White matter plasticity and maturation in human cognition
Funding information: Ontario Institute for Regenerative Medicine
Abstract
White matter plasticity likely plays a critical role in supporting cognitive development. However, few studies have used the imaging methods specific to white matter tissue structure or experimental designs sensitive to change in white matter necessary to elucidate these relations. Here we briefly review novel imaging approaches that provide more specific information regarding white matter microstructure. Furthermore, we highlight recent studies that provide greater clarity regarding the relations between changes in white matter and cognition maturation in both healthy children and adolescents and those with white matter insult. Finally, we examine the hypothesis that white matter is linked to cognitive function via its impact on neural synchronization. We test this hypothesis in a population of children and adolescents with recurrent demyelinating syndromes. Specifically, we evaluate group differences in white matter microstructure within the optic radiation; and neural phase synchrony in visual cortex during a visual task between 25 patients and 28 typically developing age-matched controls. Children and adolescents with demyelinating syndromes show evidence of myelin and axonal compromise and this compromise predicts reduced phase synchrony during a visual task compared to typically developing controls. We investigate one plausible mechanism at play in this relationship using a computational model of gamma generation in early visual cortical areas. Overall, our findings show a fundamental connection between white matter microstructure and neural synchronization that may be critical for cognitive processing. In the future, longitudinal or interventional studies can build upon our knowledge of these exciting relations between white matter, neural communication, and cognition.
1 INTRODUCTION
White matter is composed of a network of myelinated axons and related cells that support the transmission and coordination of neural signals. Its growth is a key source of brain development (Casey, Giedd, & Thomas, 2000; Dobbing & Sands, 1973; Habrand & De Crevoisier, 2001) and myelin's capacity to regenerate throughout the lifespan is a critical component of neuroplasticity (Fields, 2015; Gibson et al., 2014; McKenzie et al., 2014). Despite the robust association between white matter and cognition (Fields, 2015), very little is known about: (a) how changes in white matter may link to cognitive development, and (b) the neurophysiological basis of this link. Adaptive changes in white matter are hypothesized to facilitate cognitive processing by optimizing information transfer within and across neural networks (Fields, 2015; Nunez, Srinivasan, & Fields, 2015; Pajevic, Basser, & Fields, 2014). The relationship between the plasticity of white matter microstructure and cognitive development has received limited attention. Likewise, the impact of white matter changes on neural signaling and ultimately cognition is understudied. Recent advances addressing critical gaps within the literature now allow for greater examination of these issues.
First, myelin cannot be imaged in humans at the same resolution as model systems. As such, there is a necessary focus on “white matter” versus “myelin” plasticity in human studies. This focus reflects the spatial resolution (~mm) of Magnetic Resonance Imaging (MRI) used in clinical settings to measure white matter, where a typical volume element (voxel) can be traversed by over 100,000 myelinated axons. Thus, MRI lacks the necessary resolution and specificity to address the cellular and molecular processes that underlie plasticity. For visual purposes, voxel is usually subsampled to produce a dense fiber track image, which can lead to misrepresentation on the actual special resolution. Prior work examining white matter and cognition in children and adolescents has been limited to neuroimaging approaches that provide nonspecific information regarding white matter structure. In this article, we review novel neuroimaging modalities that provide more sensitivity to white matter tissue structure, particularly myelin.
Second, myelin plasticity cannot be manipulated in humans using the same tools as model systems. There have been numerous reviews on adult plasticity (McPhee, Downey, & Stough, 2019; Sampaio-Baptista & Johansen-Berg, 2017; Tardif et al., 2016), plasticity across the life span (Hamaide, De Groof, & Van der Linden, 2016; Kaller, Lazari, Blanco-Duque, Sampaio-Baptista, & Johansen-Berg, 2017), and including animal models (Chorghay, Karadottir, & Ruthazer, 2018). Nonetheless, here we focus our partial review on plasticity during childhood and adolescence. In children and adolescents, plasticity is examined using studies of change in white matter as a function of (a) developmental maturation thought to reflect intrinsic biological processes, or (b) extrinsic factors including learning paradigms or, in clinical settings, the administration of medication known to affect myelin formation. Intrinsic and extrinsic processes can be confounded in developmental studies, and care must be taken to account for potential operative variables in white matter plasticity. Notably, the majority of studies relating white matter to cognition in children and adolescents have used cross-sectional designs that do not provide a measure of change over time. Although we briefly discuss recent advances in cross-sectional studies that provide greater clarity regarding white matter and cognition in children and adolescents, in this article, we highlight the new longitudinal and interventional studies that provide a more direct assessment of both white matter change (i.e., plasticity) and cognitive development in children and adolescents.
Finally, there has been limited consideration of the mechanisms by which white matter structure impinges on functional changes in cognition. A useful approach for elucidating these mechanisms has been to compare healthy white matter in typically developing children with damaged tissue in children with brain insult: Such approaches have been productive in elucidating fundamental properties of neural function (Rorden & Karnath, 2004). Here, we address a potential mechanism of how structural changes to white matter tissue may yield functional changes in behavior. First, we review recent work that suggests white matter is linked to cognitive function via its impact on neural synchronization. We then test this hypothesis in a population of children and adolescents with myelin insult. Specifically, we compare a population of children and adolescents with inflammatory demyelinating disease to age-matched typically developing children, and test whether differences in the early visual neural response between the groups are predicted by differences in white matter microstructure. Finally, we use computational modeling to reproduce these findings.
Imaging white matter in humans—Novel approaches sensitive to tissue microstructure
The spatial resolution of human MRI is at the mesoscopic tissue level (~mm). As such, information on the mesoscopic tissue properties (i.e., diffusion; T1, T2 relaxation) reflect signals acquired from large assemblies of different cell types and does not provide specific information about cellular structure, including myelin and axonal membranes. Therefore, myelin structure is not imaged directly in humans, rather structure is inferred or modeled from nonspecific properties. Imaging of white matter has been recently reviewed comprehensively, so will not be covered in detail here (Lebel & Deoni, 2018; Lebel, Treit, & Beaulieu, 2017; Novikov, Fieremans, Jespersen, & Kiselev, 2018; Tamnes, Roalf, Goddings, & Lebel, 2018).
Diffusion MRI measures the random movement of water in the brain at the micron scale (Tamnes et al., 2018). Specifically, diffusion tensor imaging (DTI) models, the restriction and orientation of randomly moving water molecules in order to assess white matter structure. Using DTI, quantifiable metrics can be generated based on the displacement and directionality (anisotropy) of water molecules within the tissue. Fractional anisotropy (FA), and mean, axial and radial diffusivities (MD/AD/RD) can be derived from data obtained from diffusion-weighted images (Basser, Mattiello, & Le Bihan, 1994; Jones & Leemans, 2011). FA reflects the direction of principal diffusion, and yields a value between 0 and 1; values closer to 1 indicate highly directional diffusion (i.e., high anisotropy), as water molecules will diffuse in a restricted manner along the dominant fiber direction, whereas values closer to 0 indicate unrestricted diffusion (i.e., low anisotropy) because water molecules are unrestricted by fibers. MD represents the mean diffusion freedom of water molecules, measured in mm2/s, and represents the magnitude of water diffusion (Basser, 1995). AD, also referred to a parallel or longitudinal, reflects diffusivity along the longest axis of the ellipsoid (λ1), also measured in mm2/s (Basser, 1995). RD, also referred to as perpendicular or transverse, represents the average of diffusivity values along λ2 and λ3, the two minor axes of the ellipsoid (Basser, 1995). Lower FA and higher MD/AD/RD values are thought to reflect compromise of myelin sheath and axonal structure (Song et al., 2002).
