Implementation of an Improvement Cycle in the Compounding of Antineoplastic Therapy in a University Hospital
ABSTRACT
Background
Improving medication safety, particularly for high-risk drugs, was identified as the third Global Patient Safety Challenge by the World Health Organization in 2017. Antineoplastic agents are included in the list of high-risk medications because of their high potential to cause adverse events. This study evaluated the effectiveness of a project that was implemented to improve the antineoplastic therapy compounding process at a university hospital.
Methods
This study had a quasi-experimental before-and-after design and used a quantitative approach to investigate the effects of implementation of this project in a centralized intravenous admixture service. The compounding of antineoplastic therapy was evaluated using five quality criteria at three time points: an initial evaluation and two post-intervention re-evaluations. The results of the intervention were subjected to statistical analysis and are presented in a Pareto chart.
Results
All five quality criteria for correct compounding of antineoplastic therapy improved after implementation of the intervention, with an increase in compliance values for all criteria after the second evaluation. Improvements in criteria 1, 3, and 4 were statistically significant.
Conclusions
The implementation of the improvement project enhanced the quality criteria and caused a cultural shift in the team, with an improved view towards quality management in work processes.
Abbreviations
-
- AT
-
- antineoplastic therapy
-
- CIVAS
-
- centralized intravenous admixture service
-
- C1
-
- criterion 1
-
- C2
-
- criterion 2
-
- C3
-
- criterion 3
-
- C4
-
- criterion 4
-
- C5
-
- criterion 5
-
- SOP
-
- standard operating procedure
1 Introduction
Improving safety in the use of medicines, especially those that are high risk for adverse events, was identified by the World Health Organization as the third Global Patient Safety Challenge in its “Medication Without Harm” document published in March 2017. A medication error is defined as any avoidable event that may lead to inappropriate use of medication that has the potential to harm a patient [1]. Medication errors are common worldwide [2]. The oncology pharmacy at our institution has been striving to reduce the risk of serious and preventable medication-related harms by developing safer and more efficient systems at every step in the medication process, including prescribing, dispensing, preparation, compounding, administration, and monitoring.
Antineoplastic agents are used mainly in the treatment of malignant neoplasms. With the development of antineoplastic therapy (AT), there has been a significant increase in the survival rate of patients with malignant diseases [3]. The annual frequency of incidents related to the preparation and administration of AT is estimated to be between 2% and 5%. Therefore, there is a need to implement strategies aimed at reducing the risk of adverse events, which remains high because of the increasing complexity of health care and the requirements for greater skills and specific knowledge of the part of clinicians [4].
In a study by Ranchon et al., the pharmacy unit at a tertiary hospital in France was found to have received 6,607 prescriptions during a 12-month period corresponding to 22,138 different antineoplastic agents. In total, 449 medication errors involving 341 prescriptions were identified, which translated into an overall medication error rate of 5.2% [5]. Another study, which was performed in the oncology pharmacy at a tertiary hospital in Brazil, identified untreated health problems, incorrect administration, contraindications, prescription errors, drug interactions, incorrect dose and/or duration of AT, inadequate storage, and dispensing errors to be the main factors that interfered with the effectiveness of AT [6]. In a further study, Weingart et al. observed that although the rate of chemotherapy-related harm appeared to be lower than in comparable studies involving non-oncology patients, more research was required in patients receiving AT. Such research should characterize the nature and extent of harm and identify which interventions are best able to mitigate medication errors [7].
AT compounding is undertaken only by pharmacists with specialist expertise in oncology, requires the assistance of a pharmacy technician, and entails several steps, starting with evaluation of the prescription and ending with dispensing of the AT for administration to the patient [8]. A quality assurance system is essential for services involved in preparing AT to decrease the risk of error/harm and improve the skills of the pharmacy team. However, reports on improvement cycles are rare in the scientific literature.
The aim of this study was to evaluate the effectiveness of implementation of a program to improve AT compounding in a centralized intravenous admixture service (CIVAS) of a university hospital.
