Cardiac dysfunction associated with a nucleotide polymerase inhibitor for treatment of hepatitis C
Potential conflict of interest: Dr. Hughes is employed by Bristol-Myers Squibb. Dr. Dimitrova is employed by and owns stock in Bristol-Myers Squibb. Dr. Brown is employed by Bristol-Myers Squibb. Dr. Heath-Chiozzi is employed by and owns stock in Bristol-Myers Squibb. Dr. Yin is employed by and owns stock in Bristol-Myers Squibb. Dr. Zamparo owns stock in Bristol-Myers Squibb. Dr. Kumar is employed by and owns stock in Bristol-Myers Squibb. Dr. Manion is employed by and owns stock in Bristol-Myers Squibb. Dr. Pockros consults, advises, is on the speakers' bureau of, and received grants from Bristol-Myers Squibb. Dr. Wolf owns stock in Bristol-Myers Squibb. Dr. Hernandez consults and received grants from Bristol-Myers Squibb and consults for Vertex.
This work was supported internally by the Duke Clinical Research Institute.
Abstract
Treatment for chronic hepatitis C virus (HCV) infection is evolving from interferon (IFN)-based therapy to direct-acting antiviral (DAA) agents, yet some safety concerns have arisen involving cardiac toxicity. In this study, we sought to better understand the potential off-target toxicities of new DAAs. We retrospectively evaluated the clinical and pathological findings of the sentinel case in a phase II study that led to clinical development discontinuation for BMS-986094, an HCV nucleotide polymerase (nonstructural 5B) inhibitor. We also report on outcomes from other patients in the same study, including electrocardiogram changes, cardiovascular biomarkers, and transthoracic echocardiograms. Thirty-four patients received IFN-free BMS-986094 regimens. Six patients had left ventricular ejection fractions (LVEFs) <30%, 8 had LVEFs 30%-50%, and 11 required hospitalization for suspected cardiotoxicity. Of the patients with LVEF <50%, 6 had normalization of systolic function after a median of 20 days. T-wave inversions were the most sensitive predictor of LVEF dysfunction. B-type natriuretic peptide levels increased over time and correlated with the degree of LVEF dysfunction. Pathological analysis of cardiac tissue revealed severe myocyte damage with elongated myofibrils without gross necrosis. These findings were consistent with some results of recent primate studies that were conducted to further investigate the potential mechanisms of BMS-986094 toxicity. Conclusion: A novel nucleotide analog polymerase inhibitor developed for HCV treatment may cause a toxic cardiomyopathy. Ongoing surveillance of DAAs for cardiotoxicities may be beneficial, especially among patients at higher risk for cardiovascular disease. (Hepatology 2015;62:409–416
Abbreviations
-
- AEs
-
- adverse events
-
- BNP
-
- B-type natriuretic peptide
-
- CVD
-
- cardiovascular disease
-
- DAA
-
- direct-acting antiviral
-
- DCV
-
- daclatasvir
-
- ECG
-
- electrocardiogram
-
- FDA
-
- U.S. Food and Drug Administration
-
- HCV
-
- hepatitis C virus
-
- HIV
-
- human immunodeficiency virus
-
- IFN
-
- interferon
-
- LV
-
- left ventricular
-
- LVEF
-
- left ventricular ejection fraction
-
- Peg
-
- pegylated
-
- QD
-
- once-daily
-
- RBV
-
- ribavirin
-
- TTE
-
- transthoracic echocardiogram
-
- ULN
-
- upper limit of normal
Chronic hepatitis C virus (HCV) infection affects more than 3% (170 million) of the world's population and is a major cause of liver cirrhosis and hepatocellular carcinoma.1 Historically, HCV treatments have included pegylated (Peg) interferon (IFN)-α and ribavirin (RBV), but these agents are limited by restricted efficacy and frequent side effects.2 New regimens comprised of direct-acting antivirals (DAAs) that target different steps in the HCV life cycle are in development and some have received breakthrough therapy status by the U.S. Food and Drug Administration (FDA).3-5
One DAA in development was BMS-986094 (formerly INX-08189), a nucleotide analog HCV nonstructural 5B polymerase inhibitor.6 BMS-986094, either alone or combined with RBV, was well tolerated and exhibited dose-dependent antiviral activity in treatment-naïve HCV genotype 1–infected patients over 7 days of treatment.7 In part A of a phase II study, the combination of BMS-986094 with Peg-IFN-α and RBV appeared to be well tolerated in patients with HCV genotype 2 or 3 infection.8 After review of safety data, BMS-986094 was evaluated in IFN-free combinations with daclatasvir (DCV) and RBV in part B of this study. Subsequently, dosing was terminated after 34 patients had been dosed in part B, immediately after a patient experienced rapidly progressive heart failure and expired. Other cases of cardiotoxicity were later identified.