Although DTI metrics are nonspecific and reflect white matter organization broadly, they are still the MRI metrics most frequently used in the study of white matter in humans (Tamnes et al., 2018). Some histological verification in animal models has shown that RD is altered by demyelination and remyelination, whereas AD is altered by axonal injury but remains unaltered by myelin changes (Song et al., 2002, 2003, 2005). However, increased anisotropy in diffusion has been shown even in nonmyelinated nerves (Beaulieu & Allen, 1994). Furthermore, DTI solely characterizes the primary diffusion direction within a voxel and therefore is of limited utility in regions with multiple fiber populations, which is the majority of the brain (Lebel & Deoni, 2018). As such, DTI, as it stands, has significant limitations for measuring specific tissue microstructure.
More recent developments in diffusion imaging have been introduced to address the limitations of DTI. Diffusion kurtosis imaging (DKI), a clinically feasible extension of DTI, is sensitive to the tissue environment at the cellular scale (Jensen & Helpern, 2010; Jensen, Ja, Ramani, Lu, & Kaczynski, 2005; Tamnes et al., 2018). Furthermore, water compartment modeling can link DKI signals from intra- and extra-axonal water compartments to physical quantities characterizing the tissue (Jelescu et al., 2015). The white matter tract integrity (WMTI) model provides estimates of intra-axonal water fraction (AWF), intra-axonal parallel diffusivity (Da), and extra-axonal axial and radial diffusivities (De|| and De⊥, respectively). Such metrics can be used to make more specific inferences regarding myelin and axon structure (Falangola et al., 2014; Fieremans, Jensen, & Helpern, 2011; Jelescu et al., 2016; Tamnes et al., 2018). Notably, AWF and De⊥ have been related to inflammatory demyelination in animal models (Falangola et al., 2014; Guglielmetti et al., 2016; Jelescu et al., 2016). The neurite orientation dispersion and density imaging (NODDI) model is another water compartment-modeling approach and also provides more detailed information regarding tissue microstructure than DTI (Lebel & Deoni, 2018; Zhang, Schneider, Wheeler-Kingshott, & Alexander, 2012). More advanced acquisition techniques—that collect more comprehensive diffusion information—such as high angular resolution diffusion imaging and diffusion spectrum imaging have also been developed, but often the scan duration required for these sequences (~20–40 min) can be a limiting factor for use in children (Lebel & Deoni, 2018). We do note that with recent technological advances in MRI, including multiband or simultaneous multi-slice capabilities, scan duration for these sequences is decreasing.
Finally, there are many more MRI modalities that are sensitive to myelin largely beyond the scope of this review: For a comprehensive review, please refer the following (Alonso-Ortiz, Levesque, & Pike, 2015; Heath, Hurley, Johansen-Berg, & Sampaio-Baptista, 2018; MacKay & Laule, 2016). However, in a narrower sense, we can include magnetization transfer (MT) imaging and multi-component relaxation (MCR) time measurement. MT imaging measures the exchange of magnetization between lipid-bound and free-water protons and can provide a measure sensitive to macromolecular content, including myelin (Henkelman et al., 1993; Lebel & Deoni, 2018). MCR is a technique that is sensitive to the water trapped between the lipid bilayers of the myelin sheath (Dean et al., 2015; Lebel & Deoni, 2018; MacKay et al., 1994; O'Muircheartaigh et al., 2014) that yields an index known as the myelin water fraction (MWF), which is the fraction of the signal from the myelin water pool relative to the total water signal. MWF has shown strong correlations with post-mortem myelin-staining techniques, suggesting sensitivity to myelin (O'Muircheartaigh et al., 2014).
1.1 Developmental plasticity and cognitive maturation
1.1.1 Cross-sectional studies
The majority of studies relating white matter to cognition in children is cross-sectional and, as such, examine age-related differences across individuals. The disadvantage of this design is that it does not provide a direct measure of plasticity, as change over time in individuals cannot be evaluated using a cross-sectional sample. Recent improvements in cross-sectional studies—including the use of larger samples and imaging approaches with greater sensitivity to myelin (i.e., MCR)—have provided greater clarity regarding the impact of white matter on cognition in children and adolescents. For example, whole-brain FA from DTI was associated with visuospatial ability, independent of age, in a large sample of primary school-aged children (n = 778, age range = 6–10 years; Muetzel et al., 2015). In another study, DTI indices were used to calculate an estimate of the variability in white matter development—deemed brain age in a cohort of typically developing children, adolescents, and young adults (n = 82, age range = 6–20 years) (Ullman & Klingberg, 2017). Brain age predicted working memory and numerical ability in younger children, but not in adolescents and young adults, suggesting that variability in individual developmental timing strongly affects cognition in younger ages (6–11 years), but does not predict adolescent cognitive functioning (Ullman & Klingberg, 2017). Finally, a recent MCR study showed that voxel-wise MWF from within frontal and temporal areas predicted language abilities and that relations became more strongly associated with increasing age in infants and preschool children (n = 183, age range = 3 months–4 years; O'Muircheartaigh et al., 2014). Although cross-sectional studies are informative, their inherent insensitivity to individual differences limit their utility for examining white matter plasticity.
1.1.2 Longitudinal studies
Understanding the coupling between maturation of white matter and cognitive development requires following children longitudinally (Yeatman, Dougherty, Ben-Shachar, & Wandell, 2012). Although few such studies are evident in the great body of research examining white matter and cognition in children and adolescents, there are a few recent notable exceptions. A cohort of typically developing children, adolescents, and young adults (n = 128, age range = 8–28) was followed with DTI and cognitive assessment over the course of 5 years. White matter growth in adolescence was associated with improved performance tasks of visual processing speed and inhibitory control, whereas later growth in adulthood was associated with poorer function (Simmonds, Hallquist, Asato, & Luna, 2014). In the examination of reading in young children, longitudinal DTI studies have shown that patterns of change in white matter predicted reading ability. For example, children and adolescents with above-average reading skills initially showed FA in the left arcuate that increased over 3 years, whereas those with below-average reading skills displayed higher initial FA that declined over the same time period (n = 55, age range = 7–15 years; Yeatman et al., 2012). Furthermore, a greater improvement in DTI metrics over time within the right superior longitudinal fasciculus was observed in subsequent good versus poor readers (n = 45, age range = 4.9–12.5 years): A positive association was observed between white matter maturation and reading development (Wang et al., 2017). In preterm infants (n = 66), compromise of white matter structure in multiple brain regions at ~9 months of birth age, measured using DTI, predicted later cognitive and motor impairment at 3 years of age (Schadl et al., 2018). In a further cohort of preterm children followed with DTI imaging from shortly after birth (~7 months) to 4 years of age (n = 29 with three time points), slower changes in MD and RD within the left internal and external capsule were associated with lower IQ and language scores at 4 years of age (Young et al., 2017). Specifically, deep changes within medial white matter tracts were most related to long-term outcomes versus more lateral or superficial tracts in this population (Young et al., 2017).
Finally, longitudinal studies using MCR have provided the first insights into changing relations between MWF and cognition (Dean et al., 2015; Deoni et al., 2016). In typically developing infants and young children (n = 108, ages 2.5 months–5.5 years) increased MWF—indicative of myelination in (a) central core white matter tracts predicted motor function, (b) the posterior limb of the internal capsule, superior corona radiata, and the superior longitudinal fasciculus predicted visual function, and (c) the cerebellum, thalamus, occipital white matter, and posterior thalamic radiations predicted receptive language function (Dean et al., 2015). Furthermore, relationships between MWF and expressive language and fine motor skills changed with age in these very young children. Evidence of the dynamic nature of myelin change and cognitive function is found in a further MCR study showing differential patterns of change in MWF as a function of intelligence (Deoni et al., 2016). Specifically, children with a higher ability in language and fine motor skills exhibited slower but earlier and more prolonged increases in MWF resulting in evidence for overall increased myelination by 3 years of age compared to average and low ability children (Deoni et al., 2016).
