GemC1 is a critical switch for neural stem cell generation in the postnatal brain
Funding information Hellenic Foundation for Research and Innovation (HFRI); National Institutes of Health, the Simons Foundation, and the March of Dimes (USA).; March of Dimes Foundation; Simons Foundation; National Institutes of Health; Hellenic Foundation for Research and Innovation; General Secretariat for Research and Technology
Abstract
The subventricular zone (SVZ) is one of two main niches where neurogenesis persists during adulthood, as it retains neural stem cells (NSCs) with self-renewal capacity and multi-lineage potency. Another critical cellular component of the niche is the population of postmitotic multiciliated ependymal cells. Both cell types are derived from radial glial cells that become specified to each lineage during embryogenesis. We show here that GemC1, encoding Geminin coiled-coil domain-containing protein 1, is associated with congenital hydrocephalus in humans and mice. Our results show that GemC1 deficiency drives cells toward a NSC phenotype, at the expense of multiciliated ependymal cell generation. The increased number of NSCs is accompanied by increased levels of proliferation and neurogenesis in the postnatal SVZ. Finally, GemC1-knockout cells display altered chromatin organization at multiple loci, further supporting a NSC identity. Together, these findings suggest that GemC1 regulates the balance between NSC generation and ependymal cell differentiation, with implications for the pathogenesis of human congenital hydrocephalus.
1 INTRODUCTION
The subventricular zone (SVZ) is the largest germinal niche in the adult mammalian brain. It is located on the walls of the lateral ventricles and contains large numbers of neural stem cells (NSCs). These primary progenitors give rise to young neurons that migrate a long-distance and integrate into the neuronal network of the olfactory bulbs (Obernier & Alvarez-Buylla, 2019). Although adult NSCs retain key epithelial properties of their progenitors, radial glial cells (Mirzadeh, Merkle, Soriano-Navarro, Garcia-Verdugo, & Alvarez-Buylla, 2008), the ventricular surface in the adult brain displays a completely different pattern, as the small apical domains of NSCs are surrounded by the large apical surfaces of multiciliated ependymal cells (Mirzadeh et al., 2008). In contrast to NSCs, ependymal cells remain post-mitotic and do not possess progenitor properties under physiological conditions (Shah et al., 2018; Spassky et al., 2005).
The maintenance of the proper cytoarchitecture of the niche is of great importance for the continued production of neurons, and its disruption has been linked with the emergence of hydrocephalus (Paez-Gonzalez et al., 2011). Hydrocephalus is characterized by the abnormal accumulation of cerebrospinal fluid (CSF) in the brain ventricles and is commonly associated with elevated intracranial pressure and can be classified into two clinical forms: congenital and acquired (Kahle, Kulkarni, Limbrick, & Warf, 2016; Rekate, Nadkarni, & Wallace, 2006; Zhang, Williams, & Rigamonti, 2006). Congenital hydrocephalus is among the most common central nervous system (CNS) disorders, affecting 1–3/1000 births (Schrander-Stumpel & Fryns, 1998; Zhang et al., 2006). Multiple animal studies have been conducted to delineate the molecular etiology of hydrocephalus over the years (Fliegauf, Benzing, & Omran, 2007), whereas less is known about the genetic causes of human hydrocephalus (Kousi & Katsanis, 2016). A recent large-scale genomics study uncovered three new genes associated with congenital hydrocephalus (TRIM71, SMARCC1, and PTCH1), each of which impacts NSC fate (Furey et al., 2018).
Both NSCs and ependymal cells are generated from embryonic radial glial cells in the developing telencephalon. Lineage tracing experiments have shown that the majority of adult NSCs' precursors are produced between embryonic days (E) 13.5 and 15.5 and remain largely quiescent until they become reactivated postnatally (Fuentealba et al., 2015; Furutachi et al., 2015). A subset of radial glial cells also gives rise to ependymal cells (Merkle, Tramontin, García-Verdugo, & Alvarez-Buylla, 2004; Spassky et al., 2005). The commitment to the ependymal lineage occurs in a similar developmental stage, when radial glial cells become post-mitotic and complete their differentiation within the first two postnatal weeks (Kyrousi et al., 2015; Kyrousi, Lalioti, Skavatsou, Lygerou, & Taraviras, 2016; Kyrousi, Lygerou, & Taraviras, 2017; Spassky et al., 2005). Nevertheless, whether adult NSCs and ependymal cells are derived from a common subpopulation of radial glial cells remains poorly characterized (Kyrousi et al., 2015; Wang, Kane, Lee, & Ahn, 2014).
We and others have formerly shown that GemC1, a member of the Geminin superfamily, is implicated in cellular differentiation events of multiciliated cells in several tissues (Arbi et al., 2016; Chong, Zhang, Zhou, & Roy, 2018; Terré et al., 2016; Zhou et al., 2015), and that it is a key regulator for ependymal lineage commitment in the CNS (Kyrousi et al., 2015, 2016, 2017). The present study demonstrates a novel mutation in the GEMC1 coiled coil domain identified in a human patient with congenital hydrocephalus. Deletion of GemC1 in mice recapitulates the human phenotype and impedes ependymal cell differentiation at the initial stages of mouse radial glial cell specification. Importantly, deletion of GemC1 affects the cytoarchitecture of the SVZ niche by promoting NSC characteristics. Of note, cells lacking GemC1 display an epigenetic profile which shares significant similarity with adult NSCs in the SVZ. These findings suggest that GemC1 is a key player for the generation of NSCs and ependymal cells, while providing novel molecular insight into the pathogenesis of congenital hydrocephalus in humans.
