Incidence, risk factors, and survival of hepatocellular carcinoma in primary biliary cirrhosis: Comparative analysis from two centers†
Potential conflict of interest: Nothing to report.
Abstract
The limited information and divergent results on the prevalence, incidence, and risk factors for hepatocellular carcinoma (HCC) in patients with primary biliary cirrhosis (PBC) may be due to the low prevalence of the disease and geographical and environmental differences. Therefore, we analyzed the incidence, prevalence, survival, and risk factors for HCC in patients with PBC from two European centers (389 from Barcelona, Spain, and 327 from Padova, Italy) followed up for 9.3 ± 6.5 years. Gender, age, smoking habit, alcohol consumption, presence of hepatitis B surface antigen (HBsAg) or hepatitis C virus antibodies (anti-HCV), and advanced histological stage (III-IV) were evaluated as risk factors for tumor development. Twenty-four patients (13 from Barcelona and 11 from Padova) developed HCC. The prevalence of HCC was similar in Barcelona (3.34%) and Padova (3.36%). The incidence was 0.35 and 0.37 per 100 patient-years, respectively. Male gender, age >52 years, smoking habit, alcohol >40 g/day, HBsAg, and anti-HCV were not associated with HCC. Advanced histological stage was the only factor associated with the development of HCC (odds ratio [OR]: 5.80, 95% confidence interval [CI]: 2.34-14.38, P < 0.001). When analyzing the two series separately, male gender was associated with higher likelihood of HCC in Padova (OR: 8.09, 95% CI: 1.93-33.8, P < 0.01). The median survival after the diagnosis of HCC was 36 months. Conclusion: The prevalence and incidence of HCC is similar in Spain and Italy and the advanced histological stage is the only risk factor associated with the development of HCC in PBC. The slight disparities observed between the two series might be explained by patient features on diagnosis of liver disease. (HEPATOLOGY 2009.)
Primary biliary cirrhosis (PBC) is a progressive cholestatic liver disease, characterized by chronic inflammatory destruction of bile ducts, which eventually leads to cirrhosis.1 Hepatocellular carcinoma (HCC) is the most common primary malignant tumor of the liver2 and its incidence in PBC is not well known. Some studies have described a low incidence of HCC in PBC,3, 4 whereas in others no differences were seen in HCC development in PBC with respect to cirrhosis of other etiologies.5, 6 Case reports and other studies have shown an increased risk to develop HCC in patients with an advanced histological stage at the diagnosis of PBC.6-14 Advanced age, male sex,4, 5, 14-16 history of blood transfusion,14, 15 smoking habit, HBV and HCV (hepatitis B and C virus) infection6 have also been associated with increased probability for HCC development in patients with PBC.15-17 The limited information and the contrasting results may be explained by the low prevalence of PBC as compared with other chronic liver diseases, and by some geographical and environmental differences as well.
In this study we analyzed two large series of patients with PBC from Barcelona and Padova with the aim of assessing the incidence of HCC, to identify risk factors for development of liver cancer, and to describe the survival as compared with the predicted survival according to the Mayo model and the observed survival in an age- and sex-matched series of HCV-related HCC.
Abbreviations
Anti-HCV, anti-hepatitis C virus antibodies; HBsAg, hepatitis B virus surface antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; PBC, primary biliary cirrhosis; UDCA, ursodeoxycholic acid.
Patients and Methods
The study was carried out in two series of patients with PBC from two European centers (Liver Unit, Digestive Diseases Institute, Hospital Clínic, IDIBAPS, University of Barcelona, Spain, and Department of Surgical and Gastroenterological Sciences, Section of Gastroenterology, University of Padova, Italy) including a total of 716 patients (389 from Barcelona and 327 patients from Padova) followed for a mean of 9.3 ± 6.8 years (9.5 ± 6 and 9.1 ± 7.7 years in Barcelona and Padova, respectively). Data on the total patient population at diagnosis are shown in Table 1. The two series were similar with respect to age, gender, and severity of cholestasis but patients from Padova had more advanced histological stage. Eighty percent of patients were treated with ursodeoxycholic acid (UDCA) (mean daily dosage: 760 ± 12 mg).