Findings from these longitudinal studies provide the most compelling evidence to date that white matter plasticity is associated with cognitive development. Differential changes in white matter metrics over time predict the development of specific abilities, including motor, visual, reading, and language skills: Regional specificity in these relations is also evident. Furthermore, studies evaluating change in MWF suggest that myelination over time is a critical factor in cognitive and intellectual development (Deoni et al., 2016). Overall, the rate of growth in white matter metrics best predicts changes in cognition, and differences in growth rate are observed in individuals of varying ability. The main limitation of these observational longitudinal studies, however, is that they are correlational, and one cannot infer directionality of the associations from these observations. It is unclear whether the relations between metrics of white matter change and cognitive development reflect biological processes that support the emergence of cognition, or if the findings reflect the impact of activity-dependent learning on relevant white matter connections. Intervention studies where extrinsic variables can be manipulated are ultimately required to determine the directionality of the link between white matter growth and changes in cognition.
1.2 Extrinsically mediated white matter plasticity and cognitive maturation
The impact of the extrinsic environment or activity-dependent processes on white matter plasticity is now clear (Forbes & Gallo, 2017). For example, socioeconomic status has been shown to associate with relations between white matter structure measured by DTI and executive function in a large (n = 1,082) cross-sectional sample of children, adolescents, and young adults (Ursache & Noble, 2016). Across multiple tracts of interest, lower FA or lower tract volume was associated with reduced cognitive flexibility among children and young adults from lower income families. In contrast, children and young adults from higher income families showed preserved cognitive flexibility in the face of low white matter FA or volume.
1.2.1 Intervention studies
Observational studies of developmental plasticity do not differentiate between the impact of biological factors versus extrinsic factors on the relations between white matter and cognition. Intervention studies—where an extrinsic variable is manipulated—are required to most directly observe the impact of white matter plasticity on cognition. In this regard, a recent study (Jolles et al., 2016) examined learning-related changes in white matter tracts following 2 months of mathematics tutoring. DTI data were acquired from 18 third Grade children before and after 2 months of tutoring. Changes in regions of the superior longitudinal fasciculus following tutoring predicted individual difference in improvement of mathematics after 2 months of tutoring. Such work is compelling for linking activity-dependent changes in white matter associated with tutoring to improved mathematics performance. Without a control condition, the confounding impact of other factors influencing white matter (i.e., maturation; unobserved environmental variables) that are unrelated to the tutoring were not considered. Only controlled trials offer the ability to account for confounding effects on white matter plasticity and cognition.
Controlled clinical trials provide compelling evidence of the role white matter plasticity plays in improved cognition in children and adolescents, as changes in white matter are observed following a direct intervention design that controls for maturation and learning. We recently demonstrated activity-dependent white matter repair in children with a brain injury from their curative treatment for a brain tumor who participated in a physical exercise program (Riggs et al., 2017). Specifically, using a controlled crossover design, we showed that increased FA within the corpus callosum following a 12-week exercise program predicted increased speed of processing in these children (Riggs et al., 2017).
1.3 The impact of disease and injury on white matter—A model for linking white matter plasticity to cognition via neural communication
Very few studies in humans have considered the mechanism by which white matter connectivity mediates cognition in children and adolescents (Dubois, Adibpour, Poupon, Hertz-Pannier, & Dehaene-Lambertz, 2016). This is a critical first step in considering how white matter plasticity underlies cognitive development. White matter may adaptively coordinate the timing of signal conduction along axons over time to improve and maintain synchrony and coherence of communication within the brain (Fields, 2015). Synchrony describes when signals from two sources arrive at a common destination at approximately the same time (equal phase at some frequency), while coherence measures phase consistency between regions. Hence, white matter may have its influence on cognition via its impact on a fundamental mechanism of brain communication—neural oscillations.
Neurons are organized into functionally specialized networks (Bullmore & Sporns, 2012). Coordinated neural firing—or synchrony—within and among these networks in response to external stimuli or internal events produces neural oscillations (Hari, Parkkonen, & Hämäläinen, 2015). Synchrony of neural activation is a signature of brain communication and yields functional networks that presumably underlie behavior (Bressler, 1995; Sporns, 2002; Tononi, Sporns, & Edelman, 1994; Varela, Lachaux, Rodriguez, & Martinerie, 2001). As noted in a recent review in the area (Nunez et al., 2015), this concept was first introduced by Walter, Penfield, and Jasper (Cooper, Winter, Crow, & Walter, 1965; Jasper & Penfield, 1949; Penfield & Jasper, 1954) whereby they demonstrated that complex behavior is a combination of different rhythms; generated across various cortical areas, now known as “alpha rhythms” (Nunez, 2011; Nunez et al., 2015). Cognition is thought to emerge out of the timely coordination of such communication which is facilitated through greater synchrony between regionally distinct groups of neurons (Fries, 2005; Nyhus & Curran, 2010). Neural firing can occur at different rates, and as such neural oscillations can be divided into five major frequency bands including: Delta (0.5–3.5 Hz), theta (4–7.5 Hz), alpha (8–12.5 Hz), beta (13–30 Hz), and gamma (30–150 Hz; Buzsaki & Watson, 2012; Uhlhaas, Haenschel, Nikolic, & Singer, 2008). Because different frequency bands tend to elicit different patterns of synchrony across neural networks, each band has the potential to support specific cognitive functions (Ward, 2003). As such, white matter may affect cognition by optimizing information transfer within and across neural networks (Fields, 2015; Nunez et al., 2015; Pajevic et al., 2014).
Consistent with this, white matter structure predicts the speed of neural response in typically developing children and adults, and this relation is disrupted in injury, neurodevelopmental disorders, and disease (Berman et al., 2016; Dockstader, Gaetz, Rockel, & Mabbott, 2012; Dubois et al., 2008; Garces et al., 2014; Roberts et al., 2009; Sponheim et al., 2011; Stephen et al., 2013; Stufflebeam et al., 2008). Furthermore, children have been shown to produce weaker global alpha power but strong local alpha at various regions compared to adults, presumably due to immature cortico-cortical axon myelination (Srinivasan, 1999). Damage to white matter and impairment of its plasticity is associated with many brain disorders (Fields, 2015). Indeed, mutations in genes that regulate myelin as well as damage to white matter structure have both been implicated in a broad range of disorders associated with cognitive dysfunction (Bethune et al., 2011; Scantlebury et al., 2016; Soria-Pastor et al., 2008). Although it is widely theorized that disruption of plasticity interferes with the timing of neural signaling, and this ultimately perturbs brain synchrony and cognition—this hypothesis has received little empirical evaluation in children and adolescents.