This report is a retrospective review of adverse cardiac events and additional clinical findings noted in the index case and in patients enrolled in part B of the BMS-986094 study. We also comment on an evaluation from nonclinical studies of potential mechanisms underlying the observed cardiotoxicity and on potential implications of ongoing development of DAAs for the treatment of HCV.
Patients and Methods
Patients and Study Design
In part A, BMS-986094 was administered once-daily (QD; 25, 50, or 100 mg) in combination with weekly Peg-IFN-α and twice-daily RBV for 12 weeks. Part A enrolled 91 treatment-naïve patients with HCV genotype 1 infection. Part B was initiated after conducting the safety evaluation of part A. In this open-label study, patients received QD doses of BMS-986094 100 or 200 mg plus weight-based RBV or DCV 60 mg, or BMS-986094 50 mg plus RBV and DCV for 12 weeks. Planned recruitment was to include 20 or 40 patients per arm (120 in total) from 20 centers in U.S. treatment-naïve patients without cirrhosis 18-65 years of age infected with HCV genotypes 1, 2, or 3 were eligible for this study. Patients were excluded if they had liver disease other than HCV infection, human immunodeficiency virus (HIV) infection, QTc interval ≥450 ms or a personal or family history of torsades de pointe, creatinine clearance <50 mL/min, or serum creatinine ≥1.5 × upper limit of normal (ULN). The study was approved by local institutional review boards and was performed in accord with the Declaration of Helsinki and good clinical practice as defined by the International Conference on Harmonization and ethical principles of U.S. regulatory requirements. This study is registered with ClinicalTrials.gov (no.: NCT01425970). All patients provided written informed consent.
Safety Monitoring Pre–Index Case
Safety monitoring included the incidence, nature, and severity of adverse events (AEs) and serious AEs, discontinuations because of AEs, and clinical laboratory abnormalities, vital signs, physical exams, and electrocardiograms (ECGs). ECGs were planned at screening, baseline, weeks 2, 6, and 12, and post-treatment week 12, with evaluation by a cardiologist. Cardiac troponin I and creatinine phosphokinase were evaluated on treatment.
Safety Monitoring Post–Index Case
After dosing of study drugs was suspended, post-hoc safety evaluation changes were implemented to intensify cardiac and other safety monitoring. All patients were requested to return for immediate cardiac and full safety evaluations that included clinical exams, transthoracic echocardiograms (TTEs), ECG, and laboratory monitoring of B-type natriuretic peptide (BNP). Additional evaluations of some patients were obtained at the discretion of the treating physician and included invasive assessments of the coronary arteries and cardiac magnetic resonance imaging. Once-weekly repeat evaluations were requested and tapered on a case-by-case basis. Study sites provided available TTE and ECG data.
The on-treatment safety data for patients enrolled in part A were evaluated by an external safety monitoring committee and are available online.9 Part A patients had completed BMS-986094 therapy approximately 3-6 months preceding the index case's presentation in part B.
