Direct-acting antivirals during or after immunochemotherapy in hepatitis C virus–positive diffuse large B-cell lymphomas
Potential conflict of interest: Dr. Bruno consults and is on the speakers' bureau for AbbVie, Gilead, MSD, and Bristol-Myers Squibb. Dr. Grossi is on the speakers' bureau for Gilead and MSD. Dr. Passamonti consults, advises, and is on the speakers' bureau for Novartis. He advises and is on the speakers' bureau for Gilead. He advises Roche. Dr. Arcaini consults and advises Roche and Celgene. He advises and received grants from Gilead. He consults for Bayer. He advises Sandoz.
Abbreviations
-
- AT
-
- antiviral therapy
-
- CR
-
- complete response
-
- DAA
-
- direct-acting antiviral
-
- DLBCL
-
- diffuse large B-cell lymphoma
-
- HCV
-
- hepatitis C virus
-
- MZL
-
- marginal zone lymphoma
-
- R-CHOP
-
- rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone
Direct-acting antivirals (DAAs) revolutionized hepatitis C virus (HCV) treatment by enabling viral eradication in more than 95% of cases across all genotypes.1 Recent demonstration of lymphoma regression in 67% of patients with HCV-associated indolent lymphomas receiving exclusively DAAs clearly pointed out the etiologic relationship between HCV and specific lymphoma subtypes, typically marginal zone lymphomas (MZLs).2 Interestingly, HCV-positive diffuse large B-cell lymphomas (DLBCL) frequently display evidence of histologic transformation from previous MZL and in 26% of cases carry mutation in the NOTCH pathway, a mutational signature which is typical of splenic MZL and associated with coexisting low-grade component and poor outcome.3 In HCV-positive DLBCL, antiviral therapy (AT) is clearly insufficient and immunochemotherapy is required, although HCV eradication has been suggested to potentially improve the long-term outcome.4
Here, we report two cases illustrating DAA integration in the treatment course of HCV-positive DLBCLs (Fig. 1). The first case is represented by a 73-year-old female who was diagnosed with stage IVS, high-risk, non–germinal center DLBCL, transformed from previous splenic MZL and chronic genotype 1b HCV infection. She achieved complete response (CR) after eight cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP); but after 7 months a relapse occurred at the right arm subcutaneous tissue, liver, axillary, and iliac lymph nodes. She underwent salvage treatment with four cycles of rituximab, dexamethasone, cytarabine, and cisplatinum, which induced a second CR. However, shortly after, a new localized relapse was documented at the right arm skin and local radiotherapy was planned. Considering advanced hepatic fibrosis (Metavir score F3), it was decided to initiate concurrently AT with sofosbuvir and simeprevir. Along with sustained virological response, a lymphoma CR was obtained, which is ongoing after 1 year of follow-up.
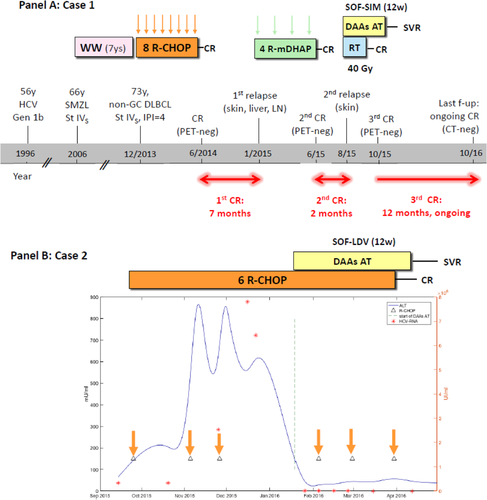
Two cases of integration of DAA-based AT either subsequently (case 1, A) or concurrently (case 2, B) with immunochemotherapy in the treatment course of HCV-positive DLBCL. Abbreviations: ALT, alanine aminotransferase; CT, computed tomography; f-up, follow-up; Gen, genotype; IPI, International Prognostic Index; LDV, ledipasvir; LN, lymph node; non-GC, non–germinal center; PET, positron emission tomography; R-mDHAP, rituximab + mini-DHAP (dexamethasone, cytarabine, cisplatinum); RT, radiotherapy; SIM, simeprevir; SMZL, splenic MZL; SOF, sofosbuvir; St, stage; SVR, sustained virological response; WW, watch and wait.
The second patient is a 33-year-old male who presented with stage IIIS, low-risk to intermediate-risk, non–germinal center DLBCL and chronic genotype 1b HCV infection (Metavir score F2). Six cycles of R-CHOP were planned. However, after the first two cycles, he developed a grade 4 acute alanine aminotransferase flare along with a rapid increase of the HCV viral load, leading to immunochemotherapy delay. The therapy was resumed only when alanine aminotransferase decreased to grade <3, but after the third cycle, transaminases raised again (grade 4) and the treatment was withdrawn. In order to assure adequate timing of treatment, it was decided to initiate DAA AT with sofosbuvir–ledipasvir. The rapid viral eradication allowed resolution of liver toxicity and restart of R-CHOP concurrently with AT without any apparent drug–drug interaction. After completion of six cycles of R-CHOP, with no more hepatic toxicity occurrence, the patient achieved positron emission tomography–computed tomography–negative CR, which is ongoing at 8 months of follow-up.
Overall, these cases suggest that, in a comprehensive curative approach to patients with HCV-positive DLBCL, viral eradication with DAAs may improve their outcome because of amelioration of the hepatic condition as well as reduction of the relapse rate. Considering the first case, the surprisingly durable CR of lymphoma obtained after interferon-free AT plus radiotherapy argues strongly in favor of a positive influence of HCV eradication on the patient outcome also in this highly difficult relapsed/refractory setting. Moreover, it may suggest that, at least in cases with evidence of histological transformation, the clearance of a postulated antigen-dependent low-grade clone may possibly eradicate the trigger of relapse. The optimal timing of DAA administration is probably after the completion of first-line immunochemotherapy and achievement of CR, although consistent clinical data are lacking. Conversely, the second case demonstrates the feasibility and efficacy of concurrent administration of DAAs with R-CHOP, which may allow completion of the scheduled course of therapy and achievement of CR also in a fraction of patients (16%-27%4) who develop severe hepatic toxicity. Interestingly, a similar strategy was demonstrated to be effective in human immunodeficiency virus–HCV coinfected patients, in whom liver toxicity of antiretrovirals is prevented by treating HCV earlier. Prospective clinical trials investigating concurrent interferon-free AT with R-CHOP in HCV-positive DLBCL are largely awaited.
REFERENCES
Author names in bold designate shared co-first authorship.