Injury to myelin may cause disruption in timing of neural signaling that maintains oscillatory neural activity within and between neural coalitions (Bells et al., 2017; Nunez et al., 2015; Pajevic et al., 2014). Hence, comparing children with white matter injury to typically developing children on measures of white matter, neural communication, and cognition is a useful means to address how white matter may mediate cognitive function. Using this approach, we recently showed a fundamental connection between white matter microstructure and neural synchronization that is critical for cognitive processing (Bells et al., 2017). Specifically, by comparing brain tumor patients with white matter compromise to typically developing children, we established that changes in the microstructure of the optic radiations and neural synchrony during visual attention predict visual-motor reaction time. Furthermore, by testing the directionality of these links through statistical modeling and verifying our findings with computational modeling, we inferred a causal relationship—namely that changes in white matter microstructure impact cognition in part by disturbing the ability of neural assemblies to synchronize. This approach is useful to establish the potential connections between white matter, neural synchrony, and cognition that can be tested in future studies using longitudinal and intervention designs. Such studies can fully elucidate the role of change in white matter on changes in cognition. In the meantime, cross-sectional studies examining pediatric populations with direct myelin insult and cognitive impairment are useful in further characterizing the relations between the condition of white matter and neural function. In that regard, children with inflammatory demyelinating disease are an ideal model to examine the relations between white matter, neural communication, and cognition.
1.4 Use of inflammatory demyelination as a model to examine the impact of white matter microstructure on neural communication and cognition
Demyelinating syndromes, both recurrent disorders such as multiple sclerosis (MS) and monophasic syndromes, such as acute disseminated encephalomyelitis, are associated with progressive structural changes in the brain and may be associated with injury and altered growth trajectory in a wide range of brain networks in youth, even after a single event. In particular, white matter microstructural changes including focal tissue damage (lesions) and alterations to normal-appearing white matter occur in youth with MS and monophasic demyelinating disorders (Amato et al., 2010; Amato, Krupp, Charvet, Penner, & Till, 2016; Aubert-Broche et al., 2014, 2017; Longoni et al., 2017). DTI has been used extensively in children and adolescents with acquired demyelinating disorders to demonstrate tissue abnormality in normal-appearing white matter (Akbar et al., 2016; Aliotta et al., 2014; Aung et al., 2018; Longoni et al., 2017; Rocca et al., 2014; Till et al., 2011; Tillman, Leach, & Pirko, 2012; Vishwas et al., 2013; Vishwas, Chitnis, Pienaar, Healy, & Grant, 2010).
Cognitive impairment in childhood (Amato et al., 2016; Julian et al., 2013) and later on in adulthood (Ruano et al., 2018) in these patients may be the result of disturbed neural communication due to white and grey matter injury (Arrondo et al., 2009). In that regard, disrupted gamma oscillations are observed in adults with MS (Arpin et al., 2017; Barratt et al., 2017; Stickland et al., 2018). Gamma (30–150 Hz) oscillations are critical for local and large-scale cortical processing (Bastos, Briggs, Alitto, Mangun, & Usrey, 2014; Fries, 2009; Roberts et al., 2013), are associated with higher-order cognitive processes such as perception (Beauchamp, Sun, Baum, Tolias, & Yoshor, 2012; Tallon-Baudry, Bertrand, Delpuech, & Permier, 1997; Tallon-Baudry, Bertrand, Delpuech, & Pernier, 1996), attention (Chalk et al., 2010; Fries, Reynolds, Rorie, & Desimone, 2001; Marshall, O'Shea, Jensen, & Bergmann, 2015; Womelsdorf & Fries, 2007), and processing of sensory stimuli (Cheyne, Bells, Ferrari, Gaetz, & Bostan, 2008) and are disturbed in psychiatric disorders (Grützner et al., 2013).
Here, we examined differences in white matter microstructure between children and adolescents with recurrent demyelinating diseases (MS, myelin oligodendrocyte glycoprotein [MOG]-associated disorders) and typically developing children and tested whether such differences predicted differences between these populations in neural synchronization associated with basic visual function. We measured white matter microstructure using DKI across the brain and along with the optic radiation as this tract is involved in early visual processing (Mori et al., 2008). We used DKI (Jensen et al., 2005; Jensen & Helpern, 2010) in combination with intracellular and extracellular microstructure parameters from the WMTI model (Fieremans et al., 2011) to measure more specific white matter microstructure changes than previously observed in DTI studies. In particular, we measured axonal water fraction (AWF), intra-axonal axial and mean diffusivity (Da|| and Da, respectively), extra-axonal axial and radial diffusivities (De|| and De⊥, respectively) and tortuosity (TORT; TORT = De||/De⊥ and is thought to be a measure of myelin volume). Furthermore, the early visual response typically shows robust synchrony in particular in the gamma frequency range (60–100 Hz) and is a marker of visual perception (Fries, 2005; Roberts et al., 2013). Hence, neural synchronization within the gamma range associated with the early visual response was assessed using magnetoencephalography (MEG). We then used path modeling to test whether recurrent demyelinating disease has an impact on visual gamma synchrony via effects on white matter microstructure. Finally, we used computer simulations to further explore the hypothesis that perturbed axonal communication due to myelin damage may be responsible for disruption of neural synchrony.
2 MATERIALS AND METHODS
2.1 Participants
We recruited consecutive children and adolescents at the Hospital for Sick Children (SickKids) with recurrent demyelinating syndromes, including MS and anti-MOG antibody (ab) positive individuals. MS patients were classified using 2017 McDonald Criteria (Thompson et al., 2018). Youth with anti-MOG-ab positive disease were identified using the following parameters: (a) presence of MOG-ab positivity using a live cell-based assay at 3 months post onset; (b) <18 years of age at onset; (c) recurrence of neuroinflammation >12 weeks after first episode. MOG-ab testing was performed through a commercial laboratory, Oxford University, following live cell-based methods previously described (Kitley et al., 2014). A comparison cohort of age-matched typically developing children and adolescents were also recruited. Detailed medical variables of the patients and participant characteristics can be found in Table 1. Institutional Research Ethics Board approval and written informed consent (or assent and consent from a parent for younger children) from each participant and/or their guardian was obtained prior to study initiation. Excluded patients had a history of (a) other neurological conditions; (b) major medical comorbidities; (c) major psychiatric comorbidity; (d) learning disability; (e) major head injury; (f) alcohol or illicit drug abuse; or (g) less than 3 months since disease onset or relapse.
| MS | MOG | TDC | p value | |
|---|---|---|---|---|
| n | 13 | 12 | 28 | |
| Sex (female/male) | 9/4 | 10/2 | 17/11 | .97 |
| Age at assessment (years) | 16.9 ± 1.0 | 12.1 ± 3.0 | 15.0 ± 2.6 | .0002 |
| Range | (15.2–18.2) | (7.5–17.8) | (9.1–19.1) | |
| Age at diagnosis (years) | 14.2 ± 1.8 | 9.6 ± 3.6 | – | .001 |
| Range | (11.7–17.4) | (2.9–13.8) | – | |
| Mean assessment—Diagnosis (years) | 2.7 ± 1.7 | 2.5 ± 2.1 | – | .49 |
| Range | (0.1–6.2) | (0.6–6.4) | – | |
| Number of clinical events | 2.3 ± 1.6 | 2.8 ± 2.4 | – | .35 |
| Range | (1–5) | (1–9) | – | |
| Number of optic neuritis episodes | 0.53 ± 0.84 | 1.3 ± 0.8 | – | .012 |
| Range | (0–2) | (0–3) | – | |
| Optic neuritis episodes—Right | 0.31 ± 0.72 | 1.0 ± 0.7 | – | .02 |
| Range | (0–2) | (0–2) | – | |
| Optic neuritis episodes—Left | 0.23 ± 0.57 | 1.1 ± 0.79 | – | .0004 |
| Range | (0–2) | (0–3) | – |
2.2 MRI protocol
All participants were scanned on a 3 T Siemens Prisma system (Siemens Medical Solutions, Erlangen, Germany) at the Hospital for Sick Children. We acquired the following MRI sequences: (a) an axial turbo spin-echo proton density [1 × 1 × 3 mm; repetition time = 2,200 ms; echo time = 10 ms; turbo factor 4]; (b) an axial T2-weighted turbo spin-echo [1 × 1 × 3 mm; repetition time = 4,500 ms; echo time = 84 ms; turbo factor 11]; (c) an axial T1-weighted, 3D magnetization prepared rapid gradient echo sequence [0.9 × 0.9 × 1.0 mm; repetition time = 1910 ms; echo time = 3.51 ms; flip angle = 9°, inversion time = 1,100 ms]; (d) a sagittal 3D FLAIR sequence [1 × 1 × 1 mm; repetition time = 6,000 ms; echo time = 356 ms; inversion time = 2,200 ms]; and (e) three sets of diffusion-weighted images using a single shot spin-echo sequence with an echo planar imaging readout [TR = 3,800 ms; TE = 73.0 ms; FOV = 244 × 244 mm, 70 slices, slice thickness = 2.0 mm, no gap, bandwidth = 1952 Hz/pixel, 2 × phase encoding polarities (anterior–posterior) along 35, 45, and 66 directions for b-values of 1,000, 1,600, and 2,600 s/mm2. Participants watched a movie through video goggles to prevent excessive head movement during the scan. In order to overlay MEG information on the MRI of the subject's brain, the three fiducial points were identified on MRI images and co-registered with the fiducial information from the MEG.
