver transplant candidates and in donor livers.8 However, NAFLD can also be present in groups of subjects that are not typically seen by hepatologists, where awareness of the disease may be lacking. One such cohort that may be affected by the increasing prevalence of NAFLD is that of healthy volunteers in biomedical research studies. Healthy volunteers play a crucial role in biomedical research and are generally considered healthy if they have no known disease as ascertained by medical history, physical examination and common laboratory tests. If the pool of seemingly healthy volunteers is actually “contaminated” by a prevalent disorder such as NAFLD, this could impact the validity of the clinical research and have consequences related to subject safety.
At the National Institutes of Health (NIH) Clinical Center, approximately 2400 healthy volunteers are enrolled annually in clinical trials. These volunteers are recruited through flyers, advertisements, listserv messages, and online searches. With the rising prevalence of NAFLD in the general population, we sought to evaluate its prevalence in the NIH Clinical Center's pool of seemingly healthy volunteers and estimate its potential impact on the validity and safety of research.
Patients and Methods
PATIENT LEVEL DATA
Retrospective de-identified subject data were retrieved from medical records through the NIH clinical research repository, Biomedical Translational Research Information System.9 This study was exempted from the need to obtain individual subject consent by the NIH Office of Human Subjects Research Protections.
The study population comprised of adult (age ≥ 18 years) subjects classified as healthy control volunteers who had been seen at the NIH Clinical Center in a clinical trial between 2011 and 2015 and had their body weight and serum alanine aminotransferase (ALT) levels measured on the same day during the study period. In total, 11,765 subjects were seen as healthy volunteers at the NIH Clinical Center during the study period, but only 4174 (36%) had simultaneous ALT and weight measurements and were considered further. Subjects with diagnoses of viral hepatitis or alcohol-related diagnoses were excluded, as well as subjects with positive serologies for hepatitis B virus, hepatitis C virus, or human immunodeficiency virus. Finally, we excluded subjects who did not participate in studies enrolling healthy volunteers during the study period (see below).
The index date for analysis was defined as the first occurrence of simultaneous weight and ALT measurement during the study period. For all subjects, we retrieved data regarding race, sex, and height, as well as additional laboratory data (within 3 months prior to the index date) including liver function tests, lipid panel, and hemoglobin A1c. In addition, we obtained all of the liver enzyme and weight measurements that were collected during the study period.
Normal ALT was defined as <31 U/L for men and <20 U/L for women10; normal body mass index (BMI) was defined as < 25 kg/m2. Subjects were stratified based on their ALT level and BMI on the index date as healthy non-NAFLD (normal ALT level and BMI), presumed NAFLD (elevated ALT level and BMI ≥25 kg/m2), and indeterminate (either elevated ALT level or overweight). The NAFLD fibrosis score was calculated based on age, hyperglycemia, BMI, platelets, albumin, and aspartate aminotransferase (AST)/ALT ratio as described previously.11
Longitudinal analyses were conducted in subjects who had more than one visit with simultaneous ALT and BMI within the study period. Safety data were analyzed using the Common Terminology Criteria for Adverse Events (CTCAE)12 using ALT as a surrogate for liver injury and were limited to subjects with more than one visit. A conservative approach was chosen using the laboratory upper limit of normal (ULN) for ALT to minimize overestimation of toxicity. Specifically, grade 1 toxicity was defined as ULN < ALT < 3 × ULN, grade 2 as 3-5 × ULN, grade 3 as 5-20 × ULN, and grade 4 as ALT > 20 × ULN. Subjects with elevated ALT levels at baseline were only considered to have an ALT-associated adverse event if there was a further rise in ALT within 90 days from the previous laboratory measurement that was >35 U/L.
