Potentiation of cannabinoid signaling in microglia by adenosine A2A receptor antagonists
Funding information: Alzheimer's Association Research Fellowship to Promote Diversity (AARF-D), Grant/Award Number: AARFD-17-503612; Ministerio de Ciencia, Innovación y Universidades (MINECO), Grant/Award Numbers: SAF2017-84117-R, SAF2016-80027-R
Abstract
Neuroprotective M2-skewed microglia appear as promising to alter the course of neurodegenerative diseases and G protein-coupled receptors (GPCRs) are potential targets to achieve such microglial polarization. A common feature of adenosine A2A (A2AR) and cannabinoid CB2 (CB2R) GPCRs in microglia is that their expression is upregulated in Alzheimer's disease (AD). On the one hand, CB2R seems a target for neuroprotection, delaying neurodegenerative processes like those associated to AD or Parkinson's diseases. A2AR antagonists reduce amyloid burden and improve cognitive performance and memory in AD animal models. We here show a close interrelationship between these two receptors in microglia; they are able to physically interact and affect the signaling of each other, likely due to conformational changes within the A2A-CB2 receptor heteromer (A2A-CB2Het). Particularly relevant is the upregulation of A2A-CB2Het expression in samples from the APPSw,Ind AD transgenic mice model. The most relevant finding, confirmed in both heterologous cells and in primary cultures of microglia, was that blockade of A2A receptors results in increased CB2R-mediated signaling. This heteromer-specific feature suggests that A2AR antagonists would potentiate, via microglia, the neuroprotective action of endocannabinoids with implications for AD therapy.
Abbreviations
-
- 2-AG
-
- 2-Arachidonoyl glycerol
-
- A2A-CB2Het
-
- A2A-CB2 receptor heteromer (or A2A-CB2 heteroreceptor complex)
-
- A2AR
-
- Adenosine A2A receptor
-
- AD
-
- Alzheimer's disease
-
- APP
-
- Amyloid precursor protein
-
- BRET
-
- Bioluminescence resonance energy transfer
-
- CB2R
-
- Cannabinoid CB2 receptor
-
- DMEM
-
- Dulbecco's modified Eagle's medium
-
- ERK
-
- Extracellular signal-related kinase
-
- GABA
-
- γ-Aminobutyric acid
-
- GPCR
-
- G-protein-coupled receptor
-
- IFN-γ
-
- Interferon γ
-
- LPS
-
- Lipopolysaccharide
-
- M0
-
- Resting microglia
-
- M1
-
- Proinflammatory microglia
-
- M2
-
- Neuroprotective microglia
-
- MAPK
-
- Mitogen-activated protein kinase
-
- NOS
-
- Nitric oxide synthase
-
- PEI
-
- Poly-ethylenimine
-
- PS
-
- Presenilin
-
- Rluc
-
- Renilla luciferase
-
- Δ9-THC
-
- Δ9-Tetrahydrocannabinol
-
- YFP
-
- Yellow fluorescent protein
1 INTRODUCTION
The presence of phagocytic cells in pathological structures of brain from Alzheimer's disease (AD) patients was already reported by Alois Alzheimer and colleagues in 1911 (Alzheimer, 1911; Alzheimer, 1991; Alzheimer, Förstl, & Levy, 1991; Stelzmann, Norman Schnitzlein, Reed Murtagh, & Murtagh, 1995). These scientists described them as CNS resident cells, that is, microglia, and not infiltrated macrophages. Recent reviews provide data on microglia involvement in the disease from results using post-mortem human samples and transgenic models of the disease (Katsumoto, Takeuchi, Takahashi, & Tanaka, 2018; Sfera et al., 2018). On the one hand, phenotypically different microglia appear at different stages of the disease and, on the other hand, it is often assumed that AD-related microglia is proinflammatory and, therefore, detrimental for the patient. Notwithstanding, microglia can be neuroprotective and, two -at least- phenotypes arise from activation of resting (or M0) cells: M1 or proinflammatory and M2 or neuroprotective. In addition, evidence exists suggesting that proinflammatory microglia can shift phenotype to end up being neuroprotective (see (Franco & Fernández-Suárez, 2015) for review). High hopes rely on the therapeutic potential of pharmacological interventions aiming at converting reactive microglia into neuroprotective cells. As in therapeutic interventions for many diseases, G protein-coupled receptors (GPCRs) appear as potential targets to skew microglial phenotype. Recent results suggest an important potential of microglial adenosine receptors in neuroprotection because expression of CD73, the ectoenzyme that produces extracellular adenosine, attenuates inflammation by promoting M2 polarization (Xu et al., 2018). Among the four subtypes of adenosine receptors, the A2A is the most promising in neuroprotection (see (Franco & Navarro, 2018) for review). Moreover, A2A receptor (A2AR) antagonists are very safe (Hauser et al., 2014; Kondo et al., 2015) and one of them, istradefylline, has been approved in Japan as adjunctive treatment in Parkinson's disease (Jenner, 2014; Jenner et al., 2009; Kondo et al., 2015; Mizuno & Kondo, 2013; Saki, Yamada, Koshimura, Sasaki, & Kanda, 2013). It is also noteworthy that A2AR antagonists afford benefits in the transgenic APPswe/PS1dE9 AD model (Faivre et al., 2018). For historical reasons related to overexpression in striatal neurons to regulate dopamine neurotransmission, studies on this receptor have focused much more on neurons than in glia.