2 MATERIALS AND METHODS
2.1 Mice knock-out strains
Inactivation of GemC1 was accomplished by the insertion of a lacZ-neo cassette between exons 2 and 3 of the GemC1 gene, as described previously (Arbi et al., 2016). Specifically, a “knockout-first” strategy that ablates gene function by the insertion of RNA processing signals without the deletion of any of the target genes was followed. The mouse line carrying the knockout (KO) allele of the GemC1 gene was generated by the trans-NIH Knock-Out Mouse Project (KOMP) and obtained from the KOMP repository. Heterozygous GemC1KO/WT or wild-type littermates were used as controls. Conditional inactivation of GemC1 was carried out using a mouse line in which exons 3 and 4 of the GEMC1 gene are flanked by loxP sites (GemC1Fl/Fl), crossed with GemC1Fl/WT;NestinCre mice. To generate GemC1 floxed allele, GemC1KO/WT mice were crossed with Flpo mice (kindly provided by Dr Anastassiadis) and subsequently with the Nestin Cre transgenic mouse line, as previously described (Spella et al., 2011). The mice were housed in the animal house of the University of Patras. All experiments involving animals were approved by the Veterinary Administrations of the Prefecture of Achaia, Greece, and were conducted in strict accordance with EU Directives.
2.2 Whole-exome sequencing and variant filtering
Three hundred and twenty seven hydrocephalus patient samples were prepared and sequenced at the Yale Center for Genomic Analysis (YCGA). Exome sequencing was performed on genomic DNA extracted from the patient blood samples using Nimblegen SeqxCap EZ MedExome Target Enrichment Kit (Roche) or the xGEN Exome Research Panel v1.0 (IDT), followed by Illumina DNA sequencing as described before (Zaidi, Choi et al. 2013). Sequencing reads were mapped onto the reference genome GRCh37/hg19 using Burrow-Wheeler Aligner-MEM (BWA-MEM) program and GATK Best Practice workflow (McKenna, Hanna et al. 2010, DePristo, Banks et al. 2011, Van der Auwera, Carneiro et al. 2013). Single-nucleotide variants and small indels were called with GATK HaplotypeCaller and annotated using ANNOVAR (Wang, Li et al. 2010) for minor allele frequency (MAF) and deleteriousness. 1,000 Genomes (August 2015), NHLBI Exome Variant Server (EVS), and ExAC (v3) (Lek, Karczewski et al. 2016) were used to annotate MAF. Deleteriousness of missense variants were predicted using Combined Annotation Dependent Deletion (CADD) algorithm (Kircher, Witten et al. 2014). We filtered for rare (MAF ≤ 5 × 10−5 in ExAC, 1,000 Genomes, and EVS databases) and high-quality variants (pass GATK Variant Score Quality Recalibration [VQSR], minimum eight total reads, genotype quality [GQ] score ≥ 20). Variants with large impact on protein structures including LoF (canonical splice-site, frameshift insertion/deletion, stop-gain, stop-loss) and deleterious missense variants (CADD score ≥ 20) were considered. Candidate variants were validated using Sanger sequencing.
2.3 In utero electroporation
In utero electroporations (IUE) were performed in mouse embryos at E14.5 or E15.5 days post coitum (dpc) as described previously (Kyrousi et al., 2015; Pilz et al., 2013). Pregnant females were anaesthetized by intraperitoneal (I.P.) injection of saline solution containing fentanyl (0.05 mg/kg), midazolam (5 mg/kg), and medetomidine (0.5 mg/kg), whereas anesthesia was antagonized by intraperitoneal injection of atipamezol (antisedan, 2.5 mg/kg) and flumazenil (anexate, 0.5 mg/kg). The uterine horns were carefully exposed and using a glass microcapillary 1 μg of the plasmid GFP-Cre (GFP.Cre empty vector was a gift from Tyler Jacks, Addgene plasmid # 20781), or pLVDest-GFP together with Fast Green (0.1%, Sigma) was injected into the lateral ventricles of the embryos. The embryos were subsequently electroporated with 5 pulses applied at 40 V for 50 ms each at intervals of 500 ms by an Electroporator ECM830 (Harvard Apparatus). Following electroporation, the embryos were placed back to the abdominal cavity and sacrificed at P7-P20, depending on the experimental setup.
2.4 Whole-mount dissection
SVZ whole mounts were obtained as previously described (Mirzadeh et al., 2008). In more detail, the brain was extracted, and the lateral ventricle was dissected from the caudal aspect of the telencephalon, and the hippocampus and septum were removed. The dissected lateral wall was fixed in 4% PFA/0.1% Triton X-100 at 4°C. After staining, the ventricular walls were further dissected from underlying parenchyma as slivers of tissue 200–300 mm in thickness and mounted on a slide.
2.5 Postnatal radial glial cells culture
For postnatal radial glial cells (pRGCs) in vitro cultures, the walls of the lateral ventricles of P0 mice were dissected, mechanically dissociated, and plated in poly-d-lysine coated coverslips in proliferation medium containing DMEM-high glucose (Gibco), 10% FBS (Gibco), 1% penicillin/streptomycin (Gibco). pRGCs were kept in proliferation medium for 3 days which was subsequently replaced by differentiation medium containing DMEM-high glucose (Gibco), 2% FBS (Gibco), 1% penicillin/streptomycin (Gibco). Cells were analyzed 5 and 12 days following differentiation (Kyrousi et al., 2015; Paez-Gonzalez et al., 2011).