PROTOCOL LEVEL DATA
Because subjects may have participated in more than one study, we retrieved all research protocols under which each subject was recruited. For each protocol, we collected inclusion/exclusion criteria, use of liver enzymes and viral hepatitis serology for screening, exclusion of significant alcohol intake, and the description of all research arms. Subjects could have participated in some studies as healthy control subjects and in others as the diseased target population; because our focus was on the impact of NAFLD on the healthy volunteer population, we excluded studies that did not enroll healthy volunteers in any of their study arms. Some subjects were classified as healthy control volunteers from participation before the study period, but during the study period participated only in trials that did not recruit healthy control subjects. These subjects were excluded.
Studies were categorized into vaccine trials, studies with pharmacological intervention, non-pharmacological intervention, and non-interventional studies. Two of the investigators (A.N. and V.T.) independently reviewed the inclusion/exclusion criteria and protocol précis for each study to assess the potential impact of recruiting NAFLD subjects on study validity. The studies were then divided into three groups of unlikely, possible, and likely impact on outcomes, based on the research question and type of intervention. For example, a hypothetical study on the metabolism of lipid-lowering medication would be judged as likely impacted by inadvertent enrollment of NAFLD subjects as healthy control subjects, whereas a functional magnetic resonance imaging study on motor function would be unlikely to be impacted. Any disagreement between the two reviewers was adjudicated in a group discussion including the senior author (YR).
STATISTICAL ANALYSIS
Descriptive statistics are shown as frequencies or described with appropriate measures of central tendency. All inferential analyses between the healthy non-NAFLD and presumed NAFLD groups were performed using parametric techniques, two-sample t-test and χ2 testing, where appropriate.
To quantify the association between ALT and weight, we performed a longitudinal analysis limited to subjects who had ≥3 separate visits during the study period (with a minimum of 4 weeks between visits) with concurrent ALT and BMI measurements. For each individual subject, a correlation coefficient r was calculated between ALT and BMI, and the distribution of individual r values was compared between subjects according to their presumed NAFLD status. All statistical analyses were performed using SPSS Statistics version 21 (IBM).
To ascertain the potential impact of undiagnosed NAFLD on clinical research protocols, the total number and percentage of presumed NAFLD subjects per protocol were calculated. Additionally, the mean number of studies per presumed NAFLD subject was also calculated. The percentage of clinical protocols that screened using BMI and ALT were also identified.
Results
STUDY POPULATION
A total of 4174 subjects were enrolled as healthy volunteers in clinical trials at the NIH Clinical Center between 2011-2015 with ALT and BMI performed on the same day (Fig. 1). 581 subjects were excluded from the analysis because they had concomitant diagnoses of chronic liver disease, viral hepatitis, sickle cell disease, hemochromatosis, or alcohol-related diseases. Another 107 were excluded for positive serologies for viral hepatitis or human immunodeficiency virus and 245 were removed for missing data. Finally, 81 subjects were excluded for not participating as healthy volunteers during the study period. The final study population comprised 3160 subjects.
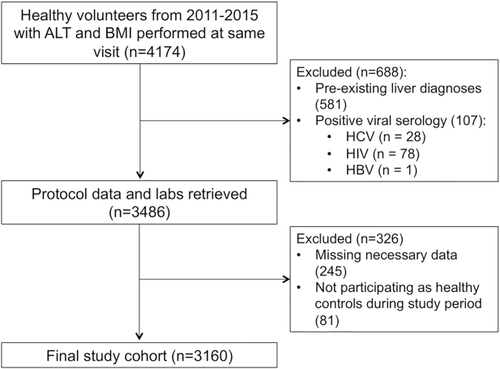
Study population.