One of the aims of this paper was to assess in microglia the role of A2A receptors, whose expression is upregulated in activated cells. In fact, in studies performed using post-mortem brains, we discovered that the A2AR is upregulated in microglia surrounding pathological hallmarks of AD (Angulo et al., 2003). Another receptor that may convey neuroprotective effects is the cannabinoid CB2 receptor (CB2R), which in the CNS is mostly expressed in glial cells. CB2R is upregulated in prefrontal cortex of AD patients and the increased expression correlates with relevant AD molecular markers, senile plaque score and Aß1-42 levels (Solas, Francis, Franco, & Ramirez, 2013). More recently, we have reported that microglia from a transgenic AD model has a similar phenotype as that found in primary microglial cultures (from wild type mice) activated by a combination of lipopolysaccharide and interferon γ (IFN-γ). Interestingly, one of the common features was an increased expression of the CB2R (Navarro et al., 2018a). It should be noted that transgenic AD models do not display significant neuronal loss and that the cognitive symptoms appear late in the life of the animal. Taking into account that (a) activation of CB2R results in neuroprotection (Navarro et al., 2016; Reyes-Resina et al., 2018) and regulates M1/M2 polarization (Zhang et al., 2015) and (b) A2AR stimulation seemingly produces pro-inflammatory mediators (see (Madeira, Boia, Ambrósio, & Santiago, 2017; Rajasundaram, 2018) for review), the aim of the present paper was to look for the interactions between microglial A2AR and CB2R with the focus placed in the design of novel therapeutic approaches to combat AD.
2 MATERIALS AND METHODS
2.1 Reagents
Lipopolysaccharide (LPS) and interferon-γ (IFN-γ) were purchased from SigmaAldrich (St. Louis, MO), CGS21680, SCH58621, JWH133, SR144528, and forskolin from Tocris Bioscience (Bristol, UK) and tetrahydrocannabinol (Δ9-THC) was provided by Phytoplant SL (Córdoba, Spain).
2.2 Expression vectors
Plasmids encoding for A2ARRluc, A2ARnRluc8, A2ARcRluc8, CB2RYFP, CB2RnYFP, CB2RcYFP, and GABABRluc and dopamine D4YFP fusion proteins were used. The human version of adenosine A2A receptor cDNA without its stop codon was obtained by PCR and subcloned to Rluc-containing vector (pRLuc-N1; PerkinElmer, Wellesley, MA) and nRluc8 and cRluc8-containing vectors (nRluc-pcDNA3.1 and cRluc-pcDNA3.1, previously created in the laboratory) using sense and antisense primers harboring unique restriction sites for HindIII and BamHI. The human version of cannabinoid CB2 receptor and ghrelin GHS-R1a receptor cDNA without their stop codons were obtained by PCR and subcloned to Rluc-containing vector (pRLuc-N1; PerkinElmer, Wellesley, MA) using sense and antisense primers harboring unique restriction sites for HindIII and BamHI or subcloned to pEYFP-containing vector (pEYFP-N1; Clontech, Heidelberg, Germany) and nRluc8 and cRluc8-containing vectors (nRluc-pcDNA3.1 and cRluc-pcDNA3.1) using sense and antisense primers harboring unique restriction sites for BamHI and KpnI.
2.3 APP transgenic mouse model of Alzheimer's disease (AD)
APPSw,Ind transgenic mice (line J9; C57BL/6 background) expressing human APP695 harboring the FAD-linked Swedish (K670N/M671L) and Indiana (V717F) mutations under the PDGFβ promoter were obtained by crossing APPSw,Ind to nontransgenic (WT) mice (Mucke et al., 2000).
2.4 Cell culture and transient transfection
HEK-293T cells were grown in DMEM medium (Gibco, Paisley, Scotland, UK) supplemented with 2 mM l-glutamine, 100 U/mL penicillin/streptomycin, MEM nonessential amino acids solution (1/100) and 5% (v/v) heat inactivated fetal bovine serum (FBS) (Invitrogen, Paisley, Scotland, UK). Cells were maintained in a humid atmosphere of 5% CO2 at 37°C. Cells were transiently transfected with the PEI (PolyEthylenImine, SigmaAldrich) method as previously described (Navarro, Borroto-Escuela, Fuxe, & Franco, 2015). To prepare mice striatal primary microglial cultures, brain was removed from C57/BL6 mice between 2 and 4 days. Microglia cells were isolated as described in (Newell et al., 2015) and grown in DMEM medium supplemented with 2 mM l-glutamine, 100 U/mL penicillin/streptomycin, and 5% (v/v) heat inactivated FBS (Invitrogen).
2.5 Immunocytochemistry
HEK-293T cells or primary microglial culture cells seeded in coverslips were treated with vehicle, with 100 nM A2AR agonist CGS21680, or with 100 nM CB2R agonist JWH133 for 3 days. On day 2, 1 μM LPS plus 200 U/mL IFN-γ treatment or vehicle were added. On day 4, cells were fixed in 4% paraformaldehyde for 15 min and washed twice with PBS containing 20 mM glycine before permeabilization with PBS-glycine containing 0.2% Triton X-100 (5 min incubation). Cells were treated for 1 hr with PBS containing 1% bovine serum albumin. HEK-293T cells were labeled with mouse anti-Rluc antibody (1/100; Millipore, Darmstadt, Germany) and subsequently treated with Cy3 anti-mouse (1/200; Jackson ImmunoResearch [red]) IgG (1 hr each). Microglial cells were labeled with a goat anti-Iba1 (1/100; ab107159; Abcam), a mouse anti-iNOS (1/100; NOS2 [C-11]: sc-7,271; SCB) or a mouse anti-arginase I (1/100; 610708; BD Biosciences) antibody, and subsequently treated with Cy3-conjugated anti-goat (1/200; 705-165-147; Jackson ImmunoResearch [red]) or anti-mouse (1/200; 715–166-150; Jackson ImmunoResearch [red]) IgG secondary antibodies (1 hr each). Nuclei were stained with Hoechst (1/100; SigmaAldrich). Samples were washed several times and mounted with 30% Mowiol (Calbiochem). Samples were observed in a Leica SP2 confocal microscope (Leica Microsystems).