2.3 MRI data preprocessing
2.3.1 Lesion masks
For participants with demyelinating syndromes (MS and MOG-associated disorders) lesions were segmented at the McConnell Brain Imaging Centre, Montreal Neurological Institute (Montreal, QC, Canada) using an automatic change detection naïve Bayesian algorithm that classifies tissue using changes within T1, T2, proton density-weighted, and FLAIR images (Elliott, 2016). After automatic lesion segmentation lesions were then reviewed and manually corrected, if necessary, by trained personnel with expertise in demyelinating lesion identification. The T1-weighted images for each participant were registered to their corresponding diffusion space using a 12-parameter affine transformation using ANTS (Avants et al., 2014).
2.3.2 Diffusion MRI preprocessing
Diffusion images were processed using the DESIGNER pipeline (Ades-Aron et al., 2018). DKI preprocessing steps including: Denoising using a principal component analysis technique (Veraart et al., 2016; Veraart, Fieremans, & Novikov, 2016), Rician bias correction within MRtrix (Version 3.0 rc2), Gibbs ringing correction (Kellner, Dhital, Kiselev, & Reisert, 2016), EPI distortion correction using topup (Andersson et al., 2003) in FMRIB's Software Library (FSL) version 5.0.11, eddy current and motion correction using eddy in FSL (Version 5.0.11; Andersson & Sotiropoulos, 2016), and signal outlier detection (Collier, Veraart, Jeurissen, den Dekker, & Sijbers, 2015). In the WMTI model, two microstructural environments are estimated (intracellular and extracellular) (Fieremans et al., 2011). White matter microstructure parameters were calculated using weighted linear least squares estimation (Fieremans et al., 2011; Tabesh, Jensen, Ardekani, & Helpern, 2011). These parameters included FA (used only for registration within the tract-based spatial statistics [TBSS] pipeline), AWF, Da||, Da De||, De⊥, and TORT.
2.3.3 White matter-based spatial statistics
Voxel-wise analysis using TBSS (Smith et al., 2006) was used to perform statistical analysis on the above parameters to test for group differences between patients with MS, MOG-associated disorders, and typically developing children. White matter parameters were aligned to the FMRIB58_FA template and skeletonized prior to statistical analysis. Registered lesion masks were input using FSL to exclude voxels that contain lesions within the patient groups using setup_mask (FSL version 5.0.11; see Figure 1 for lesion probability map). Cross-participant voxel-wise analyses were performed nonparametrically using Randomise (FSL, 5,000 permutations; Winkler, Ridgway, Webster, Smith, & Nichols, 2014) to test for the effects of group using age, age of diagnosis, sex, disease activity, disease duration, and number of optic neuritis episodes as covariates (to test the effects of age at diagnosis independent of sex on WMTI parameters). Threshold-free cluster enhancement and a familywise error corrected at p < .01 was considered significant. Statistical threshold was determined by application of the Bonferroni correction for six WMTI parameters (AWF, Da||, Da, De||, De⊥, and TORT), where pFWE < .00714 (0.05/7) was considered significant.
2.4 MEG protocol
Neuromagnetic activity was recorded using a 151-channel CTF MEG system (600 samples/s, DC-200 Hz; MISL, Coquitlam, BC, Canada) in a magnetically shielded room simultaneously with high-resolution eye tracking (Eyelink 1000, SR Research Ltd, Oakville, ON, Canada). Continuous MEG data were collected while the participants lay supine in the MEG, while stimuli were displayed on a semi-transparent screen placed 50 cm from their eyes. Visual stimuli, eye positions and trial markers (e.g., beginning of the trial) were exported and synchronized with MEG collection. Eye position was calibrated using a nine-point array that covered most of the visual field. Small coils placed at fiducial locations (nasion and preauricular points) were used to monitor head position during recording.
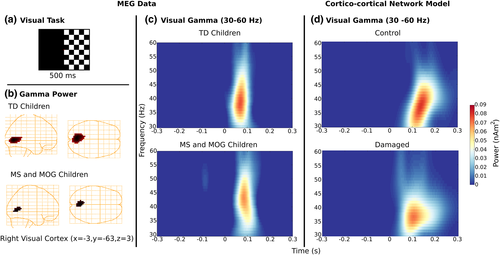
For the duration of the visual task, participants were asked to keep their right eye open and fixated on a red square in the center of the computer screen, while the left eye was patched. A black and white checkerboard pattern (polarity reversing every 300 ms) was displayed on the left or right side of the screen for 200 trials each lasting approximately 4 min (Figure 2, panel a). Fiducial locations were used to co-register source images to the subjects structural T1-weighted MR images.
2.5 MEG data preprocessing
Data were offline filtered from 40–150 Hz and segmented into epochs from −1,000 to 1,000 ms, with respect to the change in the checkerboard pattern. Trials were rejected if the head motion exceeded 5 mm or peak-to-peak changes greater than 4 pT. Localization of neuromagnetic signals was conducted using a frequency-based beamforming algorithm and single-sphere head model integrated within the BrainWave MATLAB toolbox (Jobst, Ferrari, Isabella, & Cheyne, 2018). We measured changes in induced cortical oscillations within the gamma frequency band using the synthetic aperture magnetometry (SAM) algorithm (Robinson & Vrba, 1999) in the visual cortex. Whole-brain pseudo-T difference images were created by subtracting the source power during the active time window of 300 ms duration (0–0.3 s) from a prestimulus baseline period (−0.3 to 0 s) of equal duration in the gamma (40–120 Hz) frequency band.
Source images were spatially normalized to the Montreal Neurological Institute (MNI) T1-template brain with Statistical Parametric Mapping (SPM 12: Welcome Institute of Cognitive Neurology, London, UK; Ashburner, 2009) for group averaging and alignment to the Talairach atlas.
Statistically significant pseudo-T images for each group separately were averaged and thresholded using a nonparametric permutation test adapted for beamformer source images (Singh, Barnes, & Hillebrand, 2003) and glass-heads were created to visually inspect the peak sources related to the task within the active window. Time–frequency representations (TFRs) were constructed from source waveforms at the peak location using a Morlet wavelet frequency transformation (Tallon-Baudry et al., 1997) of single-trial source activity over a frequency range of 40–140 Hz in 1 Hz steps. TFRs were converted to percent change in power relative to prestimulus baseline.