The characteristics of the study cohort are listed in Table 1. Men comprised 54% of the subjects, and the racial distribution was notable for 36% of African American descent. A total of 1732 subjects (55%) had a BMI ≥ 25 kg/m2, and 741 (23%) were obese (BMI ≥ 30 kg/m2). ALT levels were elevated in 1382 (44%) of the subjects. There were no significant differences in age and BMI between the two genders, but as expected, ALT levels were significantly higher in men (median, 28 U/L; interquartile range [IQR], 12-45 U/L) compared with women (median, 19 U/L; IQR, 8-30 U/L) (P < 0.001).
| Parameter | Total (N = 3160) | Men (n = 1709) | Women (n = 1451) |
|---|---|---|---|
| Age, years, median (range) | 30 (18-86) | 30 (18-76) | 30 (19-86) |
| Race, n (%) | |||
| White | 1558 (49) | 870 (51) | 688 (47) |
| Black | 1130 (36) | 594 (35) | 536 (37) |
| Asian | 232 (7) | 112 (7) | 120 (8) |
| Other | 240 (8) | 133 (8) | 107 (7) |
| BMI, kg/m2, mean ± SD | 26.9 ± 5.9 | 26.8 ± 4.9 | 27.0 ± 6.8 |
| ALT, U/L, median (IQR) | 23 (9-37) | 28 (12-44) | 19 (8-30) |
| AST, U/L, median (IQR) | 19 (10-28) | 21 (12-30) | 17 (11-23) |
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; IQR, interquartile range; SD, standard deviation.
NON-NAFLD AND PRESUMED NAFLD HEALTHY VOLUNTEERS
Using the previously defined classification, we classified 881 (27.9%) subjects with elevated ALT levels and BMI as presumed NAFLD (pNF), 929 (29.4%) with normal values as healthy non-NAFLD controls, and 1350 (42.7%) as indeterminate. Within the indeterminate group, 850 (26.8%) subjects had a BMI ≥25 kg/m2 and normal ALT levels, whereas 500 (15.8%) had elevated ALT levels but normal BMI.
There were several significant differences between the pNF and healthy control cohort (Table 2). The pNF subjects were approximately 9 years older than the healthy control subjects and as expected, had more metabolic abnormalities including higher total cholesterol, low-density lipoprotein cholesterol (LDL-C) and serum triglycerides and lower high-density lipoprotein cholesterol (HDL-C). There was also evidence for moderately increased insulin resistance in the pNF group with a hemoglobin A1c of 5.6% ± 0.9%. Thus, subjects in the pNF group clearly had metabolic dysfunction typical of NAFLD.
| Parameter | Healthy (n = 929) | pNF (n = 881) | P |
|---|---|---|---|
| Age, years, median (range) | 26 (18-86) | 35 (19-75) | <0.001 |
| Sex, n (%) | 0.24 | ||
| Men | 518 (56) | 516 (59) | |
| Women | 411 (44) | 365 (41) | |
| Race, n (%) | <0.001 | ||
| White | 502 (54) | 396 (45) | |
| Black | 260 (28) | 372 (42) | |
| Asian | 100 (11) | 40 (5) | |
| Other | 67 (7) | 73 (8) | |
| BMI, kg/m2, mean ± SD | 22.3 ± 1.8 | 31.2 ± 5.6 | <0.001 |
| ALT, U/L, median (IQR) | 17 (9-25) | 34 (20-48) | <0.001 |
| AST, U/L, median (IQR) (n = 1590) | 17 (11-23) | 22 (11-33) | <0.001 |
| Total cholesterol, mg/dL, mean ± SD (n = 943) | 168 ± 32 | 184 ± 40 | <0.001 |
| HDL-C, mg/dL, mean ± SD (n = 940) | 63 ± 17 | 52 ± 16 | <0.001 |
| LDL-C, mg/dL, mean ± SD (n = 940) | 89 ± 27 | 108 ± 34 | <0.001 |
| Serum triglycerides, mg/dL, mean ± SD (n = 940) | 78 ± 38 | 117 ± 77 | <0.001 |
| HbA1c, %, mean ± SD (n = 564) | 5.2 ± 0.4 | 5.6 ± 0.9 | <0.001 |
| Serum glucose, mg/dL, mean ± SD (n = 1432) | 90 ± 13 | 95 ± 22 | <0.001 |
| Platelets, K/dL, mean ± SD (n = 1750) | 240 ± 53 | 249 ± 61 | <0.001 |