2.6 Bioluminescence resonance energy transfer (BRET) assays
For BRET assays, HEK-293T cells were transiently co-transfected with a constant amount of cDNA encoding for A2ARRluc or GHS-R1aRluc and with increasing amounts of cDNA corresponding to CB2YFP. For BRET with BiFLC (Bimolecular fluorescence and bioluminescence complementation), HEK-293T cells were transiently co-transfected with a constant and equal amount of cDNA encoding for A2ARnRluc and A2ARcRluc (A2ARnRluc and cRluc or nRluc and A2ARcRluc for negative controls) and with increasing and equal amounts of cDNA corresponding to CB2nYFP and CB2cYFP (nYFP and CB2cYFP or CB2nYFP and cYFP for negative controls). Forty-eight hours after transfection cells were adjusted to 20 μg of protein using a Bradford assay kit (Bio-Rad, Munich, Germany) using bovine serum albumin for standardization. To quantify protein-YFP expression, fluorescence was read in a Mithras LB 940 equipped with a high-energy xenon flash lamp, using a 10 nm bandwidth excitation filter at 485 nm reading. For BRET and BRET with BiFLC measurements, readings were collected 1 min after the addition of 5 μM coelenterazine h (Molecular Probes, Eugene, OR) using a Mithras LB 940, which allows the integration of the signals detected in the short-wavelength filter at 485 nm and the long-wavelength filter at 530 nm. To quantify protein-Rluc expression, luminescence readings were performed (using a Mithras LB 940) 10 min after 5 μM coelenterazine h addition. The net BRET is defined as [(long-wavelength emission)/(short-wavelength emission)]-Cf, where Cf corresponds to [(long-wavelength emission)/(short-wavelength emission)] for the donor construct expressed alone in the same experiment. GraphPad Prism software (San Diego, CA) was used to fit data. BRET is expressed as milli BRET units, mBU (net BRET × 1,000).
2.7 cAMP determination
Two hours before initiating the experiment, HEK-293T cells or microglia primary cell-culture medium was exchanged to serum-starved DMEM medium. Then, cells were detached, suspended in growing medium containing 50 μM zardaverine and plated in 384-well microplates (2,500 cells/well), pretreated (15 min) with the corresponding antagonists (SCH58621 for A2AR and SR144528 for CB2R) -or vehicle- and stimulated with agonists (CGS21680 for A2AR and JWH133, and Δ9-THC for CB2R) (15 min) before adding 0.5 μM forskolin or vehicle. Readings were performed after 1 h incubation at 25°. Homogeneous time-resolved fluorescence energy transfer (HTRF) measures were performed using the Lance Ultra cAMP kit (PerkinElmer, Waltham, MA). Fluorescence at 665 nm was analyzed on a PHERAstar Flagship microplate reader equipped with an HTRF optical module (BMG Lab technologies, Offenburg, Germany).
2.8 ERK phosphorylation assays
To determine ERK1/2 phosphorylation, 40,000 transfected HEK-293T cells/well or 50,000 microglial cells/well were plated in transparent Deltalab 96-well microplates and kept at the incubator for 48 hr (transfected HEK-293T cells) or 12 days (microglia culture cells). Two to four hours before initiating the experiment, the medium was substituted by serum-starved DMEM medium. Then, cells were pretreated at 25°C for 10 min with the specific antagonists (SCH58621 for A2AR and SR144528 for CB2R) or vehicle in serum-starved DMEM medium and stimulated for an additional 7 min with the specific agonists (CGS21680 for A2AR and JWH133, and Δ9-THC for CB2R) or vehicle. Cells were then washed twice with cold PBS before addition of lysis buffer (20 min treatment in constant agitation). 10 μL of each supernatant were placed in white ProxiPlate 384-well microplates and ERK 1/2 phosphorylation was determined using AlphaScreen®SureFire® kit (Perkin Elmer) following the instructions of the supplier and using an EnSpire® Multimode Plate Reader (PerkinElmer).
2.9 ß-arrestin 2 recruitment assays
Arrestin recruitment was determined as previously described (Navarro et al., 2016, 2018b, 2018c). Briefly, BRET experiments were performed in HEK-293T cells 48 hr after transfection with the cDNA corresponding to the A2AR-YFP or CB2R-YFP and 1 μg cDNA corresponding to ß-arrestin 2-Rluc in the presence of A2AR in coexpression experiments. Cells (20 μg protein) were distributed in 96-well microplates (Corning 3600, white plates with white bottom) and were incubated with specific antagonists (SCH58621 for A2AR and SR144528 for CB2R) for 15 min and stimulated with agonists (CGS21680 for A2AR and JWH133, and Δ9-THC for CB2R) for 10 min prior the addition of 5 μM coelenterazine h (Molecular Probes, Eugene, OR). After 1 min of adding coelenterazine H, BRET between ß-arrestin 2-Rluc and receptor-YFP was determined and quantified. The readings were collected using a Mithras LB-940 (Berthold Technologies, Bad Wildbad, GE) that allows the integration of the signals detected in the short-wavelength filter at 485 nm and the long-wavelength filter at 530 nm. To quantify protein-Rluc expression, luminescence readings were also performed 10 min after adding 5 μM coelenterazine h.
2.10 Dynamic mass redistribution (DMR) assays
Cell mass redistribution induced upon receptor activation were detected by illuminating the underside of a biosensor with polychromatic light and measuring the changes in the wavelength of the reflected monochromatic light that is a sensitive function of the index of refraction. The magnitude of this wavelength shift (in picometers) is directly proportional to the amount of DMR. HEK-293T cells and microglial primary cultures were seeded in 384-well sensor microplates to obtain 70–80% confluent monolayers constituted by approximately 10,000 cells per well. Previous to the assay, cells were washed twice with assay buffer (HBSS with 20 mM HEPES, pH 7.15) and incubated 2 hr with assay-buffer containing 0.1% DMSO (24°C, 30 μL/well). Hereafter, the sensor plate was scanned and a baseline optical signature was recorded for 10 min before adding 10 μL of the specific antagonists (SCH58621 for A2AR and SR144528 for CB2R) that were recoded for 30 min followed by the addition of 10 μL of specific agonists (CGS21680 for A2AR and JWH133, and Δ9-THC for CB2R); all test compounds were dissolved in assay buffer. The cell signaling signature was determined using an EnSpire® Multimode Plate Reader (PerkinElmer) by a label-free technology. Then, DMR responses were monitored for at least 5,000 s. Results were analyzed using EnSpire Workstation Software v 4.10.