Patient Data Collection and Reporting
Data obtained through 6 months after discontinuation of dosing are reported. All ECGs were reevaluated by a blinded central reader, focusing on new postbaseline ST-segment abnormalities. These abnormalities included ST-segment depression, T-wave inversion, and T-wave amplitude loss. Each ECG was read by two cardiologists, and a third cardiologist adjudicated any discrepancies. TTEs and other imaging modalities used to evaluate cardiac function were conducted after treatment was discontinued, and the number of available evaluations varied on a patient-by-patient basis, as described in the Results. Prestudy cardiac function data were not originally specified by protocol a priori and were generally not available. Laboratory data were collected by the central laboratory during therapy or were collected in the poststudy termination period. Laboratory data from external sources (i.e., hospitalizations and outside office visits) were not included. Plasma BNP was not evaluated initially per protocol, but was evaluated retrospectively from frozen serum collected before treatment discontinuation and prospectively on serum samples collected after discontinuation using commercial assays.
Results
At the time of dosing termination on August 1, 2012, 34 patients had received therapy in part B for approximately 1-6 weeks (Table 1). Twenty patients received 200 mg of BMS-986094 with either DCV (n = 14) or RBV (n = 6), 9 received 100 mg of BMS-986094 with either DCV (n = 4) or RBV (n = 5), and 5 received 50 mg of BMS-986094 with DCV and RBV (Fig. 1). Fourteen (39%) treated patients were noted to have evidence of cardiac dysfunction, as defined by left ventricular (LV) ejection fraction (LVEF) <50%: 6 had severe LV dysfunction (LVEF <30%), and 8 had moderate LV dysfunction (LVEF 30%-50%; Fig. 1) at one or more evaluations in the 6-month period following drug discontinuation.
| Characteristic | Total (n = 34) | Cardiac Dysfunction (n = 14)a | No Cardiac Dysfunction (n = 20)b |
|---|---|---|---|
| Age, years (mean) | 52 | 52 | 52 |
| Male gender, n (%) | 23 (67.6) | 9 (64.3) | 14 (70.0) |
| Hypertension, n (%) | 13 (38.2) | 8 (57.1) | 5 (25.0) |
| Diabetes, n (%) | 3 (8.8) | 2 (14.3) | 1 (5.0) |
| Dyslipidemia, n (%) | 3 (8.8) | 1 (7.1) | 2 (10.0) |
| Smoking, n (%) | 7 (20.6) | 2 (14.3) | 5 (25.0) |
| Known CVD, n (%) | 0 | 0 | 0 |
| ECG changes (baseline) | 20 (58.8) | 13 (93.0)‡ | 7 (35.0)d |
| ACE-I/ARB at baseline, n (%) | 11 (32.3) | 7 (50.0) | 4 (20.0) |
| Beta-blocker at baseline, n (%) | 11 (32.3) | 8 (57.1) | 3 (15.0) |
| Statin at baseline, n (%) | 2 (5.9) | 1 (7.1) | 1 (5.0) |
- a Defined as ejection fraction (EF) <50% at any time.
- b Defined as no EF <50% at any time.
- c Reported abnormalities included 8 incidences of sinus bradycardia; 2 incidences each of incomplete right bundle branch block, nonspecific intraventricular conduction defect, or left atrial enlargement; and 1 incidence each of right bundle branch block, first-degree atrioventricular block, nonspecific ST abnormality, or short P-R interval.
- d Reported abnormalities included 3 incidences of nonspecific intraventricular conduction defect; 2 incidences each of sinus bradycardia or nonspecific ST abnormality; 1 incidence each of nonspecific T-wave abnormality, QTcF (corrected for heart rate using Fredericia's formula) borderline prolonged, or anterior/septal myocardial infarction criteria observed (question lead placement).
- Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
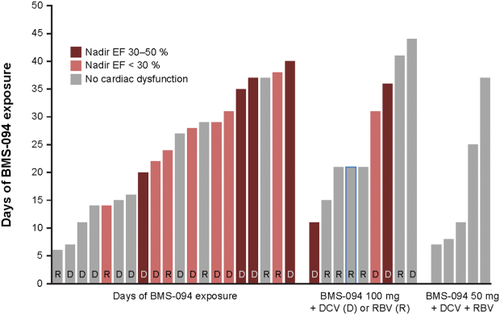
Treatment of patients with BMS-986094. Patients were treated with BMS-986094 100 or 200 mg QD, plus RBV (1,000 mg orally per day if patient weighs <75 kg or 1,200 mg orally per day if patient weighs >75 kg) or DCV 60 mg QD, or BMS-986094 50 mg plus RBV and DCV 60 mg QD. Treatment had been planned for 12 weeks, but patients were treated for between 1 and 6 weeks. Fourteen patients developed severe (EF <30%) or moderate (EF 30%-50%) systolic dysfunction, as indicated. EF, ejection fraction.
Index Case
The case that led to study drug discontinuation for all phase II subjects was a 25-year-old white male with a history of chronic HCV infection, opioid dependence, and mild depression, who received 200 mg of BMS-986094 and DCV for 40 days in part B of the study. No significant clinical events were reported during the initial three on-treatment visits (Fig. 2). This patient's week 2 ECG demonstrated a newly acquired, nonspecific ST abnormality that was considered not clinically significant at the time; creatinine and troponin levels remained within normal limits. Creatine kinase was in the normal reference range (30-294 U/L) at baseline and week 2 (141 and 227 U/L, respectively) and just above the normal reference range on weeks 1 and 4 (300 and 312 U/L, respectively). On day 39, the patient reported nausea and vomiting and was prescribed prochlorperazine. The following day, he was hospitalized for shortness of breath and was noted to have pulmonary edema, acute renal failure, and shock liver. A TTE demonstrated an LVEF <10%. Laboratory data on admission included: creatine kinase, 397 U/L (normal range: 30-200 U/L); troponin, 0.02 (normal range: <0.04); creatinine, 2.3 mg/mL (normal range: 0.7-1.3 mg/dL); alanine transaminase, 3,861 IU/L (normal range: 13-69 IU/L); and aspartate aminotransferase, 4,909 IU/L (normal range: 15-46 IU/L). Over the next 2 weeks, escalation in therapy included an intra-aortic balloon pump, extracorporal membrane oxygenation, intravenous dopamine, and continuous renal replacement therapy. Despite these measures, cardiac function did not improve and life support was withdrawn by the family approximately 2 weeks after hospitalization.
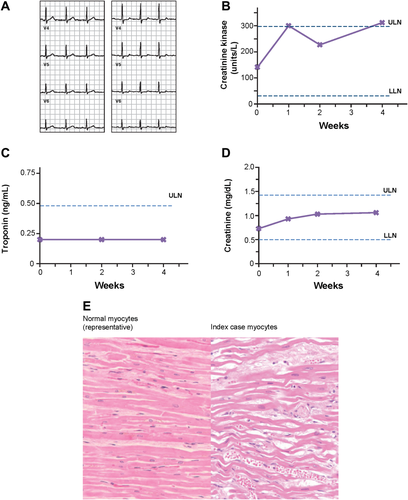
Findings from index case. (A) Representative ECGs taken at baseline (left) or at week 2 on treatment (right). (B-D) On-treatment creatine kinase, troponin I, and serum creatinine levels collected on treatment, respectively. (E) Representative hematoxylin and eosin stains of normal ventricular myocardium from an age-matched normal autopsy heart (left) and LV myocardium from the index case (right). The index case showed focal mild microscopic aggregates of mononuclear (predominantly lymphocytic) inflammatory cells in microscopic areas of recent myocyte necrosis (not shown in this image). The majority of the myocardium of both ventricles showed diffuse thinning and elongation of cardiac myocytes associated with fine interstitial fibrosis and marked interstitial edema (as illustrated in this image) with only focal, minimal microscopic areas of necrosis.