2.6 Immunohistochemistry
For hematoxylin–eosin (H&E) staining, formalin fixed, paraffin-embedded (FFPE) mouse brains were fixed in 10% formalin for 48 hr and then embedded in paraffin. Serial brain sections with 4 μm thickness were mounted on a positive charged glass slide. Automated H&E staining machine was used (Leica), and then, slides joined the cover-slipping machine.
For immunofluorescence, brains dissected from embryos and newborn mice were fixed overnight with 4% PFA. Adult brains were isolated after transcardiac perfusion and were postfixed in 4% PFA overnight at 4°C. Subsequently, brains were washed with PBS, cryopreserved using 30% sucrose, and frozen in 7.5% gelatin plus 15% sucrose. Cryosections were obtained at 10 μm thickness.
Brain coronal cryosections were postfixed with 4% PFA for 10 min, treated with 0.3% Triton X-100 for 5 min, and incubated in blocking solution containing 10% FBS, 3% BSA, 0.1% Tween 20 in 1× PBS, for 1 hr, as previously described (Spella et al., 2011). Whole mounts were incubated for 1 hr at room temperature in blocking solution, containing 10% normal goat serum in 0.1 M PBS with Triton-X100. Samples were incubated with primary antibodies in blocking solution at 4°C, overnight.
The primary antibodies used were as follows: rabbit anti-p73 (1:300, Abcam), chicken anti-GFP (1:1000, 2B Scientific), mouse anti-GFP (1:500, Molecular Probes), rabbit anti-hMcIdas (Pefani et al., 2011) (1:250), mouse anti-Foxj1 (1:500, eBioscience), rabbit anti-Foxj1 (1:500, Sigma), rabbit anti-pericentrin (1:1,000, Covance), mouse anti-pericentrin (1:1000, BD Biosciences), mouse anti-S100β (1:250, Sigma), guinea pig anti-Dcx (1:1000, Millipore), rabbit anti-BLBP (1:500, Millipore), rabbit anti-β-catenin (1:500, Sigma), rabbit anti-GFAP (1:1000, DakoCytomation), mouse anti-GFAP (1:500, Sigma), rabbit anti-Ki67 (1:1000, Zytomed), rat anti-CD31 (1:500, BD Biosciences), mouse anti-Pax6 (1:1000, Developmental Studies Hybridoma Bank), mouse anti-Ascl1 (1:100, BD Biosciences).
The following Alexa Fluor-labeled secondary antibodies (Invitrogen) were used in blocking solution for 1 hr (coronal sections, dilution 1:1000) and 24 hr (whole mounts, dilution 1:400): Alexa Fluor 488 goat anti-rabbit, Alexa Fluor 488 goat anti-chicken, Alexa Fluor 568 goat anti-mouse, Alexa Fluor 568 goat anti-mouse IgG1, Alexa Fluor 647 donkey anti-rabbit, and Alexa Fluor 647 donkey anti-mouse.
DNA was stained either with Hoechst 33258 (1:1500, Sigma), Draq-5 (1:1000, Biostatus), or DAPI (1:1500, Sigma). Sections and whole mounts were mounted in Mowiol 4-88 (Calbiochem).
2.7 Data analysis
Images derived from immunohistochemistry were recorded on a confocal fluorescence microscopy Leica TCS SP5 with a Leica DMI6000B microscope using 40× and 63× lenses. H-E photographs were taken with a Nikon Eclipse TE2000-U microscope and collected with a Nikon Digital Sight DS-L1 camera. Digital images were processed with Adobe Photoshop, Adobe Illustrator, and Fiji software. For 3D morphological characterization of NSCs, raw Z-stack images were processed with the use of Fiji. Rolling ball algorithm was used to subtract background and a 2px Median filter for highlighting the edges of the signal. Imaris 9.1 was utilized for the 3D reconstructed snapshots. Statistical significance of differences was analyzed with the non-parametric two-tailed Mann–Whitney test. All results are presented as mean and SEM.
2.8 ATAC-seq and data processing
ATAC-seq was performed as previously described (Buenrostro, Giresi, Zaba, Chang, & Greenleaf, 2013). pRGCs were isolated from control and GemC1KO/KO newborn mice and plated in poly-D-lysine coated T25 flasks. The cells were cultured under proliferating conditions (DMEM-high glucose, 10% FBS, 1% penicillin/streptomycin) to reach confluency and were, subsequently, treated with Trypzean (SigmaAldrich, T 3449) to obtain a single-cell suspension. Cells were counted and resuspended to obtain approximately 100,000 cells per sample, resuspended with ice-cold PBS, and centrifuged for 5 min at 500g. Pellets were resuspended in lysis buffer (10 mM Tris–HCl pH 7.4, 10 mM NaCl, 3 mM MgCl2, 0.1% IGEPAL) immediately followed by 10 min centrifugation at 4°C, resuspension in transposition buffer for 30 min at 37°C, and purification using the DNA Clean & Concentrator kit (Zymo; D4014) as per manufacturer's instructions. Transposed DNA was eluted in a 10 μL volume and amplified by PCR with Nextera (Illumina) primers; a maximum of 10 PCR cycles was used. Library quality control was carried out using the Agilent 2200 TapeStation System, before libraries were paired-end sequenced (75 bp read length) to a total of 25 million reads per sample on a HiSeq4000 platform (Illumina) at the CCG Sequencing Facility in Cologne. For both conditions, two biological replicates were collected from pooled mice.