2.11 In situ proximity ligation (PLA) assays
Microglia primary cultures grown on glass coverslips were fixed in 4% paraformaldehyde for 15 min, washed with PBS containing 20 mM glycine to quench the aldehyde groups and permeabilized with the same buffer containing 0.05% Triton X-100 (5 min treatment). After 1 hr incubation at 37° with blocking solution, cells were treated with specific antibodies against A2A or CB2 receptors (mouse anti-A2AR -1/100- (Millipore, Darmstadt, Germany); or rabbit anti-CB2R -1/100- (Santa Cruz Technologies)). Cells were processed using the PLA probes detecting mouse and rabbit antibodies (Duolink II PLA probe anti-Rabbit plus and Duolink II PLA probe anti-Mouse minus) and nuclei were stained with Hoechst (1/200; SigmaAldrich). Coverslips were mounted using Mowiol solution. Samples were observed in a Leica SP2 confocal microscope (Leica Microsystems, Mannheim, Germany) equipped with an apochromatic 63X oil-immersion objective (N.A. 1.4), and 405 nm and 561 nm laser lines. For each field of view a stack of two channels (one per staining) and 3 to 4 Z stacks with a step size of 1 μm were acquired. Quantification of cells containing one or more red spots versus total cells (blue nucleus) and, in cells containing spots, the ratio r (number of red spots/ cell), were determined by Duolink Image tool software.
2.12 Data analysis
The data in graphs are the mean ± SD GraphPad Prism software version 5 (San Diego, CA) was used for data fitting and statistical analysis. One- or two-way ANOVA followed by post-hoc Bonferroni test were used depending of the number of factors. Two factors were considered in the case of ligand treatments in resting or activated cells (two levels) or in the case of ligand treatments in microglia from control or transgenic mice (two levels). When pair of values were compared, the Student's t test was used. Significant differences were considered when p < .05.
3 RESULTS
3.1 A2A and CB2 receptors interact in a heterologous expression system
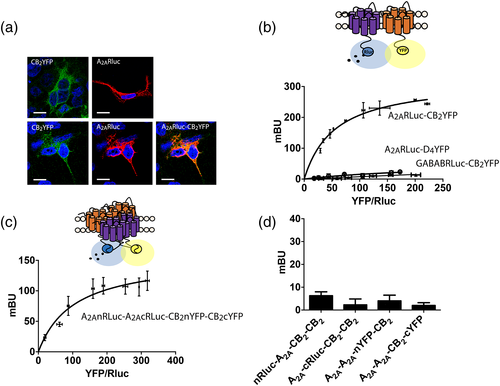
In preliminary assays we noticed that a potentially negative control for protein–protein interactions in BRET assays turned out to give a positive signal. The partner receptors were A2AR and CB2R, for which cDNAs constructs of fusion proteins containing the human full-length version and either Rluc or YFP were obtained. The cDNAs coding for - A2ARRluc and CB2R-YFP were transfected into HEK-293T cells and immunocytochemical studies were performed as indicated in Material and Methods. The results showed a high degree of colocalization; images were taken near the surface of the slide to observe a higher portion of cells' plasma membrane (Figure 1a). Confocal microscopy may suggest that receptors are close in the cell but cannot confirm receptor interactions. Accordingly, energy transfer studies were performed to establish a potential direct interaction. To achieve this objective, BRET studies were performed in cells expressing A2ARRluc and CB2RYFP. The results led to a saturable BRET curve (BRETmax of 322 ± 3 mBU and BRET50 of 55 ± 4) indicative of direct interaction, while the negative control performed by replacing A2ARRluc by GABABRluc and CB2RYFP by the dopamine D4YFP fusion proteins led to a linear unspecific signal (Figure 1b). It should be noted that BRETmax was higher than that obtained for other interacting GPCRs, something that indicates either a high level of interacting proteins or a close apposition of donor (Rluc) and acceptor (YFP) in the quaternary structure of the heteromer. There is evidence that heteromers may arrange into tetramers, and this possibility for adenosine and cannabinoid receptors was assessed by BRET combined with molecular complementation. For this purpose constructs were generated using A2AR receptors fused to either the N-terminal or the C-terminal half of the Rluc and CB2R receptors fused to either the N-terminal or the C-terminal half of the YFP. Transfection with the 4 cDNAs led to a positive and saturable BRET signal (BRETmax of 154 ± 15 mBU and BRET50 of 103 ± 9) suggesting that in the HEK-293T-based heterologous system A2A and CB2 receptors may form tetramers (Figure 1c). For negative control, assays were performed in cells expressing a combination of receptors and fusion proteins, and no BRET signal was detected in any case (see Figure 1d).
3.2 Functional characterization of A2AR-CB2R tetrameric complex in HEK-293T cells
To assess the properties of A 2A-CB 2Hets, forskolin-induced intracellular cytosolic cAMP determination, β-arrestin 2 recruitment, ERK1/2 phosphorylation and Dynamic Mass Redistribution (DMR) experiments were performed. DMR is a label-free technique used in GPCR research as it measures rearrangement of cell components (leading to mass redistribution) that is mostly mediated by G protein activation (Simon et al., 2016).
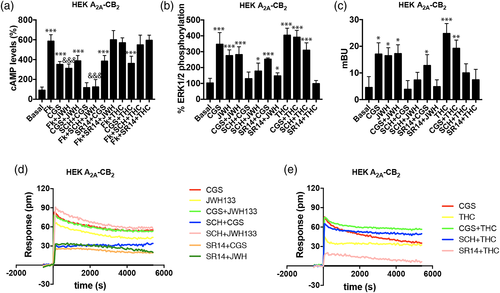
Before characterizing A2A-CB2Het functionality, the effect of receptor activation was assayed in single-transfected cells (using 0.7 μg cDNA for A2AR or 1 μg cDNA for CB2R). As expected due to Gs coupling, activation of A2AR with a selective agonist, CGS21680, led to a significant increase in [cAMP] (Figure Sup 1A). This effect was counteracted when in the same experiment cells were pretreated with a selective A2AR antagonist, SCH58621. In A2AR-expressing cells neither the CB2R selective agonist, JWH133, or antagonist, SR144528 (alone or in combination with the A2AR ligands), exerted any significant effect (Figure S1a). Similar results were obtained in MAPK signaling, β-arrestin 2 recruitment and label-free DMR assays (Figure S1b–d). In summary, A2AR-expressing cells responded only to the receptor agonist, whose effect was not blocked by CB2R ligands but by the selective A2AR antagonist.