At autopsy, pathological evaluation of the explanted heart demonstrated diffuse elongation and thinning of ventricular cells associated with fine interstitial fibrosis, very limited areas of necrosis, and limited small areas of mononuclear inflammation (Fig. 2). The degree of myocarditis appeared insufficient to account for severe biventricular dysfunction.
Cardiovascular Evaluation of Patients After Treatment Discontinuation
After dosing termination, all patients were required to have serial TTE evaluations performed locally; 80% of patients had five or more TTEs entered into the clinical database through 6 months after dosing was terminated. Of the 20 patients (including the index case) treated with 200 mg of BMS-986094, 4 had severe systolic dysfunction (defined as nadir LVEF <30%) and 7 had moderate systolic dysfunction (nadir LVEF ≥30%-<50%). Of the 9 patients dosed with 100 mg of BMS-986094, 2 had severe systolic dysfunction and 1 had moderate systolic dysfunction. None of the patients dosed with 50 mg of BMS-986094 had cardiac dysfunction. LV systolic dysfunction was observed regardless of concomitant HCV therapy (DCV or RBV). Duration of treatment on study was longer for patients with moderate or severe LV systolic dysfunction (n = 14) than for those with normal LV function (defined as LVEF >50%; n = 20): 86% of the former group of patients received ≥20 days of treatment, compared to 35% of the latter.
Figure 3 illustrates the LVEF over time for patients with abnormal TTE. The nadir LVEF occurred during the first several weeks after treatment discontinuation in most patients and, in some cases, was preceded by a normal LVEF—in 2 patients, the nadir was reached approximately 10 weeks after treatment discontinuation. Improvement of LVEF was documented in 4 of 6 patients with severe LV systolic dysfunction and in 6 of 8 patients with moderate LV systolic dysfunction. The last available LVEF was >50% in 7 of these patients. In addition to the index case, only 1 patient, treated with 200 mg of BMS-986094 and DCV, did not improve based on last data available. In this patient, cardiac evaluation revealed significant coronary artery disease requiring coronary artery bypass surgery.
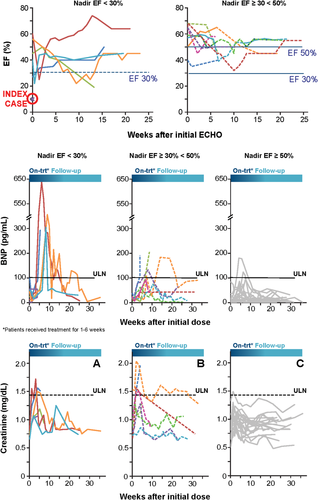
LVEF over time. (A) Patients treated with moderate or severe LV systolic dysfunction. Patients had been treated with BMS-986094 for 1-6 weeks, and the majority of subjects had the initial TTE evaluation within 1 week of discontinuing therapy. Systolic function was evaluated from the time of the initial TTE taken after discontinuation of BMS-986094. Patients with severe (nadir EF <50%) and moderate (nadir EF ≥30%-50%) LV systolic dysfunction are displayed separately. (B) Retrospective and prospective evaluation of BNP. Only BNP measurements from samples taken at the study site are included. BNP was evaluated retrospectively from frozen serum collected before treatment discontinuation and prospectively on samples collected after treatment discontinuation and are displayed from the time of initial BMS-986094 dose. The ULN for BNP is 100 pg/mL. Patients with severe, moderate, and normal (nadir EF >50%) function are displayed separately. (C) Serum creatinine changes over time. Creatinine levels are displayed from the time of initial BMS-986094 dose. The ULN for creatinine is 1.4 mg/mL. Patients with severe, moderate, and normal LV function are displayed separately. Abbreviations: ECHO, echocardiogram; EF, ejection fraction.