Each ATAC-seq .fastq file was analyzed based on the ENCODE consortium guidelines (https://www.encodeproject.org/atac-seq). In brief, sample adapters were trimmed using default parameters. Reads were mapped to the mm9 reference genome using bowtie2 (-k 4 -X2000 –local) (Langmead & Salzberg, 2012). Unmapped reads, reads mapping at low quality (MAPQ <30), reads mapping to chrM, and any unpaired reads were removed (SAMtools, sambamba, bedtools) (Li et al., 2009; Quinlan & Hall, 2010; Tarasov, Vilella, Cuppen, Nijman, & Prins, 2015). For calculating differentially accessible loci, we shifted plus-strand insertions by +4 bp and minus-strand insertions by −5 bp (as recommended by Buenrostro et al., 2013). The produced .bam (coverage) files for each sample were used without merging and THOR (http://www.regulatory-genomics.org/; parametres: -p 0.005 -f 4) (Allhoff, Seré, Pires, Zenke, & Costa, 2016) was used to identify significant differentially accessible genomic locations between knockout and the wild-type samples. Screenshots of specific genomic loci were obtained using the IGV browser (http://software.broadinstitute.org/software/igv/) (Thorvaldsdóttir, Robinson, & Mesirov, 2013). For annotating genomic coordinates of the significantly changing peaks, we used the subroutine annotatePeak from the ChIPseeker Bioconductor package (https://bioconductor.org/packages/release/bioc/html/ChIPseeker.html) (Yu, Wang, & He, 2015). Finally, Metascape (http://metascape.org/) (Tripathi et al., 2015) was used to identify the corresponding GO terms and ngs.plt for plotting coverage around specific genomic locations (https://github.com/shenlab-sinai/ngsplot) (L. Shen, Shao, Liu, & Nestler, 2014).
Picard: Broad Institute. (Accessed: February 21, 2018; version 2.17.8). “Picard Tools.” Broad Institute, GitHub repository. http://broadinstitute.github.io/picard/.
3 RESULTS
3.1 GemC1 deficiency is associated with the development of congenital hydrocephalus
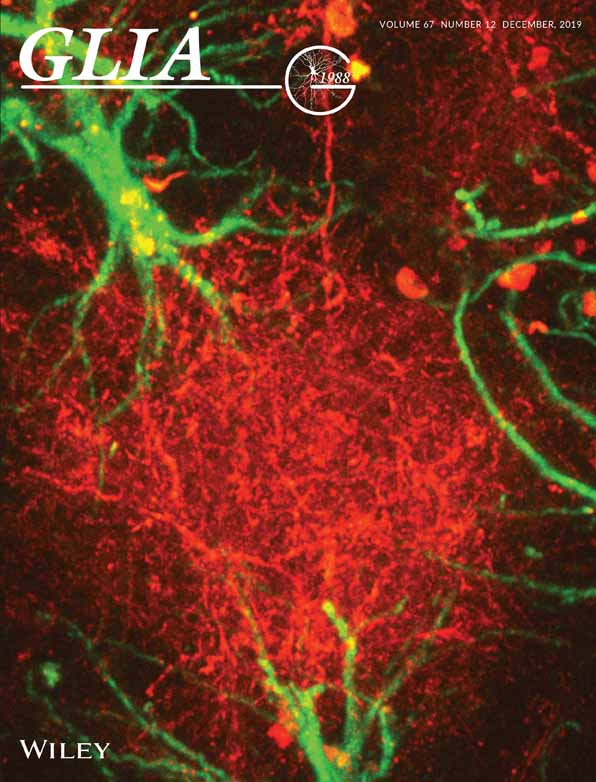
Congenital hydrocephalus is among the most common CNS disorders; however, very few genes have been associated with its development. To identify novel genes implicated in the etiology of the disorder, we performed whole-exome sequence in a cohort of congenital hydrocephalus patients (Furey et al., 2018) and identified a patient with a novel damaging mutation (Absent from in ExAC, 1,000 Genomes and EVS databases) in GEMC1 (p.Glu77Gln; Figure 1A–C). The patient was delivered by elective cesarean section due to macrocephaly and underwent neurosurgical CSF diversion (endoscopic third ventriculostomy) shortly after birth due to the presence of aqueductal stenosis. GEMC1 p.Glu77Gln is predicted deleterious by CADD (CADD score = 22.7) and maps to a highly conserved residue of the GemC1 coiled-coil domain, which is essential for the homo- and heterodimerization of Geminin family members (Caillat et al., 2015).
To elucidate the mechanisms that lead to the development of congenital hydrocephalus, we generated mice in which the GemC1 gene is either constitutively or conditionally inactivated. Mice constitutively lacking GemC1 (i.e., GemC1KO/KO) did not display any gross abnormalities during embryogenesis but they exhibited growth retardation and increased lethality in perinatal stages, in line with previous studies (Arbi et al., 2016; Terré et al., 2016). The small number of GemC1KO/KO mice surviving to adulthood exhibited a dome-shaped skull (Figure 1D), and histological analysis of brain coronal sections at postnatal day 9 (P9) revealed profound dilatation of the lateral ventricles, confirming the emergence of hydrocephalus (Figure 1F). Aqueductal stenosis was also observed (Figure 1G), thus recapitulating the phenotype observed in the human patient. GemC1KO/KO mice display a severe phenotype and the majority dies before the age of weaning, possibly due to the importance of GemC1 in other tissues. To exclude any CNS-independent phenotypes, we conditionally inactivated GemC1 in the CNS, using the Nestin Cre transgenic mouse line (herein referred to as “GemC1 cKO”). GemC1 cKO mice also appeared smaller in size and developed hydrocephalus (Figure 1E), suggesting that GemC1 is essential for normal brain development.