Experiments performed in cells expressing CB2R led to similar results that is, the selective cannabinoid receptor agonist induced CB2R function was not blocked by A2AR ligands but by the CB2R antagonist, SR144528 (Figure S1f–h). Therefore, cAMP assays were consistent with canonical coupling of CB2R to Gi and, therefore, the agonist, JWH133, led to a decrease in the cytosolic levels induced by 0.5 μM forskolin activation. The effect was nullified by preincubation with SR144528 but not by treatment with A2AR ligands. (Figure S1e). When experiments on ERK1/2 phosphorylation, β-arrestin 2 recruitment and DMR were performed, JWH133 induced a significant signal that was counteracted by the antagonist SR144528 but not by the A2AR selective ligands.
A2A-CB2Het functionality was addressed in cells transfected with cDNAs for A2AR (0.5 μg) and CB2R (0.75 μg). Selective agonists, CGS21680 and JWH133, in single treatment or in combination, were used for receptor activation and cytosolic cAMP levels, ERK1/2 phosphorylation, β-arrestin 2 recruitment and DMR were determined. cAMP determination assays were consistent with Gs coupling to A2A and with Gi coupling to CB2. However, Gi coupling was negligible upon simultaneous treatment using the two agonists, that is, the signal observed was similar to that obtained using CGS21680 (Figure 2a). This result indicates that A2AR activation blocks CB2R/Gi-mediated signaling. Next, we investigated how the antagonist of the A2AR, SCH58621, blocked agonist's effect. SCH58621 indeed blocked the effect of CGS21680 but, remarkably, it did potentiate the Gi-mediated effect elicited by JWH133. What these results suggest is that binding of SCH58621 to the A2AR potentiates CB2 receptor signaling in the A2A-CB2Het context. When analyzing the effect of CB2R antagonist SR144528, we observed a blockade of the JWH133-induced signal and no effect on CGS21680 action. Similar results were observed when DMR, that is mostly mediated by G protein-dependent pathways, was measured. Costimulation with both agonists induced an effect similar to that achieved by CGS21680, thus indicating a blockade of A2AR over CB2R/Gi-mediated actions. Consistently, the A2AR antagonist produced a potentiation in CB2R signaling (Figure 2d). To determine whether this heteromer print also holds for other signaling outputs, ERK1/2 phosphorylation and β-arrestin recruitment were assayed. Interestingly, pretreatment with the A2AR antagonist blocked the CGS21680 induced effect, but did not potentiate the cannabinoid signaling (Figure 2b,c). Thus, A2AR antagonists are increasing CB2R signaling via inhibition the adenylyl cyclase and in DMR, but not in other signal transduction pathways in which a partial cross-antagonism was detected. In both pERK1/2 and β-arrestin 2 recruitment determinations, treatment with both agonists led to a lack of synergism or additive effects (comparing with data obtained in single-agonist treatments).
3.3 Effect of the main psychoactive component of Cannabis sativa, Δ9-THC, on A2A-CB2Het functionality
The two main endocannabinoids, which act via cannabinoid receptors, are 2-arachidonoyl glycerol (2-AG) and anandamide. We selected the psychoactive component of Cannabis sativa, Δ9-THC, to test its effect when it activates the CB2R in an A2A-CB2Het context. Δ9-THC at high concentrations interacts with GPR55 but this receptor is not expressed in significant amount in untransfected HEK-293T cells (Balenga et al., 2014; Moreno et al., 2014). The effect of the natural compound was first tested in CB2R-expressing cells and the results were fully consistent with those previously reported (Navarro, Borroto-Escuela, et al., 2018a). Δ9-THC treatment over A2AR and CB2R coexpressing cells showed non-significant effect on Gi-adenylyl cyclase signaling (Figure 2a). To demonstrate if treatment with A2AR antagonists did potentiate the Δ9-THC-induced signal, our first attempt was using the label-free DMR technique. Figure 2e shows that A2A-CB2Het-expressing cells responded to Δ9-THC and that the signal was blocked by CB2R antagonist but potentiated by the A2AR antagonist. Moreover, costimulation of cells with Δ9-THC and CGS21680 produced a similar effect than that observed in single CGS21680 treatment. A similar approach, but analyzing pERK levels or β-arrestin recruitment, showed that simultaneous treatment with Δ9-THC and the A2AR agonist did not result in any synergic or additive effect and that Δ9-THC-induced effect was partially blocked by the A2AR antagonist (partial cross-antagonism).
3.4 Occurrence and functionality of A2A-CB2Hets in primary cultures of activated microglia
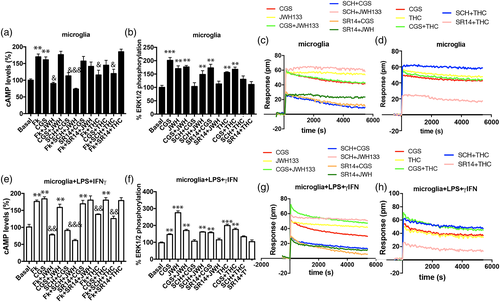
Since microglial activation leads to upregulation of CB2 and A2A receptor expression (Carlisle, Marciano-Cabral, Staab, Ludwick, & Cabral, 2002; Concannon, Okine, Finn, & Dowd, 2015, 2016; Correa et al., 2010; Gyoneva et al., 2014; Madeira, Rashid, Ambrósio, Santiago, & Langmann, 2018; Maresz, Carrier, Ponomarev, Hillard, & Dittel, 2005; Mecha, Carrillo-Salinas, Feliú, Mestre, & Guaza, 2016; van der Putten et al., 2009), our next aim was to identify the heteromer in microglia and any differential expression of A2A-CB2Hets in resting versus activated microglial cells. In resting microglia the different signal transduction read-outs led to results that were similar to those found in cotransfected HEK-293T cells. By measuring cytosolic cAMP levels (Figure 3a), ERK1/2 phosphorylation (Figure 3b) and DMR (Figure 3c,d) we observed that both A2AR and CB2R agonists produced a characteristic signal and a non-additive effect when used in combination; the signal observed was similar to that obtained with CGS21680. The A2AR antagonist enhanced CB2R signaling via Gi (cAMP determinations and DMR read-outs), and a partial cross-antagonism was detectable in ERK1/2 phosphorylation. On the other hand, the CB2R antagonist had no effect over A2AR signaling when measuring cAMP levels and MAPK phosphorylation but decreased DMR label-free readings. Interestingly, Δ9-THC treatment induced a significant decrease in cAMP levels. Taking into account the data obtained in cells only expressing the CB2R, it is likely that this effect is derived from Δ9-THC stimulation of cannabinoid CB1 receptors present in these cells (Navarro, Borroto-Escuela, et al., 2018a). However, in ERK1/2 phosphorylation and DMR techniques, coestimulation showed a non-additive effect. Moreover, while the A2AR antagonists potentiated Δ9-THC effect on DMR, SCH58621 partially blocked MAPK pathway activation. Off target effects due to expression of proteins able to be affected by THC such as GPR55, GPR18 or peroxisome proliferator-activated receptor gamma cannot be ruled out.