| Characteristic | N = 716 |
|---|---|
| Age (years) | 51.7 ± 0.6 |
| Female (%) | 93.2 |
| Pruritus (%) | 44.9 |
| Jaundice (%) | 15.7 |
| Ascites (%) | 6.4 |
| Encephalopathy (%) | 0.8 |
| Variceal bleeding (%) | 3.1 |
| Total bilirubin (mg/dL) | 1.5 ± 0.2 |
| ALT (U/L) | 99 ± 5 |
| Alkaline phosphatase (U/L) | 743 ± 38 |
| γ-GT (U/L) | 316 ± 15 |
| Albumin (g/L) | 40.2 ± 0.3 |
| Prothrombin index (%) | 93.8 ± 0.7 |
| AMA-positive | 91.2 |
| Histological stage (%) | |
| I | 50.8 |
| II | 20.8 |
| III | 18.7 |
| IV | 9.7 |
The diagnosis of PBC was established if the patients fulfilled two of the following criteria: alkaline phosphatase at least 1.5 times the upper normal levels, and positive antimitochondrial antibodies and compatible liver biopsy. Patients were regularly assessed every 4-6 months by clinical and analytical procedures. An abdominal ultrasonography was also performed in all the patients diagnosed after 1981 to disclose the development of HCC with time intervals of 6 months in the patients diagnosed before 1999 and at intervals ranging from 6 to 24 months depending on the histological stage in the patients evaluated after this year. Histological stage was classified according to Ludwig and Scheuer's criteria in Spain and Italy, respectively. The clinical, biochemical, immunological, and histological characteristics were also recorded when the diagnosis of HCC was established.
HCC was diagnosed by abdominal ultrasonography and confirmed by other imaging procedures (computed tomography [CT], magnetic resonance imaging [MRI], and arteriography) and histology (fine-needle aspiration cytology or liver biopsy). The following features as potential risk factors for the development of HCC were recorded: male gender, tobacco consumption, alcohol intake higher that 40 g/day, as well as the presence of hepatitis B surface antigen (HBsAg), antibodies against HCV, and advanced histological stages (stages III and IV). Age over 52 years (the median age of the series at diagnosis of PBC) was also considered a potential risk factor for HCC.
Survival free of liver transplantation was analyzed from the moment of PBC diagnosis until death or transplant. Survival curves were compared between patients who did or did not develop HCC following a cohort of patients with PBC from Barcelona who did not develop HCC. The survival after the diagnosis of HCC was also calculated and compared with the survival of a series of an age- and sex-matched population of 62 patients with HCC related to HCV cirrhosis, and with the predicted survival according to the Mayo model. The predicted survival for the first 7-year period was obtained for each patient according to the risk score at diagnosis of HCC16 and all individual curves were averaged to obtain the overall predicted survival curve.
Statistical Analysis.
Data are expressed as mean ± standard error of the mean. The chi-square test with Yates correction or Fisher's tests were used to analyze differences in discontinuous variables and Student's t test was used to compare continuous variables. The independent effect of significant variables associated with development of HCC was assessed using backward stepwise logistic regression. The criteria for entering and removing variables were ≤0.05 and ≥0.1, respectively. Survival, related to follow-up time, was analyzed using the Kaplan-Meier method and compared using the log-rank test. A value of P ≤ 0.05 was considered statistically significant.
Results
Twenty-four patients (12 female and one male from Barcelona and eight female and three males from Padova) developed HCC. The diagnosis of HCC was made in all but one patient during follow-up. In the remaining patient the tumor was diagnosed after transplantation in the explanted liver (incidental). The demographic, clinical, biochemical, and histological features of these patients with HCC at diagnosis of PBC and diagnosis of HCC are summarized in Tables 2 and 3. At diagnosis of HCC all the patients had advanced histological stage or cirrhosis (stage IV) demonstrated by a previous liver biopsy, features of severe portal hypertension such as esophageal varices, ascites, hepatic encephalopathy, imaging procedures compatible with advanced disease (abdominal CT or MRI), and examination of surgical specimens from segmental resection or liver explants after transplantation.