Next, the ventricular walls of the lateral brain ventricles from adult GemC1KO/KO or GemC1 cKO survivors were isolated and subjected to immunofluorescence for acetylated α-tubulin (cilia marker) and β-catenin (marker of cell boundaries). In the ventricular wall of control mice, clusters of multiple cilia were present on the apical surfaces of cells, whereas in the absence of GemC1 cells carrying multiple cilia were not detected (Supporting Information Figure S1A, Figure 1H). To further assess the population of mature ependymal cells, we performed immunostaining for S100β and pericentrin. Under physiological conditions, a large proportion of cells facing the ventricle displayed high S100β expression around their cell body and accumulation of pericentrin, a cellular profile which is characteristic for mature ependymal cells (Mirzadeh et al., 2008). However, we could hardly detect any cells with this expression pattern in GemC1KO/KO brains, further suggesting that ependymal cells are absent (Figure 1I).
Given that ependymal cells of the murine SVZ are derived from a subpopulation of radial glial cells specified between E14.5 and E16.5 dpc (Kyrousi et al., 2015; Spassky et al., 2005), we examined their progenitors during embryogenesis. We performed immunofluorescence for McIdas, which is one of the earliest known markers for committed radial glial cells to the ependymal lineage (Kyrousi et al., 2015) and Foxj1, an established marker for the early steps of multiciliated ependymal cell differentiation (Jacquet et al., 2009). In the control mice, a few McIdas-expressing cells were identified in the medial wall at E16.5 dpc (Supporting Information Figure S1B), whereas their population was expanded later during development (Supporting Information Figure S1C), as previously described (Kyrousi et al., 2015). Foxj1 expression was also evident at E18.5 and further expanded after birth (Supporting Information Figure S1D and E, respectively). At the initial steps of ependymal cell maturation (P0), periventricular cells in the walls of lateral ventricles were found to express McIdas (3%) and Foxj1 (10%), whereas in the absence of GemC1, we could hardly detect any McIdas+ or Foxj1+ cells. Our findings show that the commitment to the ependymal lineage has already been impeded during early stages of mouse embryogenesis, suggesting a pathogenic mechanism for the development of congenital hydrocephalus in humans.
3.2 Radial glial cells lacking GemC1 acquire a NSC fate
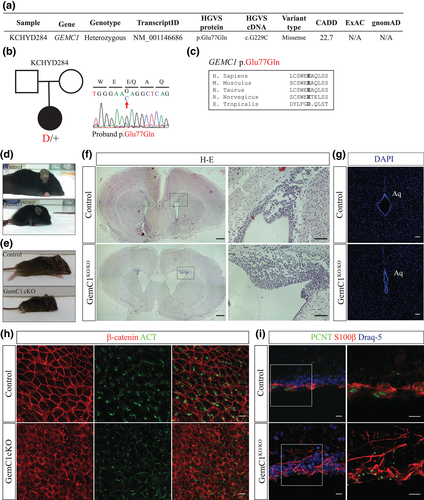
Radial glial cells lacking GemC1 are unable to differentiate into ependymal cells; which cellular fate do GemC1KO/KO radial glial cells acquire? Whole-mount immunofluorescence was performed on ventricular walls of the lateral ventricles, using GFAP, a marker of NSCs and astrocytes, and β-catenin to delineate cell boundaries, confirming the formation of pinwheel structures of cells surrounding GFAP-expressing cells under physiological conditions (Mirzadeh et al., 2008) (Supporting Information Figure S2A). However, we failed to detect rosette-like structures in the absence of GemC1, whereas expansion of cells expressing GFAP throughout the wall of the lateral ventricles was observed.
To examine this further, we performed immunofluorescence on brain coronal sections derived from P7 control and GemC1KO/KO mice using anti-GFAP. Our analysis revealed that in the medial wall of the lateral ventricles obtained from control mice, cytoplasmic expression of GFAP was detected in cells with a cuboidal morphology, consistent with the emergence of an ependymal cell population. However, in the absence of GemC1, GFAP+ cells with long processes that resemble radial glia were detected (Figure 2A). To further elucidate the cellular identity of GemC1KO/KO cells, expression of BLBP, a marker of radial glial cells and NSCs, was examined (Figure 2B). The population of BLBP-expressing cells lining the medial wall of the lateral ventricles was increased by two-fold in GemC1KO/KO mice (Figure 2D).
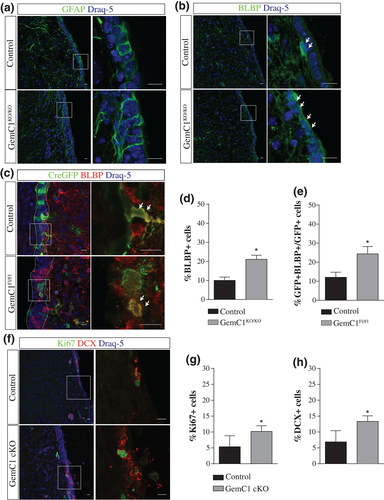
Tο exclude the possibility that changes in the cytoarchitecture of the niche due to hydrocephalus could provoke the cellular alterations observed in GemC1KO/KO mice, we also specifically deleted GemC1 from a small subset of radial glial cells during embryogenesis. A Cre-GFP plasmid was introduced in the developing brain of GemC1Fl/Fl E15.5 dpc embryos, using in utero electroporation, whereas GemC1Fl/WT embryos were used as control. Mouse brains were analyzed at P7, and Cre recombination was monitored by GFP expression. To examine whether the ependymal cell population is affected, we performed immunofluorescence experiments using anti-p73 antibody, a transcription factor that was highlighted for its critical role in ependymal cell generation (Fujitani et al., 2017; Gonzalez-Cano et al., 2016), acting upstream of Foxj1 (Marshall et al., 2016; Nemajerova et al., 2016). In the control mouse brains, 30% of GFP+ cells were co-expressing p73, whereas p73+ cells were rarely detected in GemC1Fl/Fl electroporated brains, representing only 1.7% of GFP+ cells (Supporting Information Figure S2B, C). These results recapitulate the phenotype described for the constitutive or conditional inactivation of the GemC1 gene, pointing to the lack of committed cells into the ependymal cell lineage.