To analyze the A2A-CB2 heteroreceptor complex formation and function in activated microglia, primary cultures were treated for 48 hr with 1 μM LPS and 200 U/mL IFN-γ. The phenotype of activated microglia was assessed by immunocytochemical detection of a microglia marker, ionized calcium binding adaptor molecule 1 (Iba-1) and of M1 and M2 markers, respectively, arginase 1 (Arg-1) and inducible nitric oxide synthase (iNOS). The results demonstrated an important increase in iNOS and Arg-1 fluorescence signal when cultures were previously treated with CGS21680, while a small non-significant decrease in Iba-1 fluorescence was observed by JWH133 pretreatment (Figure 4b–e). No differences in cell shape due to the different treatments were observed upon using a maker or membranes (AlexaFluor 488-conjugated wheat germ agglutinin). These results suggest that A2AR activation is proinflammatory and probably detrimental for microglial survival and, in contrast, that CB2R agonists exert a protective action. Cell survival was analyzed by estimating cell death in activated cells treated with A2AR or CB2R ligands. Cell death due to LPS plus IFN-γ treatment (circa 40%) was slightly increased when cells were treated with the A2AR agonist and significantly decreased when the CB2R agonist was used (Figure 4a). These results indicate a protective role of CB2R activation on survival of activated microglial cells.
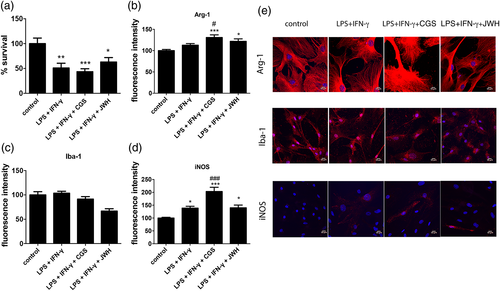
Results of cAMP determination obtained in microglia treated with LPS plus IFN-γ, showed increases upon treatment with the A2AR agonist whereas CB2R activation by JWH133 engaged Gi in forskolin-treated cells, being the effect higher than that obtained in resting cells (Figure 3e). A2AR activation blocked CB2R/Gi signaling since cells treated with both agonists responded as cells treated with CGS21680. The heteromer print was also detectable in activated cells. In fact, pretreatment with the A2AR antagonist blocked A2AR-mediated signal while it potentiated CB2R-mediated signal using JWH133. The CB2R antagonist blocked CB2R function but had no effect over A2AR activation (similar results were obtained in resting cells).
DMR results (Figure 3g,h) were similar to those obtained in cAMP determination assays, but a lack of synergy/additive effect was observed upon determination of pERK levels (Figure 3f). Furthermore a partial cross-antagonism was detectable in activated cells; in fact, the A2AR antagonist partially blocked CB2R signaling towards the MAPK pathway. The results obtained in activated microglia show an increase in A2AR and CB2R signaling compared to resting cells and the occurrence of A 2A-CB 2Hets as deduced from identification of the heteromer print(s).
3.5 A2A-CB2Het expression and function in the APPSw,Ind transgenic mouse model of Alzheimer's disease
Alzheimer's disease (AD) pathological hallmarks include β-amyloid plaques and aggregates of aberrantly phosphorylated tau protein (intracellular neurofibrillary tangles). Whereas the role of reactive microglia in the brain of patients is not well understood, it is known that the expression of A2A and CB2 receptors is upregulated in the brain of patients. Such upregulation comes mainly from expression in microglial cells. We took advantage of the APPSw,Ind transgenic mouse AD model to better understand the role of A2A-CB2Hets in activated microglia. A2A-CB2Het expression was addressed in hippocampal microglia obtained from two-day-old pups that were individually cultured. Each culture was assigned to either APPSw,Ind or control after genotyping. To check for receptor complex expression, an in situ proximity ligation assay (PLA) was developed using specific antibodies against A2AR and CB2R. While only 12% of cultured cells from control animals showed red fluorescence dots, which correspond to clusters of heteroreceptor complexes, the number of cells stained in cells from APPSw,Ind transgenic animals increased to 58%. Furthermore, the number of red dots/cell significantly increased from 1.8 to 4.3 dots/cell (Figure 5a,b). These results indicate a clear increase in A2A-CB2Het expression in the microglia isolated from the APPSw,Ind transgenic animals. Consequently, we questioned about the functional implication of this complex in the first AD stages; it should be noted that young APPSw,Ind animals do not display any cognitive impairment.