| Albumin, mg/dL, mean ± SD (n = 1331) | 4.4 ± 0.3 | 4.1 ± 0.4 | <0.001 |
| NAFLD fibrosis score, mean ± SD (n = 1358) | −3.29 ± 0.04 | −2.50 ± 0.04 | <0.001 |
| <−1.455, n (%) | 661 (98.1) | 573 (83.8) | |
| −1.455-0.675, n (%) | 12 (1.8) | 105 (15.3) | |
| >0.675, n (%) | 1 (0.1) | 6 (0.9) |
- Abbreviations: BMI, body mass index; HDL-C, high-density lipoprotein; LDL-C, low-density lipoprotein; NAFLD, nonalcoholic fatty liver disease; pNF, presumed NAFLD., Serum TG: serum triglycerides, HbA1c: Hemoglobin A1c
Within the indeterminate group, subjects with elevated BMI had an intermediate metabolic phenotype with glucose, HbA1c, cholesterol, LDL-C, HDL-C, and triglyceride levels that were worse than healthy control subjects but better than pNF subjects. These subjects were also older than healthy control subjects (Supporting Table S1). Interestingly, subjects with high ALT levels but normal BMI had, for the most part, metabolic indices that were not different from healthy control subjects.
To verify that elevated ALT at the index visit was not incidental, we evaluated the persistence of ALT elevation in subjects with more than one visit throughout the study period. In 205 pNF men and 145 pNF women with a median of 3 (range, 2-52) visits, elevated ALT persisted in 79% and 81% of subjects, respectively. Furthermore, we calculated the correlation between ALT levels and BMI among 252 subjects who had three or more concurrent ALT and BMI measurements. ALT was positively correlated with BMI in the majority of the 154 pNF subjects (median individual r = 0.28) and as expected, their r values were significantly different from 0 (P < 0.0001). On the other hand, in the 98 healthy control subjects, correlations did not differ from 0 (median r = 0.09, P = 0.56, P = 0.04 for the difference between groups) (Fig. 2). Thus, in the pNF group ALT is clearly associated with weight.
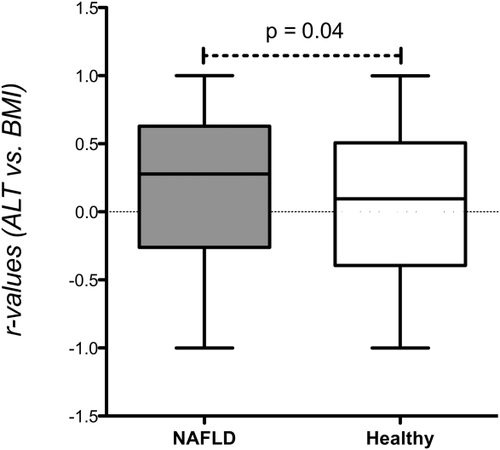
Distribution of individual correlation coefficients (r) for the association of ALT and weight in 252 subjects with at least three concurrent measurements (154 with presumed NAFLD and 98 healthy control subjects). P = 0.04 for the difference between groups.
The NAFLD fibrosis score (NFS) was suggested as a noninvasive index to assess disease stage in patients with confirmed NAFLD.11, 13 Data for its calculation were available in 684 subjects in the pNF group, of whom only 6 (0.9%) had NFS > 0.675, consistent with advanced fibrosis (Table 2).
Subjects could potentially participate in more than one trial. In fact, 42% of the healthy control subjects and 36% of the pNF subjects participated in two or more trials, and the range was 1-29 trials per subject. The average number of clinical trials was 2.1 ± 2.4 for pNF subjects and 1.9 ± 1.6 for healthy control subjects (P = 0.43).
CLINICAL TRIALS AND EFFECT OF PRESUMED NAFLD
A total of 149 clinical trials enrolled healthy volunteers from our study population, and these trials were all reviewed in detail. A total of 101 trials were non-interventional, 10 had a non-pharmacologic intervention, 23 included pharmacological intervention, and 15 were vaccine development studies. Presumed NAFLD subjects comprised 31% of subjects enrolled in non-interventional studies, 24% of interventional study subjects, and 25% of those enrolled in vaccine trials.