2 Methods
This study used a quasi-experiment before-and-after design with a quantitative approach to investigate the implementation of an improvement project developed in the period from September 2021 to February 2022 in a university hospital located in the city of Natal, Rio Grande do Norte, Brazil. The improvement project is a strategy involving quality evaluation and reflection on the work process, identification of a quality problem, decision of the criteria to measure the quality level, collection of the necessary data to evaluate and discuss the results, and definition of actions and quality improvements [9]. In this sense, the service recipients were the adult patients under antineoplastics drugs in the hematology and clinical oncology departments, while the providers were the team of pharmacy technicians and pharmacists.
The study followed the model and norms adopted by the Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) [10]. The research was conducted at the CIVAS of a university Hospital Pharmacy Sector in Brazil, qualified as a High Complexity Assistance Unit in Oncology. The team that works in the CIVAS comprises two pharmacists and three pharmacy technicians.
2.1 Population and Sample
Samples were selected at three time points: at the beginning of the months of September 2021, November 2021, and February 2022. The collection was performed consecutively until the desired sample size of 60 manipulated ATs prescribed for oncology patients was reached, based on feasibility for evaluation and decision-making, without requiring a precise confidence interval, at a level of ± 5%. The selection was based on a temporal criterion.
Quality improvement studies often consider smaller sample sizes as their main goal to inform managerial decision-making for health service enhancement. In this context, utilizing sample size tables to estimate a proportion, the number 60 allows for a sufficiently precise estimate within a 95% confidence interval, sufficient for decision-making, irrespective of the compliance level of the selected candidates [11].
2.2 Steps of the Study
In this study, an improvement project was implemented following six methodological steps: (1) identification and prioritization of the quality problem; (2) analysis of the problem's causes; (3) development of quality evaluation criteria; (4) initial compliance evaluation; (5) improvement intervention targeted at the most problematic criteria; and (6) quality level re-evaluation to verify the intervention's effectiveness, followed by a second re-evaluation after 6 months [12].
2.2.1 Step 1: Identifying and Prioritizing the Quality Problem
An analysis of the opportunities for improvement was performed with the professionals who worked at the CIVAS via meetings with the team, where the entire process was mapped, from the arrival of the medical prescription to care. At the end of the voting, five quality problems/opportunities for improvement related to AT were chosen: (1) improvement in the AT compounding process; (2) dispensing of oral antineoplastic drugs; (3) improvement in the safety of AT prescriptions; (4) improvement in the flow of the AT prescriptions; and (5) improvement in the communication between the professionals of the multidisciplinary team. Subsequently, the prioritization matrix was applied, an essential strategy for identifying critical points significantly impacting patients where the team could implement changes to enhance safety. This concluded that the most impactful opportunity for improvement on patient safety would be: “Improvement in the AT compounding process.”
2.2.2 Step 2: Analysis of the Causes of the Problem
Subsequently, the same team built a cause-and-effect diagram, where hypothetical causes of possible failures in the AT compounding process were identified using brainstorming techniques. The causes were grouped into four generic categories: professionals, infrastructure, work processes, and inputs.
2.2.3 Step 3: Development of Quality Evaluation Criteria
In this way, causes for possible errors in the AT compounding process were identified in meetings with the team, in which brainstorming techniques and the creation of a cause-and-effect diagram were used. The validities were analyzed, and reliability was tested through a pilot study with two independent raters (Table 1).
| Criterion | Exception | Clarifications |
|---|---|---|
| C1. The pharmacist must evaluate every medical prescription. | After evaluation, the pharmacist must stamp and sign the prescription. | |
| C2. All AT must be correctly identified. | The AT identification label must contain the following information: patient's full name without abbreviations, hospital registration, patient's location, qualitative and quantitative composition of all components, total volume, date and time of compounding, administration care (route and time of infusion), expiration date, temperature conditions for conservation and transport, and identification of the manipulator with professional board registration. The label for identification must also contain a description of the type of tubing or syringe appropriate for the administration of the drug, which must be affixed to the syringe or large-volume parenteral solution (saline) to be used for administration. |
|
| C3. All tubing must be correctly filled with the large volume parenteral solution (saline). | AT dispensed in syringe | The tubing, which is connected to the saline or glucose solution, must be filled before adding the antineoplastic drug. This activity must be performed to not leave air bubbles in the lumen of the tubing. |
| C4. All supplies must be separated by the patient before they are transferred to the compounding area. | Medications for the same patient must be separated in an individualized basket to organize the compounding process. | |
| C5. All manipulated AT must be visually inspected. | Visual inspection to verify the presence of leakage and/or foreign bodies must be performed. |
- Abbreviation: AT, antineoplastic therapy.