ST-segment abnormalities were frequently noted in ECGs of patients with TTE evidence of LV dysfunction. A retrospective evaluation identified three main abnormalities: ST depressions, T-wave inversions, or loss of T-wave amplitude. ST depression appeared to be the most specific and least sensitive finding: It was observed only in 2 patients with severe cardiomyopathy. T-wave inversions appeared to be most sensitive and were observed in 86% of cases and 20% of patients with normal LVEF. Loss of T-wave amplitude was observed in 79% of cases and in 60% of patients with normal LVEF.
Increases in BNP were observed in patients with severe and moderate systolic cardiomyopathy and in patients with normal LVEF (Fig. 3). Based on available retrospective and prospective data, the following patients had BNP >100 pg/mL: 5 of 6 with LVEF <30%, 4 of 8 patients with LVEF 30%-50%, and 2 of 20 with LVEF >50%. The index case did not have BNP increase >100 pg/mL during therapy, and no BNP data were collected during his hospitalization.
The index case and other patients experienced renal function changes based on serum creatinine levels (Fig. 3). Most patients, regardless of severity of systolic dysfunction, experienced increased serum creatinine, which improved over time after treatment discontinuation. The highest recorded serum creatinine value remained within the normal reference range in the majority of patients. In addition to the index case, who required renal replacement therapy, 1 patient required temporary hemodialysis.
Discussion
This is the first clinical report of cardiotoxic changes associated with a novel nucleotide analog polymerase inhibitor developed for the treatment of chronic HCV infection. Although most participants did not have symptomatic heart failure, the frequency of cardiac dysfunction after exposure suggests possible drug-related cardiotoxicity. The most common findings were ST-segment and T-wave abnormalities on the surface ECG. Plasma BNP levels were raised over baseline, but, in most cases, were not significantly elevated above normal reference ranges. Most cases exhibited recovery of cardiac function after ending BMS-986094 treatment. Pathological analysis of the myocardium from the index case showed profound sublethal myocyte injury with very limited areas of necrosis and no appreciable infiltration of polymorphonuclear cells, consistent with, but not diagnostic of, a toxic cardiomyopathy. In the earlier phase of this study (part A; BMS-986094 combined with Peg-IFN and RBV), there was no clear cardiac signal. This may have been resulted, in part, from the lower doses utilized in this earlier phase. In addition, subjects had been off BMS-986094 therapy for 3-6 months at the time the index cases had been reported and the initial echocardiograms were performed to assess cardiac function. Although standard ECG monitoring performed in part A did not identify cardiac signals at the time the study was ongoing, retrospective review of these ECGs focusing on nonspecific ST/T-segment abnormalities described in this article suggested a possible dose relationship, although the data were limited to draw any conclusions.
As recently reported, a series of nonclinical in vitro and in vivo investigative studies were conducted for further characterization of potential mechanisms associated with BMS-986094 cardiotoxicity.10-12 In monkeys, the investigators described partial-to-complete reversibility of dose- and time-related ventricular dilatation, decreased ventricular function, and ST-segment changes.12 These nonhuman primate observations mirrored some of the clinical findings associated with BMS-986094 described in this article. The reversible nature of the events suggests transient, rather than permanent, injury to the cardiac myocytes. The actual mechanism remains unknown, but these studies indicate that BMS−unknown_hyphen;986094 did not appear to be a direct mitochondrial toxicant; however, some potential changes in cardiac energy utilization were described.11 In vitro, BMS-986094 was associated with concentration- and time-related cytotoxicity in human cardiomyocytes.13