Moreover, the expression of BLBP in electroporated cells along the medial wall of the lateral ventricle was examined (Figure 2C). BLBP+ cells were increased by two-fold in the absence of GemC1 (12.1% and 24.3% of the total GFP population in control and GemC1Fl/Fl mouse brains, respectively; Figure 2E). The increased percentage of BLBP-expressing cells raised the question of whether these cells display proliferative and neurogenic capacity, key features of NSCs. We evaluated neurogenesis and proliferation in the medial wall, as it appeared less affected by the emergence of hydrocephalus (Figure 2F). Under physiological conditions, the medial wall of P20 mice is comprised by a thin epithelium which is mainly covered by ependymal cells. As neurogenesis is limited in the medial wall, the percentages of Ki67 and DCX expressing cells in the control brains, were very low (5.3% and 6.8%, respectively). Interestingly, the percentages of Ki67+ (10.2%) and DCX+ (13.3%) cells in the GemC1 cKO brains were almost two-fold higher (Figure 2G, H, respectively) in support of increased neurogenesis and proliferation in the absence of GemC1.
Given that adult neurogenesis takes place at much higher levels in the dorsal-lateral and lateral walls of the lateral ventricles, we next sought to determine how the deletion of GemC1 affects the cellular populations in this subregion. Adult NSCs have been described as apical GFAP+ cells that are intercalated within the ependymal layer and are in close contact with the blood vessels (Doetsch, Caillé, Lim, García-Verdugo, & Alvarez-Buylla, 1999; Shen et al., 2008). To eliminate the possibility of hydrocephalus affecting neurogenesis in GemC1KO/KO mice, we performed targeted deletion of GemC1 in single cells by introducing a Cre-GFP plasmid (and GFP as a control) through in utero electroporation in the developing cortex of E14.5 dpc GemC1Fl/Fl mouse embryos (Figure 3). We measured the percentage of electroporated cells (GFP+) which co-express GFAP and were in contact with the vasculature, as it was determined by CD31 immunoreactivity. Our results revealed that in the absence of GemC1, the percentage of GFP + GFAP+ cells in contact with blood vessels was two-fold higher, suggesting that cells lacking GemC1 in the lateral ventricles displayed NSC characteristics (Supporting Information Figure S3).
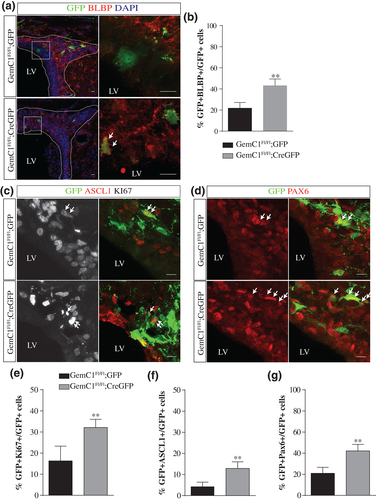
In addition, our analysis showed that the percentage of cells expressing BLBP was higher in the absence of GemC1 (21.7% and 43% of the total GFP population in the control and GemC1-deleted cells, respectively; Figure 3A, B), in line with our findings in the medial wall of GemC1KO/KO mice. To further investigate the cellular identity of GemC1 deleted cells, we assessed their proliferative potential. Our analysis revealed that in the absence of GemC1, the percentage of proliferating cells (Ki67+) was approximately 32%, whereas under control conditions, the corresponding percentage was 16%, indicating that the number of proliferative cells was increased in the absence of GemC1 (Figure 3C, E). Moreover, we assessed the neurogenic potential of these cells by examining the expression of Ascl1 and Pax6, two transcription factors that have been characterized as neural fate determinants in the postnatal brain (Urbán & Guillemot, 2014) (Figure 3C, D). Upon control conditions, the percentage of GFP + Ascl1+ was low (4.3%), whereas upon GemC1 inactivation, their percentage was increased threefold (12.9%) (Figure 3F). In line with the increased percentage of Ascl1+ cells, the population expressing Pax6+ was also found increased in the absence of GemC1 (20.9% and 42.1% of GFP- and CreGFP-electroporated cells, respectively; Figure 3G). Furthermore, our analysis showed that the majority of Ascl1+ cells was proliferating (Ascl1 + Ki67+). Our results suggest that cells lacking GemC1 reveal a neurogenic potential and maintain a proliferating potential.
Our findings highlight the importance of GemC1 in regulating the balance between NSC and ependymal cell generation, with implications in the process of adult neurogenesis.