- Source: Own research.
The criteria were assessed in terms of the percentage of compliance by a professional who was not involved in the process. After the initial evaluation, it was possible to detect failures in the compounding process and, thereafter, to develop strategies to mitigate these failures.
Data were collected at the CIVAS using a spreadsheet on compliance with the criteria to be assessed. This was divided into three aspects. The initial evaluation took place before the intervention in September 2021 and was followed by another evaluation 1 month post-intervention in November 2021 and a final evaluation 6 months post-intervention in February 2022.
2.2.4 Steps 4 and 5: Development of the Intervention
First, we performed an initial evaluation of compliance and implemented a targeted improvement intervention for the most problematic criteria.
After the first collection of data, the CIVAS team met to discuss the results and plan the necessary interventions. The set of interventions that emerged from this meeting was distributed in the form of an affinity diagram that included the following three actions to be implemented. (1) Reorganization of work processes: a standard operating procedure (SOP) was developed for correct filling of the intravenous lines containing saline or glucose solution. The SOP was prepared by the team, validated by our institutional quality sector, implemented as a standard, and published on the hospital's intranet to address a gap in hospital protocols. Subsequently, the team was instructed to handle AT only after the pharmacist reviewed the medical prescription, even if this resulted in a delay in the start of administration. (2) Team training: based on the guidelines contained in the SOP for correct filling of the tubing, the pharmacy technicians were trained by CIVAS pharmacists for 2 h daily over 3 consecutive days. The importance of attention to performance of activities and avoiding distractions that can generate errors was underscored. (3) Acquisition of materials: Plastic baskets were used as containers to transfer materials and medications from the CIVAS antechamber to the restricted compounding area. Acquisition of a large number of baskets allowed separation of AT supplies for individual patients. After the interventions, the second data collection was performed to determine if there were any changes in the quality of AT compounding.
2.2.5 Data Analysis
The point estimate was calculated alongside a 95% confidence interval for the level of compliance with the criteria of the selected samples, determining the values of absolute and relative improvements for each criterion. Point estimation is used to determine a single value that estimates the parameter analyzed in the sample and allows conclusions to be drawn about a population based on the information from the sample.
A one-tailed hypothesis test was performed by calculating the Z-value to determine the statistical significance of the detected improvement. The null hypothesis (absence of improvement) was met when the p-value was > 0.05.
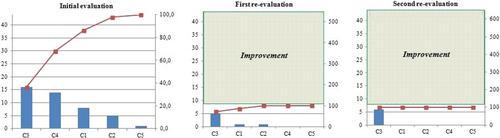
We used before-and-after Pareto charts to show the results because this method provides a complete graphic representation that facilitates prioritization of intervention strategies. In each chart, the abscissa contains bars for the various criteria evaluated from the most frequent to the least frequent for noncompliance. The left ordinate axis shows the absolute number of noncompliant cases, while the right axis shows the corresponding relative frequency calculated as a percentage associated with the total number of noncompliant cases in the evaluation [12].
Several strategies were used to control for confounding factors, including narrowing of the study sample based on well-defined and established inclusion criteria. The researcher also ensured the collection of complete and accurate data; to reduce subjectivity, quality issues were identified and prioritized in consultation with the health care team working at the study site.
3 Results
The results of the initial evaluation (before the intervention, September 2021), the first re-evaluation (post-intervention, November 2021), and the second re-evaluation (post-intervention, February 2022) are presented below.