| Characteristic | Overall N = 24 | Barcelona N = 13 | Padova N = 11 |
|---|---|---|---|
| Age (years) | 51.6 ± 2.0 | 52.2 ± 3.1 | 50.9 ± 2.6 |
| Fatigue | 11 (45.8) | 5 (38.5) | 6 (54.5) |
| Pruritus | 10 (41.7) | 4 (30.8) | 6 (54.5) |
| Melanoderma | 2 (8.3) | 2 (15.4) | 0 (0.0) |
| Weight loss | 3 (12.5) | 2 (15.4) | 1 (9.1) |
| Jaundice | 8 (33.3) | 3 (23.1) | 5 (45.5) |
| Ascites | 4 (16.7) | 1 (7.7) | 3 (27.3) |
| Encephalopathy | 1 (4.2) | 1 (7.7) | 0 (0.0) |
| Esophageal varices | 6 (30.0) | 2 (15.4) | 4 (57.1) |
| Total bilirubin (mg/dL) | 1.5 ± 0.8 | 1.5 ± 0.8 | 1.4 ± 0.7 |
| ALT (U/L) | 102 ± 15 | 101 ± 24 | 104 ± 14 |
| Alkaline phosphatase (U/L) | 745 ± 140 | 801 ± 208 | 670 ± 185 |
| γ-GT (U/L) | 354 ± 96 | 368 ± 139 | 330 ± 122 |
| Albumin (g/L) | 39.6 ± 1.4 | 39.0 ± 2.1 | 40.5 ± 1.5 |
| Prothrombin index (%) | 89.6 ± 2.7 | 92.8 ± 2.9 | 83.2 ± 5.3 |
| Hemoglobin (g/dL) | 12.2 ± 0.4 | 11.7 ± 0.4 | 12.9 ± 0.5 |
| Leukocytes (x 109/L) | 5.5 ± 0.5 | 6.1 ± 0.7 | 4.8 ± 0.7 |
| Platelets (x 109/L) | 149 ± 17 | 160 ± 23 | 134 ± 28 |
| AMA positive | 21 (95.5) | 11 (100.0) | 10 (90.9) |
| ANA positive | 10 (47.6) | 5 (45.5) | 5 (50.0) |
| Histological stage | |||
| I | 7 (31.8) | 7 (53.8) | 0 (0.0)* |
| II | 4 (18.2) | 2 (15.4) | 2 (22.2) |
| III | 3 (13.6) | 2 (15.4) | 1 (11.1) |
| IV | 8 (36.4) | 2 (15.4) | 6 (66.7) |
| Mayo risk score | 5.4 ± 0.2 | 5.1 ± 0.2 | 5.9 ± 0.5 |
| Child-Pugh score | 5.7 ± 0.2 | 5.5 ± 0.2 | 6.2 ± 0.5 |
- Values in parentheses indicate percentage.
- * P < 0.05.
| Characteristic | Overall N = 24 | Barcelona N = 13 | Padova N = 11 |
|---|---|---|---|
| Age (years) | 65.5 ± 2.1 | 65.8 ± 3.0 | 65.0 ± 3.1 |
| Fatigue | 10 (50.0) | 5 (38.5) | 5 (71.4) |
| Pruritus | 7 (35.0) | 2 (15.4) | 5 (71.4)* |
| Melanoderma | 1 (5.0) | 1 (7.7) | 0 (0.0) |
| Weight loss | 3 (15.0) | 3 (23.1) | 0 (0.0) |
| Jaundice | 11 (55.0) | 5 (38.5) | 6 (85.7)* |
| Ascites | 12 (66.7) | 9 (69.2) | 3 (60.0) |
| Encephalopathy | 6 (31.6) | 4 (30.8) | 2 (33.3) |
| Total bilirubin (mg/dL) | 5.4 ± 6.7 | 5.2 ± 7.4 | 5.4 ± 4.9 |
| ALT (U/) | 65 ± 10 | 60 ± 11 | 73 ± 21 |
| Alkaline phosphatase (U/L) | 532 ± 105 | 652 ± 149 | 311 ± 71 |
| γ-GT (U/L) | 158 ± 41 | 199 ± 56 | 70 ± 16* |
| Albumin (g/L) | 36.1 ± 1.9 | 34.4 ± 1.9 | 39.3 ± 4.0 |
| Prothrombin index (%) | 74.3 ± 3.7 | 77.4 ± 5.0 | 69.2 ± 5.1 |
| Creatinine (mg/dL) | 0.9 ± 0.1 | 1 ± 0.1 | 0.8 ± 0.1 |
| Hemoglobin (g/dL) | 10.9 ± 0.4 | 10.8 ± 0.5 | 11.1 ± 0.7 |
| Leukocytes (× 109/L) | 5.1 ± 0.6 | 6.0 ± 0.9 | 3.6 ± 0.4 |
| Platelets (× 109/L) | 128 ± 24 | 97± 15 | 179 ± 57** |
| α-fetoprotein (ng/mL) | 634 ± 436 | 712 ± 645 (12/13) | 478 ± 315 (6/11) |
| Mayo risk score | 6.4 ± 0.5 | 6.8 ± 0.6 | 6.6 ± 0.7 |
| Child-Pugh score | 7.5 ± 0.5 | 7.7 ± 0.6 | 7.0 ± 0.9 |
| MELD score | 11.1 ± 1.6 | 11 ± 1.8 | 11.8 ± 4.1 |
- Values in parentheses indicate percentage.