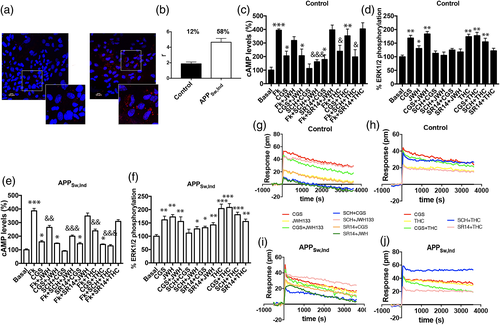
As per cAMP determination assays, A2A-CB2Het functionality when CGS21680 was used was similar in the two groups, transgenic (white bars) and controls (black bars). In contrast, the effect of the CB2R agonist, JWH133, was potentiated in samples from transgenic animals. In agreement with the features of A2A-CB2Het, coactivation produced a similar signal to that obtained by only A2AR activation (Figure 5c–e). When cells were pretreated with the selective antagonists, the heteromer print was detected; in fact, SCH58621 not only inhibited CGS21680-mediated but also potentiated JWH133-mediated signaling. This was evident in cells from control and APPSw,Ind animals. In contrast, SR144528, the CB2R antagonist, blocked JWH133- but not CGS21680-induced signaling (Figure 5c,e). These results were qualitatively similar in cAMP assays when comparing transgenic and control animals. Results concerning DMR were also similar to those here described for cAMP determination assays (Figure 5d–f).
In contrast the comparison of data from cells obtained from both types of animals led to differential finings in pERK1/2 assays. Analysis of MAPK activation in cells from transgenic mice showed a marked effect of JWH133 and a lack of additive effects upon costimulation. Again there was no potentiation of CB2R function induced by the A2AR antagonist (Figure 5g–j). Similar results were obtained when the CB2R was stimulated with Δ9-THC instead of using JWH133. Finally, the partial cross-antagonism in the pERK signal was detected in cells from transgenic and from control animals.
To sum up, the results obtained demonstrate a marked increase in CB2R-mediated effect that correlates with an increase in A2A-CB2Het expression in microglia isolated from the transgenic APPSw,Ind mice model. In this model the CB2R signaling in microglia is reduced when A2AR is activated but, importantly, potentiated via Gi coupling in the presence of A2AR antagonists.
4 DISCUSSION
Adenosine and cannabinoid receptors appeared early in evolution as they sense metabolic products to respond to basic needs of cells/organisms. Of the four adenosine receptors, the A2AR has attracted wide interest due to several factors. The receptor is found in all organs but with variable levels of expression. It is highly expressed in the striatum and for this reason it was rapidly considered a therapeutic target in Parkinson's disease. Selective A2AR antagonists are very safe and one of them, istradefylline, has already been approved for prescription to parkinsonian patients (Jenner, 2014; Jenner et al., 2009; Kondo et al., 2015; Mizuno & Kondo, 2013; Saki et al., 2013). Perspectives of A2AR antagonists are also high to potentiate immunological innate mechanisms to combat cancer (Hatfield & Sitkovsky, 2016; Ohta et al., 2012).
The results in this paper show that A2AR antagonists may be useful in neuroprotection but acting in microglia and not in neurons. The success of the first-in-class A2AR antagonist in the therapy of PD comes from work in neurons (see (Fuxe, Marcellino, Genedani, & Agnati, 2007; Strömberg, Popoli, Müller, Ferré, & Fuxe, 2000; Tanganelli et al., 2004) for review). Similarly, it is assumed that the neuroprotective potential attributed to this class of compounds is mediated by A2AR in neurons (Ferreira et al., 2015; Kaster et al., 2015; Meng et al., 2019). Preventing neuronal death is a difficult issue to be addressed in humans (Franco & Navarro, 2018) and in fact, istradefylline was approved to address symptoms but not to modify the course of PD (Kondo et al., 2015; Mizuno & Kondo, 2013; Navarro et al., 2015; Saki et al., 2013). Glial, and particularly, microglial GPCRs may be instrumental to afford neuroprotection in neurodegenerative diseases. It has been recently proposed that A2AR antagonists could also act on neurons to counteract neurodegeneration in Parkinson's disease by interfering with potentiation by agonists of fibrillar alpha-synuclein accumulation in A2AR-rich dorsal striato-pallidal GABA neurons. The fibrillar alpha-synuclein may then reach the surrounding vulnerable dopaminergic terminals via extracellular vesicle mediated volume transmission (Borroto-Escuela & Fuxe, 2019).
Of the two cannabinoid receptors, the CB1 is highly expressed in neurons although it is also present in glial cells; in contrast the expression of the CB2 receptor is much lower in the CNS than in the periphery (Alexander et al., 2017; Rico et al., 2016). Resting microglia express more CB1R than CB2R and cannabinoids help to impede cell activation. Interestingly, A2A and CB2 receptors have in common that they are upregulated upon microglial activation (Aires et al., 2019; Benito et al., 2005; Concannon et al., 2015, 2016; Fishman et al., 2009; Maresz et al., 2005; van der Putten et al., 2009). Evidence comes from in vitro assays, for instance in primary cultures of microglia from rodent brain, and also from data obtained using brain samples from patients suffering from CNS diseases coursing with neuroinflammation. As earlier commented, A2AR is present in microglia surrounding plaques in AD brains but not in microglia from nondemented age-matched control brains (Angulo et al., 2003). Upregulation of the CB2R is evident in both the brain of AD patients (Benito et al., 2003, 2008; Grünblatt et al., 2007; Ramírez, Blázquez, Gómez del Pulgar, Guzmán, & de Ceballos, 2005; Solas et al., 2013) and in cultures of microglia treated with LPS plus IFN-γ (Aso & Ferrer, 2016; Koppel et al., 2013; López et al., 2018; Mecha, Carrillo-Salinas, Feliú, Mestre, & Guaza, 2016; Navarro et al., 2018a; Savonenko et al., 2015; Wu, Hocevar, Foss, Bie, & Naguib, 2017). The results here presented show that microglia cells significantly express a functional unit constituted by A2A-CB2Hets whose expression was markedly increased in activated cells.