3.1 Initial Evaluation and Re-Evaluations After the Intervention
Among the 60 ATs evaluated, the criteria that showed the lowest compliance were C3 (filling the tubing), C4 (supplies separated by the patient), and C1 (medical prescription evaluated). By observing the results in Table 2, it is possible to infer that there was a significant improvement in compliance with the criteria assessed when comparing the results pre- and post-intervention. By applying the calculation of Z for a 5% statistical significance, a statistical difference was identified between the evaluations in criteria C1, C3, and C4. A greater compliance with these criteria was observed in the second and third evaluations as compared to the initial evaluation. Regarding criteria C2 and C5, although these showed greater compliance in the re-evaluations, this difference was not statistically significant.
| Initial evaluation % (n) | First re-evaluation% (n) | Second re-evaluation% (n) | |||
|---|---|---|---|---|---|
| Criterion | Fulfills | Fulfills | Fulfills | Absolute improvement % | p-value |
| C1 | 87 (52) | 98 (59) | 100 (60) | 13 | 0.012 |
| C2 | 92 (55) | 98 (59) | 100 (60) | 8 | 0.067 |
| C3 | 73 (44) | 92 (55) | 90 (54) | 17 | 0.003 |
| C4 | 77 (46) | 100 (60) | 100 (60) | 23 | < 0.001 |
| C5 | 98 (59) | 100 (60) | 100 (60) | 2 | NS* |
| Total | 100 (60) | 100 (60) | 100 (60) |
- * NS, not statistically significant.
3.2 Opportunities for Improvement
When evaluating the opportunities for improvement using the Pareto chart (Figure 1), we observed that the C3, C4, and C1 criteria were the most problematic after the first evaluation, accounting for 86.4% of all detected failures. Furthermore, 44 instances of noncompliance were recorded during the initial evaluation, seven of which persisted at the time of the first re-evaluation and six at the time of the second re-evaluation, indicating resolution of 37 deficiencies.
3.3 Unexpected Beneficial Effects
During the study, we also addressed other important safety and quality issues in our service. These included affixing a red label marked “High surveillance” on all antineoplastic agents that were handled, labeling syringes used in AT compounding with the name of the agent to prevent mix-ups, and modifying the labeling of drugs with similar spellings or sounds, such as using capital letters and bold font to differentiate agents with similar names, for example, CARBOplatin and CISplatin.
4 Discussion
Overall, the results of this study demonstrate an improvement in an AT compounding service. These interventions required low financial cost but provided significant improvement in the compounding quality and could be easily incorporated into the service routine.
Regarding criterion C1 namely “every medical prescription must be evaluated by the pharmacist,” we determined that an oncology-qualified pharmacist's review and validation of the AT is crucial to manage medication risks, by detecting, solving, and preventing drug therapy issues. This approach enables the pharmacist to guarantee treatment safety and efficacy, minimize financial loss, and lessen errors in AT preparation [13]. The participation of a multi-professional team, in which professionals work together to ensure safe assistance to the patient, is of paramount importance [14].
Although prescription errors are well documented in the literature, a study performed at a hospital in southern Brazil demonstrated the vulnerability of the prescribing system, even when computerized, and the importance of safeguards and organized processes to avoid errors [15], underscoring the importance of evaluation of all prescriptions by a pharmacist with specialist expertise in oncologic medication.
A 10-week observational study at a university hospital in France, which assessed the clinical impact of pharmaceutical interventions based on analysis of prescriptions for injectable antineoplastic agents by pharmacists, demonstrated that the interventions, mostly aimed at recommending dosage adjustments, showed potential to prevent prolonged hospitalizations, permanent disabilities, and various neurological, hematological, renal, gastrointestinal, and cutaneous toxicities [16].
These findings reinforce the importance of pharmaceutical evaluation in the prescribing process, evidencing its clinical significance in ensuring safer and more effective treatments for cancer patients.
Regarding criterion C2, “all AT must be correctly identified,” the compliance rate in the initial evaluation was 92%. Although there was no direct intervention on this criterion, the rates in the subsequent re-evaluations rose to 98% and 100%, respectively. It is advised to identify the drug name, dosage, volume, route of administration, and start and end date [4]. Beyond identifying the drug used in AT, it is essential to verify the patient's identity, check for allergies, assess the drug's toxicity profile, determine whether any medications can mitigate potential complications, and monitor the patient before, during, and after administration of AT [4].