3.3 Ablation of GemC1 induces widespread chromatin accessibility changes promoting NSC identity
It is tempting to speculate that this positive regulation in neurogenesis could be due to increased numbers of NSCs in the postnatal brain. To further investigate the acquisition of a NSC fate in the absence of GemC1, we examined the chromatin landscape of GemC1KO/KO cells. To eliminate the possibility that intrinsic factors within the SVZ microenvironment could affect the cell population examined in GemC1KO/KO mice, we took advantage of a primary cell culture assay, in which pRGCs can differentiate into SVZ niche cells in vitro (Kyrousi et al., 2015; Paez-Gonzalez et al., 2011). Indeed, this system recapitulated the in vivo phenotype, as GemC1KO/KO pRGCs failed to express Foxj1, lacked accumulation of pericentrin and did not generate clusters of cilia even after several days in culture (Supporting Information Figure S4). Utilizing this culture assay, we examined changes to chromatin landscape in GemC1KO/KO pRGCs by probing chromatin accessibility genome-wide using ATAC-seq (Buenrostro et al., 2013). Following data mapping and analysis, we readily observed widespread changes in accessibility. For example, we observed that ependymal-associated genes, such as Foxj1, displayed a “closed” chromatin organization in the absence of GemC1. On the contrary, genes linked with a NSC signature appeared to adopt a more “open” organization, like Blbp (also known as Fabp7) (Figure 4A).
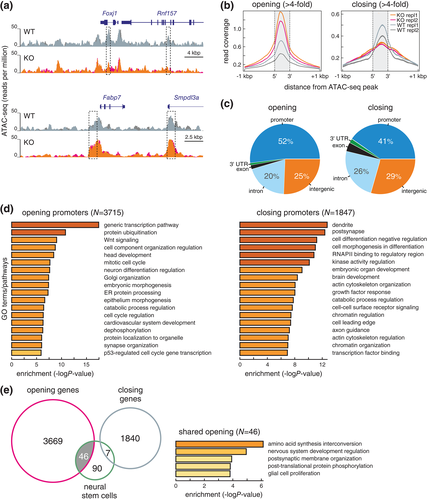
Proceeding with the analysis, we focused on those ATAC-seq peaks that exhibited significant changes of >fourfold in GemC1KO/KO compared to wild-type cells (see Methods for details). Using such stringent criteria, changes predominantly concerned gene promoters and could be further subdivided in peaks showing increased or decreased accessibility (Figure 4B, C). Of ATAC-seq peaks that showed significantly increased accessibility, >50% were found at gene promoters and 25% at distal intergenic sites (Figure 4C, left). An approximately 40 and 30% of peaks showing chromatin compaction mapped to promoters and distal intergenic sites, respectively (Figure 4C, right).
Notably, GemC1KO/KO cells showed more than twice as many promoters with a more open chromatin landscape, compared to those with a less accessible one (i.e., >3,700 compared to ~1850 promoters; see Supporting Information Table S1). In line with the aforementioned results, “opened” promoters in knockout cells were related to genes linked to cell cycle reactivation and neural differentiation, whereas “closed” promoters involved genes associated with transcriptional and chromatin regulation, as well as with development of dendrites/synapses or axon guidance (Figure 4D).
We compared genes exhibiting differential accessibility with signature genes of NSCs from the mouse SVZ (derived from single-cell RNA-seq data; Zywitza et al., 2018). Strikingly, 1/3 of these signature genes (listed in Supporting Information Table S1) could be seen “opening up” upon GemC1KO/KO and concerned genes with functions relevant to neural system development and glial cell proliferation (Figure 4E).
Taken together, GemC1 modulates the specification of radial glial cells toward either the NSC or the ependymal cell lineage by reprogramming their gene expression profile. Mechanistically, we propose that radial glial cells lacking GemC1 display higher accessibility in gene promoters that are linked with the adult NSC lineage, such as BLBP. We suggest that the balanced expression of GemC1 is essential for the proper assembly of the adult SVZ niche both in mice and humans, and thus, dysfunction of GemC1 may cause brain disorders, such as hydrocephalus.
4 DISCUSSION
Congenital hydrocephalus, one of the most common congenital brain abnormalities, is characterized by an expansion of the CSF-filled ventricular compartment. A limited number of genes has been associated with its development, with a recent study uncovering three new genes associated with congenital hydrocephalus in humans (TRIM71, SMARCC1, and PTCH1), which are highly expressed in multiciliated ependymal cells and impact the NSC fate (Furey et al., 2018). Here, we show that constitutive or brain-specific deletion of GemC1 results in the development of severe congenital hydrocephalus in mice. We show that lack of GemC1 expression impedes early commitment of radial glial cell events toward the ependymal lineage and acquire adult NSC characteristics instead.
Radial glial cells consist a population which generates all the major cellular components of the adult cortex, including the adult NSCs and multiciliated ependymal cells (Lim & Alvarez-Buylla, 2016; Spassky & Meunier, 2017). Given that GemC1 deletion prevents radial glial cells to acquire the ependymal fate, we hypothesized that these cells might acquire an alternative fate. Indeed, deletion of GemC1 resulted in an increased number of cells expressing the NSC markers GFAP and BLBP and displaying a radial glia-like morphology. The upregulation of NSC characteristics raised the question of whether the absence of GemC1 could positively regulate neurogenesis. Under physiological conditions, adult neurogenesis takes place both in the lateral wall, adjacent to the striatum, and the medial wall, next to the septum, albeit in lower rates (Mizrak et al., 2019). A higher number of Ki67+ and DCX-expressing cells was identified in the medial wall of GemC1 cKO brains, suggesting that neurogenesis is positively regulated in the absence of GemC1. However, we cannot exclude that GemC1 deletion affects differentially each subregion of the niche. To further establish that ablation of GemC1 affects neurogenic potential, we specifically inactivated GemC1 in a small fraction of radial glial cells during embryogenesis both in the medial and the dorsal-lateral wall. Our results in the medial wall confirmed GemC1KO/KO data, as elevated numbers of BLBP+ cells were identified. Further analysis in the dorsal-lateral wall revealed that the absence of GemC1 favors the generation of cells displaying a morphology reminiscent of adult NSCs. Moreover, we observed increased number of cells expressing Ascl1 and Pax6, two crucial regulators of neurogenesis (Andersen et al., 2014; Brill et al., 2008; Urbán & Guillemot, 2014). Taken into account that these transcription factors are expressed both in the active adult NSCs and their progeny (Dulken, Leeman, Boutet, Hebestreit, & Brunet, 2017; Llorens-Bobadilla et al., 2015), our data suggest that GemC1 deletion may not only favor the generation of NSC-like cells but also positively regulate their lineage progression toward more differentiated progeny. In line with this hypothesis, GemC1-deleted population showed an increased proliferating capacity.