2.6 Partial least squares—Path modeling
We employed Partial Least Squares, Path Model analysis, using PLSpm in R to measure the performance of our path model (number of bootstraps = 5,000; Sanchez, 2013). For this model, we hypothesize that the presence of a demyelinating syndrome (Group: MS and MOG-associated disorders versus typically developing children) had direct and indirect effects on visual gamma neural synchrony associated with visual perception via differences in white matter microstructure of the optic radiations. We first tested the accuracy of the model using a number of criteria: (a) examining the relationship between the latent constructs and their associated measures, known as loading; (b) Cronbach's alpha; (c) Dillon–Goldstein's rho. Finally, the discriminant validity of the measurement model was tested by evaluating whether the cross-loading for each measure relative to the other latent constructs were larger than the loadings obtained from measures belonging to other latent constructs. Next, the quality of the structural model was evaluated in four ways (a) significance of the regression fit (t test), (b) R2 determination coefficients of the endogenous variables (low R < 0.2, moderate 0.2 < R < 0.5), (c) average variance extracted (AVE), and (d) goodness-of-fit. The goodness-of-fit measure (GoF) for our PLS path modeling is proposed as the geometric mean of the average communality and the average R2. We used the following guidelines for judging a good fit: GoF > 0.36 (Tenenhaus, Vinzi, CHatelin, & Lauro, 2005); bootstrap percentile confidence intervals for path weights (β; 95% percentile), and R2 did not contain zero.
2.7 Computational modeling
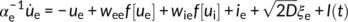
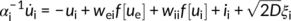
The activation function f determines the response of a population to recurrent inputs from other populations, as is defined by f[U] = (1 + exp[−25 U])−1. Both excitatory and inhibitory populations receive recurrent inputs from each other (via the coupling gains wee, wie, wei, wii), constant inputs ie, i and Gaussian white noise of variance D. With chosen parameters, the set of equations above exhibits gamma-like oscillations at about 50 Hz, although this peak frequency can be adjusted to fit the data.
We used this model to evaluate the influence of the temporal coincidence of visual inputs on evoked gamma responses (see Figure 3). To do this, we considered a stream of visual stimuli whose arrival times would be impacted by myelin damage along with the optic radiations. These inputs are considered to be relayed from the retina through the lateral reticulate nucleus (LGN) via the optic radiations. Specifically, excitatory neurons were driven by a stream of afferent stimuli, delivered with timings τk, that is, 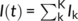 , where Ik = Ioδ(t − τk)/K for some intensity Io. These individual pulses where set to a duration of 10 ms. In the spirit of previous work from the authors (Bells et al., 2017), inputs were delivered with timings statistics that reflect myelin integrity. Specifically, the conduction timings τk were sampled from a gamma distributionΓ(α, θ), whose order α was increased to represent myelin damage (reflecting more variable conduction velocities). The higher the order, the more variable is the conduction times and thus the less coincident are the inputs (see Figure 3). This results in a diminished perturbation amplitude on cortical populations and thus decreases net gamma power evoked in the response. In both controls and compromised cases, the overall intensity of the visual inputs remains the same, but only the effective dispersion of the timings changes, leading to variable evoked gamma responses. We analyzed the power of the responses at visual stimulation onset using the same method as the human MEG data. The computational MEG signal M(t) was modeled as the mean activity of the excitatory population subjected to observational Gaussian white noise of intensity S, that is, M(t) = ue(t) + S ξ(t), with S = 0.01 (refer Table 2 for the parameters used in the model).
, where Ik = Ioδ(t − τk)/K for some intensity Io. These individual pulses where set to a duration of 10 ms. In the spirit of previous work from the authors (Bells et al., 2017), inputs were delivered with timings statistics that reflect myelin integrity. Specifically, the conduction timings τk were sampled from a gamma distributionΓ(α, θ), whose order α was increased to represent myelin damage (reflecting more variable conduction velocities). The higher the order, the more variable is the conduction times and thus the less coincident are the inputs (see Figure 3). This results in a diminished perturbation amplitude on cortical populations and thus decreases net gamma power evoked in the response. In both controls and compromised cases, the overall intensity of the visual inputs remains the same, but only the effective dispersion of the timings changes, leading to variable evoked gamma responses. We analyzed the power of the responses at visual stimulation onset using the same method as the human MEG data. The computational MEG signal M(t) was modeled as the mean activity of the excitatory population subjected to observational Gaussian white noise of intensity S, that is, M(t) = ue(t) + S ξ(t), with S = 0.01 (refer Table 2 for the parameters used in the model).
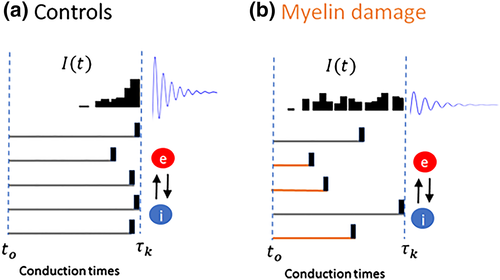
| Symbol | Definition | Value |
|---|---|---|
| αe | Membrane rate constant—Excitatory cortical cells | 2.5 |
| αi | Membrane rate constant—Inhibitory cortical cells | 2.5 |
| wee | e → e synaptic connection strength | 1.6 |
| wei | e → i synaptic connection strength | 2.0 |
| wie | i → e synaptic connection strength | −3.6 |
| wii | i → i synaptic connection strength | −1.3 |
| ie | Bias input to excitatory population | 0.0 |
| ii | Bias input to excitatory population | −0.2 |
| Io | Visual stimuli amplitude | 8 |
| D | Noise variance | 0.0001 |
| dt | Integration time step | 1 ms |
3 RESULTS
3.1 Medical and demographic comparisons
There were no significant differences between the healthy control and patients groups with respect to sex [Kruskal–Wallis Test: Χ2(1, N = 53) = 0.001, p = .97] and between MS and MOG with respect to disease duration [Χ2(1, N = 25) = 0.48, p = .49] and number of clinical events [Χ2 (1, N = 25) = 0.887, p = .35]. Significant differences between the groups with the respect to age [Χ2 (1, N = 53) = 17.325, p = .0002] and between MS and MOG with respect to age of diagnosis [Χ2 (1, N = 25) = 10.65, p = .001], number of optic neuritis episodes on the left [Χ2 (1, N = 25) = 6.26, p = .0004], right [Χ2 (1, N = 25) = 5.2823, p = .02], or both optic nerves [Χ2 (1, N = 25) = 5.2823, p = .012] were found.
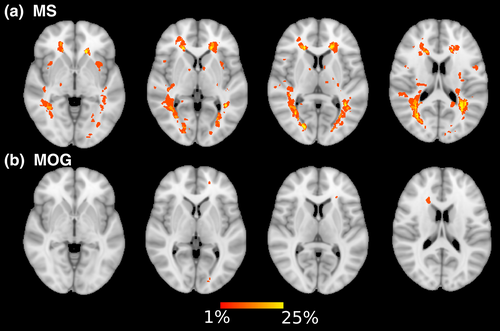
3.2 Lesion masks in children with demyelinating syndromes
We calculated a lesion probability map to demonstrate the number of lesions within our patient groups, MS and MOG, throughout both hemispheres (Figure 1).