In terms of criterion C3, specifically “all tubing must be correctly filled with a large volume of saline solution,” air bubbles in the lumen of the infusion line, depending on their size, can prevent administration of medication. This may lead nurses to open the infusion system in an attempt to address the issue, causing spillage of AT and occupational exposure of staff members to these agents [17]. A study conducted in a chemotherapy service at a university hospital in France detected several errors during AT compounding, one of the most common being inadequate filling of the infusion line [18].
Regarding criterion C4, “all supplies be separated by the patient before being transferred to the compounding area,” an initial evaluation identified a compliance rate of 77%. After the acquisition of more baskets for the separation of supplies, compliance was 100% in both re-evaluations. Moreover, these baskets should be monitored for supply separation, infusion bag surfaces, gloves, and shelves for storage of medications to ensure the safety of health professionals who manipulate the AT [19].
Criterion C5, stating “all manipulated AT must be visually inspected,” already demonstrated high compliance initially. Although no intervention was performed on this criterion, compliance in subsequent evaluations reached 100%. Thus, ensuring correct AT identification, inspecting the therapy before infusion, and checking the medication's appearance is crucial [4].
Additionally, we wish to emphasize the importance of informing the patient and companion about the therapy. Educational materials can be developed to include information on diagnosis, treatment objectives, expected treatment duration, administration scheme, drug interactions, both rare and common AE, the potential impact of AT on sexuality and fertility, guidance on when to contact the health team or seek immediate care, and follow-up plans [14].
The improvements identified in this study have the potential to benefit other healthcare facilities by serving as a model for improving AT compounding services. The low-cost interventions and their adaptability to different operational contexts make them highly replicable for other healthcare services. By implementing similar interventions, facilities can improve patient safety, optimize resource utilization, and standardize processes, contributing to higher quality of care.
This study had some limitations. First, the investigation and application of the intervention were only at the research site; therefore, these results may only reflect a local phenomenon, affecting the interpretation and generalizability of the results. Another limitation is the performance of two evaluation moments to monitor the improvement the criteria. Several published studies provide data on the incidence of errors in chemotherapy. However, it is difficult to compare these data due to the variety of study designs and methods and the diversity of the parameters used.
5 Conclusions
This study demonstrated an improvement in all five criteria of quality in the AT compounding after the implementation of interventions, with an increase in the values of compliance with all criteria after the second evaluation. Statistical significance was observed in the criteria of medical prescription evaluated by the pharmacist (C1), filling of the tubing (C3), and supplies separated by the patient (C4).
The implementation of the improvement project was considered effective as it triggered improvements in AT compounding. Finally, we conclude that multifaceted interventions can bring about positive changes in the quality of AT compounding and that periodic monitoring is required to maintain the level of quality achieved and to perform new interventions when appropriate.
Author Contributions
Luciana Moreira Dantas Barreto: conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Joyce Karolayne dos Santos Dantas: data curation (equal); formal analysis (equal); investigation (equal); writing – original draft (equal). Tâmara Taynah Medeiros da Silva: investigation (equal); supervision (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Louise Constancia de Melo Alves Silva: supervision (equal); validation (equal); visualization (equal); writing – original draft (equal). Kauanny Vitoria Gurgel dos Santos: methodology (equal); validation (equal); visualization (equal); writing – original draft (equal). Laura Lima Souza: visualization (equal); writing – original draft (equal). Daniele Vieira Dantas: conceptualization (equal); methodology (equal); project administration (equal); writing – original draft (equal). Rodrigo Assis Neves Dantas: conceptualization (equal); methodology (equal); project administration (equal); resources (equal); supervision (equal); validation (equal); visualization (equal); writing – review and editing (equal).
Acknowledgments
The authors have nothing to report.
Ethics Statement
The study protocol was approved by the Research Ethics Committee of the Federal University of Rio Grande do Norte under the Opinion and Certificate of Ethical Appreciation (49005521.8.0000.5292) and performed in accordance with the ethical guidelines stated in the Declaration of Helsinki. Service staff were informed of the research aims, method of data collection, and benefits and risks.
Consent
Consent was obtained from all study participants for the use and storage of their data. Participants were also assured that their data would be treated confidentially and anonymized.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
This study has been entered in the Kaggle repository (https://doi.org/10.34740/kaggle/dsv/7343811). Data is available on request from the authors.