- * P < 0.05; **P < 0.01.
The mean interval between the diagnosis of PBC and the development of HCC was not significantly different in the two centers (13.8 ± 1.8 years in Barcelona and 15.1 ± 2.6 years in Padova). This period was longer in patients in the early histological stages (16.2 ± 1.7 years) than in those with advanced stages III and IV (11.9 ± 2.3 years), but similar in males and females. In most cases the tumor was unifocal (46%) or with fewer than three nodules (31%), and was multinodular or diffuse in the remaining cases (23%). The largest diameter of a single nodule was 4.2 ± 2.3 cm. Portal venous thrombosis was observed in three cases. Serum α-fetoprotein levels, measured at the time of the diagnosis of HCC, were normal in eight patients and elevated in 11 patients (mean 625 ± 1756 ng/mL). No information was available in the remaining five patients.
The prevalence of HCC in PBC was similar in the two series (3.34% in Barcelona and 3.36% in Padova) with an overall prevalence of 3.35 cases per 100 patients. The incidence of HCC was 0.36 cases per 100 patient-years (0.35 and 0.37 in Barcelona and Padova, respectively). Male gender, older age (>52 years), smoking habit, alcohol intake (>40 g/day), and HBsAg and anti-HCV positivity were similar in patients with and without HCC. Patients who developed HCC had more advanced histological stage at diagnosis of PBC than those without HCC (odds ratio [OR]: 5.80, 95% confidence interval [CI] 2.34-14.38, P < 0.001) (Table 4). Logistic regression analysis identified stage 4 as the only risk factor associated with HCC. The risk factors were somewhat different in the Barcelona and Padova series, because gender risk was only observed in patients from Padova (OR: 8.09, 95% CI: 1.93-33.8, P < 0.01) (Tables 5 and 6).
| PBC-HCC N = 24 | PBC no HCC N = 692 | OR (95% CI) | P | |
|---|---|---|---|---|
| Male sex | 4 (16.7) | 45 (6.5) | 2.87 (0.94–8.77) | n.s. |
| Age ≥52 years | 12 (52.2) | 353 (51.0) | 1.04 (0.46–2.41) | n.s. |
| Smoking | 1 (4.2) | 62 (9.0) | 0.44 (0.06–3.33) | n.s. |
| Alcohol >40 g/day | 1 (4.2) | 36 (5.2) | 0.79 (0.10–6.03) | n.s. |
| HbsAg-positive | 1 (4.2) | 2 (0.3) | 15 (1.31–171) | n.s. |
| Anti-HCV–positive | 3 (12.5) | 23 (3.3) | 4.15 (1.15–14.94) | n.s. |
| Stage III-IV | 11 (50.0) | 193 (27.9) | 2.58 (1.10–6.06) | 0.04 |
| Stage IV | 8 (36.4) | 62 (9.0) | 5.80 (2.34–14.38) | 0.0001 |
- Values in parentheses indicate percentage; n.s., not significant.