The direct interaction of the two receptors was confirmed in primary cultures of microglia, which displayed the A2A-CB2Het print and heteromer clusters detected by PLA. One of the often-found heteromer prints, the cross-antagonism, was unidirectional (antagonist of A2AR blocked the functionality of the CB2R ) and not detected in all analyzed signals. In addition, a new heteromer print, not properly described for any other heteromer, consisted of antagonist-mediated potentiation of the Gi-mediated signal arising at the partner receptor. The results were consistent and similar in both the heterologous system and in activated cells from primary cultures. Indeed, blockade of the A2AR resulted in higher Gi-mediated coupling of the CB2R. This result contrasts with that obtained in heteromers formed by A2A and A1 receptors. As in the A2A-CB2Het, one of the receptors is coupled to Gs and another to Gi but it results that A2AR activation leads to blockade of Gi-coupling while antagonism releases the brake on CB2R-medaited signaling. The latter does not occur in the case of the A1-A2AHet; different heteromers coupled to Gs and Gi behave differently. It is logical that an heteromer with two receptors for a same molecule, adenosine, have antagonistic properties; in fact the A1-A2AHet is an adenosine sensor acting via Gi at low adenosine concentrations and via Gs at high concentrations of the nucleoside; the mechanism of action, recently deciphered, relies on the long intracellular C-terminal domain of the A2AR (Ciruela et al., 2006; Navarro et al., 2016, 2018).
Previously reported upregulation of A2AR and CB2 receptors in the brain of AD patients is here mimicked in the transgenic APPSw,Ind animal, where it correlates with a significant increase in the expression of A2A-CB2Het complexes and exhibition of its particular properties. Upregulation of A2A-CB2Het in activated microglia appears as detrimental as activation of adenosine receptors nullifies the benefits of CB2R activation. Then it is predicted that any neuroinflammatory response coursing with increases of adenosine, for instance in stroke or in decreased adenosine-to-ATP conversion due to reduced glucose consumption, would exacerbate neurodegeneration (Fan, Dawson, & Dawson, 2017; Stockwell, Jakova, & Cayabyab, 2017). Accordingly, our results suggest that A2AR antagonists would revert this situation, first of all because adenosine could not act on the cognate receptors and secondly, because the A2A-CB2Het print consists of increased CB2R signaling when the partner receptor is blocked. As earlier mentioned, istradefylline, the first in class drug approved for the treatment of Parkinson's disease (Jenner, 2014; Jenner et al., 2009; Kondo et al., 2015; Mizuno & Kondo, 2013; Saki et al., 2013), opens the door to the use of A2AR antagonists in other therapeutic interventions. Our results suggest that such antagonists could be useful in microglia-mediated protection of neuronal death associated to AD. It should be also noted that among the few natural receptor antagonists one is the centrally acting molecule most consumed in the world, caffeine. Importantly, caffeine is a non-selective adenosine receptor antagonist, mainly acting on A1 and A2A receptors (Ukena et al., 1986; Varani et al., 2000). Our results would predict that caffeine would be beneficial in cases of aberrant microglial activation or in cases when microglia is prone to be activated, as it would facilitate neuroprotective cannabinoid action on upregulated CB2R. Further experimental work is needed to demonstrate this hypothesis but it is worth highlighting that some epidemiological studies have found a correlation between caffeinated coffee consumption and reduced risk of suffering AD disease. Despite some initial controversy, robust prospective studies have been performed that provided reliable promising results. The “Canadian Study of Health and Aging,” which made a follow-up of the cognitive and health status of >6,000 individuals concluded that the risk of AD was reduced by physical activity and coffee and wine consumption whereas other factors/conditions (among other: smoking, stroke and heart disease) showed no association with disease risk (Lindsay et al., 2002). Coffee intake may be related to preventing neuronal death as the risk to suffer from another neurodegenerative disease such as PD is also reduced by coffee/caffeine intake (Ross et al., 2000). The hypothesis of neuroprotection mediated by A2AR blockade in microglia correlates with findings in one of the few reports in vivo addressing the role of these receptors in microglia. Interestingly, motility, investigated in the 5xFAD mouse AD model, was increased in microglia around amyloid plaques, while application of an A2AR antagonist, preladenant, reverted hypermotility (Gyoneva, Swanger, Zhang, Weinshenker, & Traynelis, 2016). Also noteworthy is the work showing that retinal death in glaucoma models can be reverted by blocking the microglial A2AR, which in turn “suppresses elevated pressure-induced inflammation, oxidative stress, and cell death in retinal cells” (Aires et al., 2019; Rodrigues-Neves et al., 2018).
It is already known that cannabinoid signaling limits exacerbated microglial activation upon acute N-methyl d-aspartate-induced injury (Eljaschewitsch et al., 2006). Interestingly, in 2007, a review considered that cannabinoids had potential in AD as they seemingly reduced amyloid-induced oxidative stress and tau phosphorylation (Campbell & Gowran, 2009). The receptors mediating such responses were not known but it was assumed that they were those expressed by activated microglia. From studies in microglia activated following stroke, CB2 is the receptor that appears as more important in regulating cell activation (Schmidt, Schäfer, Striggow, Fröhlich, & Striggow, 2012; Zarruk et al., 2012). Actually, from a therapeutic point of view, targeting CB1 has poor prospects due to the widespread distribution in brain and in both neurons and glia, to the psychotropic properties of agonists (phytocannabinoids or synthetic CB1R agonists) and also due to the side effects occurring after administration to humans of rimonabant, a selective CB1R ligand (Sam, Salem, & Ghatei, 2011). Recently it has been demonstrated that activation of CB2R by paeoniflorin, one of the major constituents of Paeonia lactiflora, regulates the polarization of microglia/macrophages in hippocampus (CA1 region) in vascular dementia models, (Luo et al., 2018)). In conclusion, our data support the view that, via microglia, A2AR antagonists may enhance the action of cannabinoids in CB2R and the final output is neuroprotective. Accordingly, microglial A2AR appear as targets to delay progression of neurodegenerative diseases. Two scenarios may be useful to afford neuroprotection (a) A2AR antagonists enhancing endocannabinoid action and (b) A2AR antagonists enhancing the action of exogenously administered cannabinoids.
ACKNOWLEDGMENTS
This work was supported by grants from the Spanish Ministry of Innovation and Competitiveness: Ref. No. SAF2017-84117-R and SAF2016-80027-R; they may include EU FEDER funds, and by the U.S. Alzheimer's Association, grant Ref. No. AARFD-17-503612.
CONFLICT OF INTEREST
Authors declare that Phytoplant Research SL provided Δ9-THC. None of the authors have shares of the company. Apart from Verónica Sánchez de Medina and Carlos Ferreiro-Vera, who work at Phytoplant Research, none of authors received any salary/stipend from the company.