3.3 White matter microstructure is compromised in children with demyelinating syndromes
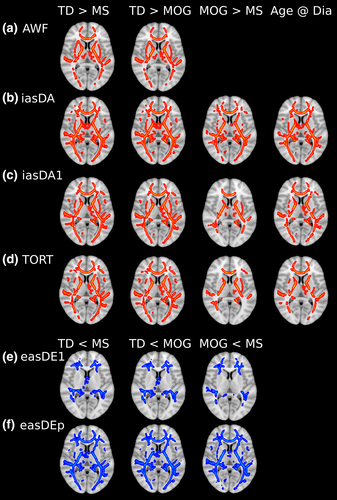
We used a voxel-wise approach to test for group differences in 6 WMTI metrics: AWF, Da||, Da, De||, De⊥, and TORT. We found no difference within these metrics as a function of disease duration, disease activity, or number of optic neuritis episodes. Consequently, these variables were not used in the final TBSS analysis. Typically developing children displayed higher AWF, Da||, Da, and TORT relative to children with demyelinating syndromes (MS- and MOG-associated disorders) (Figure 4a–d). In contrast, typically developing children had lower De||, and De⊥, relative to children with demyelinating syndromes (Figure 4e,f). In comparison, children with MOG-associated disorders displayed higher AWF, Da||, Da, and TORT and lower De||, and De⊥, relative to children with MS. Axial related indices, Da||, Da, and TORT, correlated with age of clinical disease onset, which changed at a greater rate at younger ages. Significant voxels of group difference from the thresholded voxel-wise images within the left and right optic radiation for the WMTI metrics were extracted and carried forward to for use in the path modeling model.
3.4 Visual gamma synchrony is perturbed in children with demyelinating syndromes
Differential power images using the SAM beamformer revealed gamma activity time-locked to the checkboard-switch, beginning approximately at onset and staying active until after 0.1 s (Figure 2). In Figure 2, group-averaged visual gamma activity for patients and typically developing children separately is localized to visual cortex and presented on template glass-brain plots (Talairach coordinate: x = −3, y = −63, z = 3, p < .01) (Figure 2, panel b). Visual gamma power (30–60 Hz) reached a maximum following the checkerboard switch (~0.05 s; Figure 2, panel c). Analysis of the effects of group, age at scan, age of diagnosis, number of optic neuritis episodes bilaterally, on the left and right on gamma power was performed using a square-root transformation and a MANOVA (Pillai's Trace = 0.30, F = 2.95, df (2, 51), p = .017. Maximum gamma power relative to baseline was stronger for typically developing children than children with demyelinating syndrome (F [2,51] = 15.237, p = .0003). Follow-up univariate ANOVAs also revealed that gamma power differed as a function of age of symptom onset (F [2,51] = 10.99, p = .0018), duration of disease (F [2,51] = 11.91, p = .0011). Furthermore, gamma power differed as a function of number of optic neuritis episodes bilaterally (F [2,51] = 5.75, p = .02), on the left (F [2,51] = 5.75, p = .02) and right (F [2,51] = 4.07, p = .0049). Thus, peak values for gamma in V1 were carried forward to our path model.
3.5 White matter microstructure influences reaction time via effects on neural synchrony
Finally, we tested the relations between groups and WMTI microstructure in predicting visual gamma using Partial Least Squares–Path Model analysis (Sanchez, 2013). Within our hypothesized model, we suggested that children with demyelinating syndrome have compromised white matter microstructure within their optic radiation, which in turn impacts neural synchrony within the visual cortex (Figure 5a). Thus, we constrained our hypothesized path model to include a mean of the voxels that passed thresholds for all WMTI indices within the optic radiations to predict visual gamma (Visual γ) synchrony within the visual cortex. Besides group status (i.e., children with demyelinating syndrome vs. typically developing children), WMTI indices along the optic radiations, and visual gamma within the visual cortex, we also included age, age of symptom onset, duration of disease, number of clinical events, and number of optic neuritis episodes as variables in the model. In particular, we tested if WMTI metrics influenced MEG gamma synchrony (number of bootstraps = 5,000).
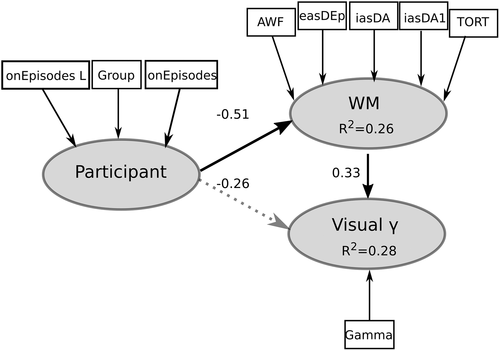
First, the accuracy of a measurement model that included the following latent constructs was tested: Participant (indicators are age, age of disease onset, sex, and group status), white matter (indicators are AWF, Da||, Da, De||, De⊥, and TORT; note De||, De⊥, loading on the latent variable were changed to be similar as the other white matter indices) within the ORs, and Visual γ (indicators are peak gamma percent change values). All indicators had a loading of greater than 0.8 on their respective latent constructs, except age, age of symptom onset, disease duration, and De|| where loadings were 0.32, 0.66, 0.77, and 0.67, respectively. Hence, these measures were removed from the model. All remaining measures corresponded well with their latent constructs (Cronbach's alpha >0.85, Dillon–Goldstein's rho >0.9). Furthermore, the discriminant validity of the measurement model was good: Cross-loading for each measure relative to the other latent constructs were larger (>0.1) than the loadings obtained from measures belonging to other latent constructs.
We observed within our hypothesized model that participant predicted white matter (β = −0.50 [−0.75, −0.36 CI95], t = −4.0 p = .0005) and white matter predicted Visual γ (β = 0.33 [0.02, 0.61 CI95], t = 2.25 p = .03; Figure 5). Coefficients of determination (R2) for each latent variable were white matter = 0.26 (0.10, 0.49 CI95) and Visual γ = 0.25 (0.15, 0.42 CI95). The direct effect of participant on Visual γ was not significant (β = −0.26 [−0.48, 0.007 CI95], t = −1.78, p = .08). Decreased AWF, TORT, and intra-axonal diffusivity (Da||, Da) and increased extra-axonal radial diffusivity (De⊥), predicted reduced Visual γ. The GoF for our PLS path modeling was 0.46. AVEs were 0.90 and 1.0 for white matter and Visual γ, respectively, which were all larger than the recommended 0.5 cut-off (Sanchez, 2013) and greater than the correlations between constructs (<0.80). Thus, acquired demyelinating syndromes in children appear to have a direct effect on white matter microstructure and an indirect effect on Visual γ synchrony via impact of microstructure.
3.6 The influence of signal integrity on oscillatory power
To better understand one potential mechanism underlying these findings and explore our hypothesis further, we analyzed a computational model of gamma response in V1 where the coincidence of feedforward visual inputs was disrupted as a consequence of myelin compromise of the optic radiations. This was done to model the influence of disrupted connectivity on downstream visual populations. Specifically, we evaluated the impact of input coincidence on the power of gamma oscillations in both excitatory and inhibitory cortical populations. To assess the effect of myelin integrity on the propagation of visual sensory signals, we increased the dispersion of the axonal conduction times of afferent visual inputs by increasing the order of the gamma distribution from which those where sampled (see section 2).
As seen in Figure 1d, our simulations reveal that, despite the overall amplitude of the visual inputs being the same, overall gamma power was highly sensitive to the temporal structure of these inputs. Less coincident visual inputs were found to decrease the salience of the gamma power amplification observed after stimulation onset, which corroborated our experimental findings (compare Figure 1c,d for experimental versus computational findings). Taken together, our computational simulations highlight the key role played by the temporal organization of signals between neural assemblies and outline the important role of myelin (and signal) integrity in the generation and preservation of coherent oscillatory activity.