| Characterstic | PBC-HCC N = 11 | PBC no HCC N = 316 | OR (95% CI) | P |
|---|---|---|---|---|
| Male sex | 3 (27.3) | 14 (4.4) | 8.09 (1.93–33.8) | 0.008 |
| Age ≥52 years | 5 (50.0) | 174 (55.1) | 0.81 (0.23–2.87) | n.s. |
| Smoking | 1 (9.1) | 42 (13.2) | 0.65 (0.08–5.23) | n.s. |
| Alcohol >40 g/d | 1 (7.7) | 32 (10.1) | 0.88 (0.11–7.16) | n.s. |
| HbsAg-positive | 1 (9.1) | 2 (0.6) | 15.70 (1.31–187.9) | n.s. |
| Anti-HCV–positive | 2 (18.2) | 17 (5.4) | 3.90 (0.78–19.52) | n.s. |
| Stage III-IV | 7 (77.8) | 119 (37.7) | 5.79 (1.18–28.36) | 0.03 |
| Stage IV | 6 (66.7) | 35 (11.1) | 16.62 (3.98–69.48) | < 0.001 |
- Values in parentheses indicate percentage; n.s., not significant.
| Characteristic | PBC-HCC N = 13 | PBC no HCC N = 376 | OR (95% CI) | P |
|---|---|---|---|---|
| Male sex | 1 (7.7) | 31 (8.2) | 0.92 (0.11–7.37) | n.s. |
| Age ≥52 years | 7 (53.8) | 179 (47.6) | 1.28 (0.42–3.89) | n.s. |
| Smoking | 0 (0.0) | 20 (5.3) | 0.64 (0.03–11.22) | n.s. |
| Alcohol >40 g/day | 0 (0.0) | 4 (1.1) | 3.06 (0.15–59.90) | n.s. |
| HBsAg-positive | 0 (0.0) | 0 (0.0) | NA | |
| Anti-HCV–positive | 1 (7.6) | 6 (1.6) | 5.13 (0.57–46.11) | n.s. |
| Stage III-IV | 4 (30.8) | 74 (19.7) | 1.81 (0.54–6.05) | n.s. |
| Stage IV | 2 (15.4) | 27 (7.2) | 2.35 (0.49–11.15) | n.s. |
- NA, not applicable; n.s., not significant. Values in parentheses indicate percentage.
Nineteen patients (79.8%) were treated with UDCA before the development of HCC, and the mean interval between diagnosis of PBC and the time of starting UDCA treatment was 10 ± 1.2 years. Four patients received UDCA for 1 year, six patients for 5 to 10 years, and three patients for more than 10 years. No data on the duration of UDCA treatment were available in the remaining six patients. The mean daily dosage of UDCA was 800 ± 24 mg. Treatment with UDCA was similar in patients with and without HCC (79.2 and 79.9%, respectively).
Patients with HCC had significantly shorter cumulative survival free of transplantation as compared with those without HCC (P = 0.004) (Fig. 1). Thirteen patients were not submitted to any specific therapy for HCC (54.2%), two patients were treated with tamoxifen, four with locoregional procedures (percutaneous ethanol injection or transarterial chemoembolization), two with surgical resection, and three patients with liver transplantation. The median survival of the patients after the diagnosis of HCC was 36 months (range 9.9-62.1 months). In patients with a single nodule the median survival was significantly higher than those with more than one nodule (P < 0.05). The survival of patients with PBC and HCC was not significantly different from the survival of the age- and sex-matched HCV-related HCC, because of the good survival observed in transplanted patients. However, when excluding the transplanted patients the probability of survival was significantly different in patients with PBC than in those with HCV-related HCC (P < 0.001) (Fig. 2). In these patients the actual survival was significantly lower than that predicted by the Mayo model (Fig. 3).
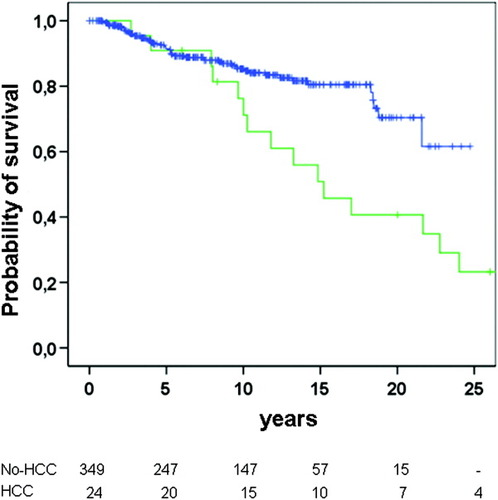
Probability of survival free of transplantation in patients with PBC according to the development of hepatocellular carcinoma (green line: HCC; blue line: no HCC) (P < 0.001).