The hypothesis that GemC1 deficiency favors adult NSC identity is further supported by the altered chromatin landscape of GemC1-deficient cells. Ependymal-associated genes like FoxJ1 present a “closed” chromatin organization in the absence of GemC1, whereas other genes linked with NSC signature appeared to adopt a more “open” organization. A characteristic example is that of the Blbp promoter (also known as Fabp7), which was found to be more “open,” consistent with our in vivo data indicating its high expression in GemC1KO/KO cells. Other examples of NSC-like genes that seem more “active” upon GemC1 deletion include GFAP and Gja1 (Dulken et al., 2017) and Ttyh1, which is characterized as a positive regulator of Notch signaling pathway (Kim et al., 2018). Moreover, several Notch elements (such as Notch1, Jag1, Dll1, and Rbpj) normally expressed in adult NSCs, acquire more “accessible” chromatin organization upon GemC1 deletion. This corroborates with previous findings that suggest an antagonistic role between GemC1 expression and the Notch signaling pathway in the acquisition of ependymal and NSC fate, respectively (Kyrousi et al., 2015). Furthermore, our ATAC seq analysis suggests that multiple signaling pathways, including Wnt, as shown by the GO analysis, and BMP reveal increased accessibility. Our results indicate that several of the extrinsic and intrinsic signals suggested to play an important role in the regulation of NSCs and adult neurogenesis present an active chromatin conformation supporting further the acquisition of NSC fate in the absence of GemC1 (Lim & Alvarez-Buylla, 2016). In accordance with the acquisition of a NSC fate, genes linked to cell cycle regulation exhibit an “open” chromatin conformation in GemC1KO/KO cells, which is in sharp contrast with the expected epigenetic profile of post-mitotic ependymal cells and supports the idea of a cell type with stem cell characteristics.
The clinical manifestation of congenital hydrocephalus in humans is heterogeneous and several molecular mechanisms may underlie the congenital hydrocephalus pathology. We provide evidence that GΕmC1 is associated with human congenital hydrocephalus, as we detected a novel damaging mutation in GEMC1 in a patient with congenital hydrocephalus. Importantly, structural characterization of GemC1 has highlighted Glu77 specifically as a critical amino acid for the correct conformation of the coiled-coil (Caillat et al., 2015). Based on earlier analysis, this mutant is expected to show altered ability to form homo and heterodimers with the Geminin family members (Caillat et al., 2015). It was previously shown that GemC1 forms heterodimers with McIdas and Geminin, whereas Geminin acts antagonistically to GemC1 in the regulation of transcriptional activation of key transcription factors for multiciliogenesis (Arbi et al., 2016; Caillat et al., 2013; Caillat et al., 2015; Kyrousi et al., 2015; Patmanidi et al., 2017; Terré et al., 2016). As Geminin was previously shown to be implicated in stem cell self-renewal and differentiating decisions (Karamitros et al., 2015; Spella et al., 2011), antagonistic roles for Geminin and GemC1 in determining the ependymal cell lineage are likely. Although the human genetic findings we provide will require further validation by sequencing other congenital hydrocephalus patients, the fact that ependymogenesis in mice and humans shares many common characteristics (Coletti et al., 2018; Pressler & Auvin, 2013), suggests that the mechanism defined here in mice may be operative in some forms of human congenital hydrocephalus.
Finally, recent findings by Furey et al., associated human congenital hydrocephalus to novel mutations in genes implicated in the regulation of neural progenitor cell fate (Furey et al., 2018), raising the idea of a new potential pathogenic mechanism underlying the human disease. Our results provide further evidence that GEMC1 loss-of-function could also contribute to the development of hydrocephalus in humans through increased generation of NSCs. Our data reinforce the idea that fate decisions between NSCs and ependymal cells are closely linked and provide novel ideas on the fundamental understanding of the disease pathogenesis with potential implications for congenital hydrocephalus treatment.
4.1 Data sharing
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ACKNOWLEDGMENTS
We thank the Advanced Light Microscopy Facility of the Medical School at the University of Patras and the Experimental Animal Facility of the University of Patras for support with experiments. We are also grateful to Dr. Vasiliki Bravou and Sofia Nikou for their assistance in histology; Marianna Iliadou for helping with the ATAC-seq experiments; Marina Arbi and all the members of our laboratories for helpful discussions. We thank Meletios Verras for professional assistance with graphical abstract design and the Advanced digital Microscopy Facility of the Institut de Recerca Biomèdica de Barcelona (IRB) for 3D reconstruction images. This study was supported by the General Secretariat for Research and Technology (GSRT) and Hellenic Foundation for Research and Innovation (HFRI) and by State Scholarships Foundation (IKY). K.T.K. is supported by the National Institutes of Health, the Simons Foundation, and the March of Dimes Foundation (USA). The authors declare no competing interests.