Only 51 of the trials required metabolic evaluation at screening, including baseline lipids and insulin sensitivity, and most were focused on comparing the healthy volunteers with patients with specific metabolic derangements, including diabetes mellitus and dyslipidemia. The primary study question was related to energy metabolism in 27 trials.
For each clinical trial, we reviewed the study précis, research question, inclusion/exclusion criteria, and characteristics of nonhealthy study groups, if relevant, and assessed the potential impact of inadvertently enrolling a subject with NAFLD as a presumably healthy control. We estimated that there would be no impact on 98 (66%) of the trials, a potential impact on 41 (28%) trials, and a likely impact on 10 trials (7%). Examples of likely impacted trials include studies that investigated the effect of nutritional or pharmacological interventions on lipid metabolism, studies assessing the effects of weight loss on inflammatory markers, and studies associating bile acid secretion with energy metabolism. On average, the percentage of control patients who met pNF criteria per trial was 29%, and this did not differ between trials with predicted unlikely, possible, or likely impact (Table 3). Overall, liver enzymes or BMI were evaluated as part of the exclusion criteria in only 26.2% and 25.6% of trials, respectively, and only 14% used both. Exclusion based on liver enzymes or BMI was more likely in trials with potential or likely impact (Table 3), but even within the trials likely to be impacted by enrollment of NAFLD, the use of liver enzymes at screening was only 70%. The cutoff value of liver enzymes used as an exclusion criterion was typically defined as 2 × or 5 × ULN, without defining the ULN value. This type of cutoff was used in 36 trials for ALT and 26 trials for AST. Only three studies specified numeric cutoffs for ALT, two using an ALT of 41 U/L or AST of 34 U/L and one using an ALT of 80 U/L with no gender differentiation.
| All Trials | Unlikely Impacted Trials | Possibly Impacted Trials | Likely Impacted Trials | |
|---|---|---|---|---|
| Number of trials | 149 | 98 | 41 | 10 |
| Number of subjects per trial, mean (range) | 42 (1-693) | 38 (1-693) | 57 (1-505) | 26 (2-77) |
| Percent pNF subjects per trial, mean (range) | 29 (0-100) | 29% (0-100) | 29 (0-88) | 28 (0-50) |
| Use of liver enzymes as exclusion criterion, n, % | 39 (26) | 24 (25) | 11 (27) | 4 (40) |
| Use of BMI as exclusion criterion, n (%) | 38 (26) | 13 (13) | 18 (43) | 7 (70) |
- Abbreviations: BMI, body mass index; NAFLD, nonalcoholic fatty liver disease; pNF, presumed NAFLD.
EFFECT OF PRESUMED NAFLD ON PATIENT SAFETY
To determine whether pNF was associated with liver-related adverse events, we assessed subjects' ALT levels over time. Using the CTCAE criteria for adverse events, pNF had a total of 15 ALT-based adverse events during the study period with three grade 3-4 ALT elevations (Fig. 3). These reflect a median rise in ALT of 59 U/L (range, 37-420 U/L) over a median of 15 days (range, 1-74 days). In comparison, healthy control subjects had only four adverse events during this period with only one grade 3-4 adverse event (P < 0.06 for comparison with pNF) with a median ALT rise of 55 U/L (range, 41-168 U/L) over a median of 18 days (range, 7-86 days). The magnitude and timing of ALT increases that met the adverse event criteria did not differ between pNF and healthy control subjects. Only one hepatology consultation was requested during the study period for a pNF subject with ALT elevation of 137 to 177 U/L within a 3-week period.