Further evaluation of the potential mechanisms of cardiac dysfunction and its relevance to other DAAs requires further study. Of note, the cardiotoxicity of BILN 2061 (Boehringer Ingelheim), a reversible inhibitor of HCV NS3/NS4A serine protease, halted its clinical development. Initial pathological examination suggested mitochondrial dysfunction with minimal myonecrosis, although metabolomic assessments were not performed.14 Consistent with our findings for BMS-986094, biomarkers of cardiac dysfunction (i.e., cardiac troponins and BNP) were insensitive measures of BILN 2061-associated myocardial injury, and for both agents, cardiac function normalized over time. Currently, it remains unclear whether cardiotoxicity is a general effect of DAAs. To date, we are unaware of unexpected cardiac events in patients treated with other investigational therapies with a similar chemical or molecular structure to BMS-986094. Nevertheless, ongoing studies are limited in size, and, without the appropriate monitoring for cardiac safety signals, an agent presumed to have less cardiotoxicity could potentially yield increased cardiac dysfunction when used in a larger population of patients—particularly in those with underlying heart disease or who are older and at risk for cardiovascular disease (CVD). Notably, 75% of U.S. patients with HCV were born between 1945 and 1965, which is the demographic with the highest prevalence of CVD.15 For HCV DAAs currently on the market since 2011, a review of the FDA Adverse Event Reporting System quarterly reports at the time of this publication did not reveal any new reports of serious cardiac signals for these novel therapies.16
Appropriate screening and monitoring methods to identify cardiac dysfunction remain unclear. In this study, several traditional cardiac screening tests were performed and only a small fraction of patients with evidence of cardiac dysfunction experienced symptoms of heart failure. Plasma levels of BNP, a sensitive marker of myocardial stress, were modestly elevated in the majority of patients with cardiac dysfunction, and no patients had elevated troponin levels. Therefore, these biomarkers likely have little utility as screening methods. Nonspecific ECG abnormalities were noted in cases of cardiac dysfunction, but were only apparent in hindsight, and it remains unknown whether they have the associated sensitivity or specificity needed for screening. Therefore, screening methods need to be developed for use in patients treated with agents that may increase cardiotoxicity.
Moving forward, postmarketing surveillance for novel DAAs may be needed for cardiovascular safety given that the population is shifting to an older demographic increasingly at risk for CVD.17 Although BMS-986094 development was discontinued based on the seriousness of the clinical events reported in the phase II study, it could be postulated that safety issues from a less-toxic medication might only become apparent after the drug is marketed and used to treat larger patient populations. With the introduction of the Critical Path Initiative,18 the FDA highlighted the need to establish more-definitive systems to detect and respond to drug toxicity involving patients, regulatory agencies, and industry. Postmarketing approaches to identifying potential toxicities include use of registries, electronic safety monitoring systems, and patient-reported outcomes.
In the case of BMS-986094, patients exposed to BMS-986094 in phase I and II trials have been offered the opportunity to be enrolled into a registry (Longitudinal Assessment of Cardiovascular and Renal Health in Patients with Hepatitis-C [CARE-Hep C]; NCT01846494) created to understand the incidence of cardiac and renal dysfunction related to the study medication and to arrange for appropriate referral in cases of cardiac dysfunction. This registry may improve our understanding of the BMS-986094 safety profile and may also help determine the incidence of HCV-associated cardiac dysfunction. Furthermore, CARE-Hep C could serve as a template for the observation of patients undergoing treatment with novel DAAs.
In conclusion, we report that a novel polymerase inhibitor developed for the treatment of chronic HCV infection may cause a toxic cardiomyopathy. The majority of patients with echocardiographic evidence of cardiac dysfunction did not have heart failure symptoms and none had significant abnormalities in cardiac troponin or BNP levels. Several patients developed concomitant renal failure and some required hemodialysis. Results of prospective nonclinical mechanistic studies suggest that BMS-986094 is not a direct mitochondrial toxicant, but that alteration of cardiac energy generation or utilization may be involved. Ongoing and future clinical trials of DAAs may benefit from careful surveillance for cardiac and renal abnormalities.
Acknowledgment
Professional medical writing and editorial assistance was provided by Carolyn Carroll, Ph.D., an employee of Bristol-Myers Squibb.