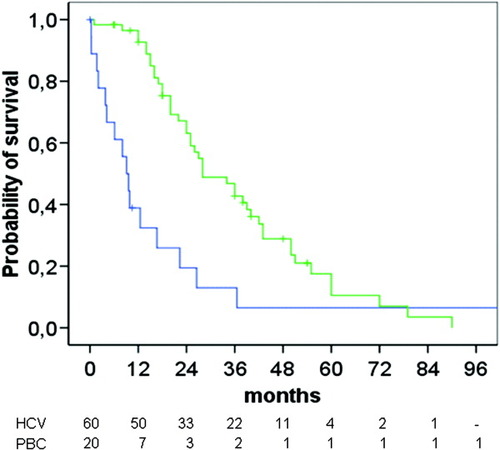
Survival of the patients (PBC) after developing HCC (blue line) as compared with the survival of patients with HCC of HCV etiology (green line). (P = 0.001).
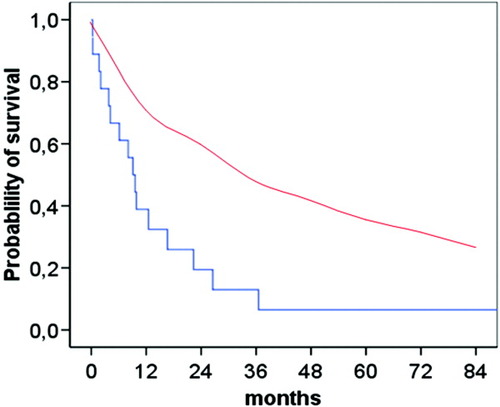
Survival of the patients after developing HCC (blue line) as compared with the predicted survival of the patients estimated by the Mayo model (red line). Log-rank test P < 0.001.
Discussion
The uncertain data on the prevalence and incidence of HCC in PBC results from the few studies performed over the years undertaken to analyze the development of liver cancer in patients with this chronic cholestatic disease.3, 6-14 Actually, the first case reports of primary liver cancer in PBC were published in 1979.7 In this and other early reports, cancer development in PBC was considered very unusual and mainly observed in males, so it was concluded that gender was a risk factor for HCC in this disease.5, 7, 18 Since then, other studies have been published,6, 8, 17, 19-21 most of them lacking in sufficient long-term follow-up and, as a consequence, decreasing the probability of cancer development, or reported as case-control studies.10 Moreover, the relatively low number of patients included in some studies is another compounding factor as well as the geographical influence.
To our knowledge, this long-term follow-up study carried out in two referral European centers for PBC in Spain and Italy assembles the largest series of patients with PBC evaluated for the longest period of time in order to detect the potential risk factors for primary liver cancer in PBC. A further objective of the present study was to compare the similarities and differences observed in these two series to disclose the real incidence and the risk factors associated with HCC, which can be overwhelmed when studying rather small cohorts of patients. The two series were equivalent, not only in terms of number of patients evaluated, but also regarding the severity of the disease and the follow-up period, which were alike. This similarity resulted in a comparable prevalence and incidence of HCC carcinoma in Spain and Italy, which, on the other hand, are in the range reported in other studies performed in Western countries when analyzing lower numbers of patients and shorter periods of follow-up.4, 15 Thus, the prevalence of HCC in PBC is 3.35 cases per 100 patients, a figure that confirms earlier reported prevalences by the same groups. A higher prevalence has been observed when analyzing only patients with advanced disease.4, 17 Moreover, this is a unique study that specifically reports the incidence of HCC in PBC, being 0.35 cases per 100 person-years. This incidence is lower than that calculated from other studies, a dissimilarity that can be explained, in part, by the longer follow-up in the current report (mean: 9.8 years; range 2.3 to 30 years) as compared with shorter periods in the other studies (from 3.3 to 7.3 years).4, 10, 15, 17
Advanced histological stage was the only factor associated with a high risk for developing HCC in PBC. This is in accordance with other studies that indicated that HCC only appears in patients with endstage disease and particularly when they have cirrhosis.4, 6, 10-14 All the patients indeed had cirrhosis when the liver cancer was diagnosed, as demonstrated by different procedures in addition to histological assessment. Moreover, 90% of the patients had features of portal hypertension such as ascites, variceal upper gastrointestinal bleeding, or hepatic encephalopathy, thus indicating the fact that HCC develops in patients with advanced disease. However, we failed to find any other association with development of HCC in PBC, including tobacco smoking, alcohol intake higher than 40 g/day, or the presence of B and C virus hepatitis markers.6 This seems reasonable taking into account the low number of patients with PBC exposed to tobacco smoking and moderate alcohol intake. However, our data on the markers of previous viral infection does not support earlier reports indicating that blood transfusion14, 15 and anti-HCV6 are