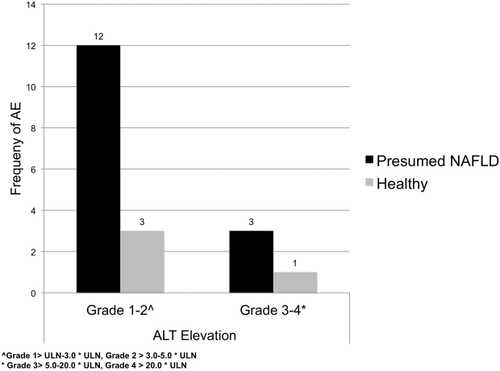
Frequency of adverse events for ALT in healthy and presumed NAFLD volunteers during the study period.
Discussion
Over the past two decades, the prevalence of obesity and NAFLD has increased substantially in United States.4, 14, 15 Therefore, it is not surprising that obese subjects are increasingly seen within the pool of volunteers to clinical research.16 In this study, we extended this finding by demonstrating that NAFLD as well is very prevalent in this population and is present in at least one fourth of the subjects enrolled as healthy volunteers in clinical trials at the NIH Clinical Center, over a wide spectrum of trial designs.
Although we did not have radiographic or histologic confirmation of diagnosis, the vast majority of subjects we defined as presumed NAFLD are likely to indeed have an excess accumulation of liver fat for several reasons. First, our study population is reflective of the overall demographics of the Washington metropolitan area as reported by the US Census17 and the overall 28% prevalence of presumed NAFLD is in line with reported rates from other US-based population studies.3, 4, 18 Second, the presumed NAFLD cohort had, as expected from subjects with NAFLD, a significantly worse metabolic profile compared with subjects we classified as truly healthy and to the indeterminate subjects, with higher total cholesterol, higher serum triglycerides, higher LDL-C and lower HDL-C along with evidence of insulin resistance. Third, the majority of subjects demonstrated persistence of ALT elevation upon repeated testing, confirming that they were not diagnosed based on an isolated event, and further supported by an association of ALT with changes in BMI. Finally, the definition we have used has been acceptable and used in other population studies in the past.19-24 In fact, it is likely that we are underestimating the prevalence of NAFLD in our population, because ALT is not a very sensitive marker. A more sensitive non–imaging-based index would be the Fatty Liver Index25, 26; however, to calculate the Fatty Liver Index, gamma-glutamyltransferase and waist-to-hip circumference values are needed, neither of which is routinely performed in the setting of a nonmetabolic clinical trial. In fact, in our entire cohort, gamma-glutamyltransferase and waist-to-hip circumference together were available for fewer than 30 subjects. Thus, it is likely that most of the subjects we classified as pNF based on elevated enzymes and BMI truly had NAFLD as the underlying cause of their laboratory derangements, though it is possible that others, especially from the high BMI indeterminate group, also had NAFLD. Using the NAFLD fibrosis score, we estimate that the majority of subjects in the pNF group do not have advanced fibrosis, as expected in this cohort of seemingly healthy people.
Recruitment of subjects with NAFLD and obesity as controls in clinical trials can affect study validity in multiple ways. It is well known, and supported by our data, that subjects with NAFLD typically have features of insulin resistance, dyslipidemia, and other components of metabolic syndrome. Thus, they would be inappropriate controls for studies assessing these features. Similarly, NAFLD is also inherently associated with alterations in energy metabolism27 and with atherosclerosis,28, 29 suggesting it should be considered when screening subjects to relevant trials.
The expression of hepatic uptake and efflux transporters, as well as that of xenobiotic-metabolizing Cyp450 enzymes is altered in NAFLD and nonalcoholic steatohepatitis, and resultant alterations in drug disposition have been demonstrated in animal models30-32 and human studies.33, 34 In fact, even obesity by itself can affect drug metabolism and bioavailability.35 Thus, with any trials using a medication or agent that undergoes hepatic metabolism, recruitment of subjects with obesity and/or NAFLD can affect the results.
An additional concern is subject safety. Although animal models suggested increased susceptibility of fatty livers to various noxious stimuli, to date no human study demonstrated increased incidence of drug-induced liver injury in subjects with pre-existing NAFLD.28, 36 However, if patients with chronic liver diseases like NAFLD do develop drug-induced liver injury, they are likely to have worse outcomes.36-38 We did detect an increased rate of markedly elevated liver enzymes (CTCAE grade 3-4) in subjects with presumed NAFLD, and these typically occurred over a relatively short period (median of 14-18 days). These findings could reflect an association with study interventions but could also be independent of the studies the subjects participated in and reflect other, unrelated causes (i.e., acute infection, alcohol consumption, etc). Our study design and the use of de-identified data did not allow for definitive differentiation between the two possibilities; however, the short time frame does suggest an association with study procedures, as we would expect non–study-related causes to be spread over a longer time period. Notably, these rises in liver enzymes did not elicit the need for hepatology consultation in most subjects, suggesting the subjects were asymptomatic. Given the inherent limitations, we cannot infer that pNF subjects are at a higher risk for liver injury in clinical trials, but awareness and careful monitoring seem to be warranted.
The ALT cutoff we used in our study is based on the 95th percentile of healthy volunteers with normal BMI, lipids, and blood glucose,10 is consistent with recent guidelines from the American College of Gastroenterology,39 and has been used by other studies.20 The ULN reported by clinical laboratories is typically higher than this cutoff, varies widely between facilities, is not affected by assay methodology or device, and is dependent predominantly on the selection of healthy subjects to define the reference range at each facility.40 ALT levels that are greater than the cutoff we used have been shown to be associated with abnormal metabolic and atherogenic profiles, even when lower than a common laboratory ULN of 40 U/L.41 Approximately one fourth of the clinical trials we evaluated attempted to avoid recruiting patients with liver disease, typically by using a cutoff of 2-5 times the laboratory ULN for inclusion into studies. Obviously, this has resulted in inadvertent inclusion of a large proportion of subjects with NAFLD. None of the trials have used the strict and gender-specific normal ALT cutoff, suggesting poor awareness on the part of clinical investigators of the impact of the accurate reference range.
The main limitations of our study, as discussed above, are the use of de-identified data without actual chart review and the reliance on indirect measures (anthropomorphic and laboratory) to define NAFLD. In addition, although we have data on racial distribution, ethnicity was not accurately captured in our database, limiting our ability to assess the impact of Hispanic origin in our study population, given the increased prevalence of NAFLD in Hispanic-Americans.4, 18 Finally, our study only encompasses healthy volunteers who had simultaneous liver transaminases and BMI measurements and can only be assumed to represent the whole population of healthy volunteers.
Overall, the significance of this study is to increase awareness and vigilance among clinical researchers. Subjects with NAFLD should be included in trials that aim to reflect the general population, both for practical purposes (given the high prevalence of NAFLD) and for generalizability. However, strictly speaking, these subjects should not be considered healthy and are inappropriate controls for trials assessing metabolic pathways or drugs and devices that could be affected by hepatic dysfunction or NAFLD. Our data are not necessarily aimed at investigators designing a trial with NAFLD as the research question, given that hepatologists are likely to be aware of the high prevalence of NAFLD, proper liver enzyme cutoffs, poor sensitivity of ALT, and potential impact of NAFLD. Non-hepatologists, on the other hand, are far less aware of the disease and its features, and tend to erroneously rely on marked elevations in liver enzymes as a trigger for evaluation and referral.42, 43 Increasing awareness among the broad community of non-hepatologist clinical investigators to the stricter ALT thresholds is crucial in this regard.
In conclusion, NAFLD is a common and an often-overlooked disease in subjects recruited as healthy control volunteers to clinical trials. Use of a strict ALT cutoff of 19 U/L in females and 30 U/L in males may help overcome concerns for the presence of NAFLD in seemingly healthy control subjects and improve research validity.
Acknowledgments
We would like to thank Dr. Bryan Curtin for assisting with difficult questions.