potential risk factors for HCC in patients with PBC. The lack of influence of these markers of viral infection in the development of HCC in PBC is further reinforced by the fact that no association between the presence of HBsAg and anti-HCV antibodies was observed independently in Spain and in Italy. However, it should be pointed out that anti-HCV antibodies was a rather significant risk factor for HCC in the Italian series. These differences can be related to the variability in the prevalence of anti-HCV antibodies in the general population of these two Mediterranean countries, being much higher in Italy.22, 23
Our study also fails to demonstrate that male gender is a risk factor for HCC in PBC when considering the overall series from Italy and Spain, thus totaling the highest series published. This is opposed to results reported in other studies,4, 5, 14-16 a discrepancy that can be explained from the duration of follow-up and the number of patients evaluated. Certainly, male gender was a risk factor for HCC in the series from Italy but not in the Spanish one. Hence, this result highlights that the number of patients at risk is critical in order to obtain a valid conclusion.
Older age is another feature that has been anticipated as a risk factor for liver cancer development in PBC patients. In the overall series and when the analyses were performed separately in the Italian and in Spanish series, no effect was observed regarding age. This is in conflict with the data reported in other studies, which suggest that older age equal or greater than 70 is one of the variables entering into the model for HCC development in PBC.14-16 In this study only 6 of the 24 patients with HCC (25%) were 70 years or older, thus questioning that age is a critical feature for developing HCC. Probably what is more relevant is the duration of the disease and the progression toward cirrhosis. Thus, in our series the gap between the diagnosis of PBC and the development of HCC was 12 years, a period of time much longer than that reported in other studies.4, 17
Additional data observed in this study was the survival of patients after the diagnosis of HCC, which was lower than that observed in an age- and sex-matched control group of patients with HCC and HCV infection. It is difficult to explain the reasons for such survival variability, which apparently does not depend on the extension of the liver tumor. Perhaps it can be partially explained by the fact that most patients with PBC had very advanced disease and with features of decompensated cirrhosis. On the other hand, it should be pointed out that the mean survival of PBC and HCV patients with HCC was alike when the four liver-transplanted patients with PBC and HCC were included in the analysis of survival. Looking at the survival curves, it is evident that PBC patients die early after HCC diagnosis, but then the prognosis is good, mainly because of the long-term survival of PBC transplanted patients who were still alive 8 years after liver replacement.
Another point deserving a comment is the fact that apparently UDCA treatment has no effect on the development of HCC in PBC, because the lack of UDCA treatment was not a risk factor for HCC. Certainly, this is not accurate because there was a long period of time between the diagnosis of PBC and the beginning of UDCA treatment. Therefore, it can be suggested that the lack of UDCA treatment in these patients probably resulted in faster progression of the disease and as a consequence higher probability for developing cirrhosis, which is the only risk factor for HCC.
In conclusion, HCC can be the final event in patients with PBC. The liver cancer development mainly depends on the progression of the disease because it only occurs in patients with advanced histological stage and features of portal hypertension. No other risk factors for cancer development have been detected. Therefore, the surveillance of HCC in patients with PBC should be restricted to those with clinical features and imaging data consistent with disease progression and in those with very long follow-up.