4 DISCUSSION
Using multimodal neuroimaging, we provide novel evidence for associations between local white matter—including estimates of myelin and axonal microstructure—and local gamma oscillations in visual cortex. Our main findings are (a) children and adolescents with demyelinating disorders showed evidence of myelin and axon compromise and reduced gamma power during a visual task and (b) differences in WMTI metrics within the optic radiations between patients and typically developing children predict peak gamma oscillations within visual cortex. As few studies have investigated the links between white matter microstructure and neural function, our results lay the groundwork for future multimodal longitudinal studies across healthy and diseased populations where this behavior and activity may be altered. We have furthermore investigated one plausible mechanism at play using a computational model of gamma generation in early visual cortical areas.
Our clinical sample is an ideal population in which to examine the relationship between myelin and neural communication. First, our findings are consistent with previous DTI work showing that children and adolescent with demyelinating syndromes, mainly MS, display compromise of nonlesional normal-appearing white matter (Akbar et al., 2016; Longoni et al., 2017). However, we extend this work using a DKI-WMTI approach that is more specific to white matter microstructure than DTI. In particular, we observed that children and adolescents with MS and MOG displayed decreased AWF, myelin volume (TORT), and intra-axonal diffusivity (Da|| and Da) as well as increased extra-axonal radial diffusivity (De⊥) along with the optic radiations and throughout normal-appearing white matter while controlling for age of the participant. To the best of our knowledge, this is the first study to demonstrate white matter microstructure compromise in normal-appearing white matter in MOG-associated disorders. Future studies looking at the longitudinal changes with children with demyelinating disorders will help address the disease impact on developmental plasticity.
The WMTI markers have been shown to be related to myelin in histological studies. AWF and extra-axonal radial diffusivity (De⊥) are sensitive to both demyelination and remyelination in cuprizone animal models (Falangola et al., 2014; Jelescu et al., 2016). Furthermore, intra-axonal axial diffusivity (Da|| and Da) is decreased during acute inflammation in the cuprizone model (Guglielmetti et al., 2016). AWF and intra-axonal radial diffusivity (Da|| and Da) also reflect axonal density changes due to injury (Chung et al., 2018).
Post-mortem MS studies have shown myelin and axonal injury within lesions: However, axons appear to be preserved in remyelinated areas (Schultz et al., 2017): Crucially, if axons are not remyelinated, axonal degeneration occurs (Lee, Biemond, & Petratos, 2015; Simons, Misgeld, & Kerschensteiner, 2014; Singh et al., 2017). Post-mortem data from MOG lesions demonstrated demyelination with partial axonal preservation (Zhou et al., 2017). Thus, the differences in WMTI metrics between children and adolescents with MS and MOG versus typically developing children may reflect the impact of the disease on both myelin and axon structure and highlights the role of axonal injury accompanying myelin injury.
Second, gamma oscillations during visual task performance were perturbed in our patient group relative to typically developing children. These gamma oscillations were not only influenced by group status but by age of symptom onset, disease duration, and number of optic neuritis episodes, but not age at scanning. By comparing such a clinical population to typically developing children, we were able to reveal differences within neural synchrony—providing a platform to model the impact of myelin and axonal damage across different diseases on neural oscillations during development. Our findings are consistent with findings in adults with MS, where gamma disruption have been observed using MEG during visual task performance (Barratt et al., 2017; Stickland et al., 2018). Gamma oscillations may reflect neural input to visual cortex from the retina via the lateral geniculate nucleus (Castelo-Branco, Neuenschwander, & Singer, 1998; Sadakane et al., 2006; Solomon, Lee, & Sun, 2006). Recently, Murty, Shirhatti, Ravishankar, and Ray (2018) demonstrated in humans using EEG and nonhuman primates using local field potentials that slow (~20–40 Hz) gamma emulated long-range processing and fast (40–70 Hz) gamma was systematic to short-range processing. (Barratt et al., 2017; Stickland et al., 2018). Our findings may reflect core deficits in visual processing that may influence broader cognition in children and adolescents with MS and MOG-associated disorders—and appear to reflect disrupted local neural communication.
Damage to myelin and axons alters conduction velocities and decreases temporal synchrony within neural ensembles by increasing the variance in conduction velocity, resulting in impaired neural function (Bells et al., 2017; Fields, 2015; Nunez et al., 2015; Pajevic et al., 2014). In past DTI work, decreased FA in pediatric MS patients was associated with increased connectivity within the resting state visual network (Akbar et al., 2016). Here, using path modeling, we observed that the impact of inflammatory demyelination (as reflected by group differences between patients and typically developing children accounting for disease activity and eye-specific optic neuritis) had indirect effects on gamma oscillations in the visual cortex during visual stimulation—through compromise of myelin and axon structure within the optic radiations. Based on these findings, we conclude the injury of the optic radiations within our patient population may play a contributing factor in decreased gamma power within visual pathway. That is, the decreased gamma power that we observed may reflect alterations in conduction speeds associated with white matter compromise of the optic radiations within patient groups. These findings motivate us to propose that regional neuronal communication is modulated by local white matter microstructure. To explore this mechanism further, we developed and analyzed a computational model of gamma generation in the visual cortex, and deliberately altered the temporal structure of the inputs, to reflect myelin damage along with the optic radiations. Our simulations aligned with our experimental findings and gamma power was indeed seen to be hindered by disrupted input timing.
Our current experimental and computational findings, as well as past work (Bells et al., 2017), show a fundamental connection between white matter microstructure and neural synchronization that appears critical for cognitive processing. Although studies comparing patients and healthy cohorts are essential in delineating such a connection, our patient based cross-sectional studies ultimately do not provide evidence of the role of myelin plasticity in neural communication and cognition. Future multimodal imaging studies are required, including longitudinal and intervention studies, to follow-up on the exciting relations observed between white matter, neural communication, and cognition.
5 SUMMARY
5.1 Rate of change in white matter imaging metrics—Evidence of mesoscopic plasticity
Based on findings from longitudinal and intervention studies, estimates of change in white matter, including slope or difference scores provide the most useful metrics of plasticity. The key contributions of longitudinal studies are clear from these studies, in that it is the trajectory of white matter change, or plasticity, that predict cognitive function, and in particular, the rate of change in different white matter regions predicts the development of different cognitive abilities. Faster rates of change throughout childhood and adolescence appear most adaptive in predicting both faster rate of cognitive development and final ability (Wang et al., 2017; Yeatman et al., 2012; Young et al., 2017). How protracted the change in white matter is, however, also important: Slower and more protracted myelination in childhood predicts improved intellectual growth (Deoni et al., 2016). Furthermore, there may be critical periods where greater plasticity—as reflected in increased rates of growth—support specific cognitive functions (Simmonds et al., 2014).
5.2 Connecting cellular plasticity to mesoscopic plasticity
With the advent of multimodal imaging, we can fully characterize the structural and functional dynamics of brain networks that underlie white matter plasticity and cognitive development. Furthermore, novel imaging methods and approaches have allowed greater information about cellular or molecular properties in humans. However, these approaches still do not have cellular resolution nor straightforward access to manipulating myelin—such as those that exist in animal work involving novel transgenic approaches where myelin plasticity can be inducibly manipulated. A key for future research will be multi-disciplinary collaboration that links the study of myelin plasticity and behavior in animal models to analogous studies of white matter and cognition in humans. This work can be coupled using computational modeling. With the ability to develop and manipulate simulations of cortical microcircuits and large-scale brain networks, the impact of white matter perturbations on synchrony and timing of cortical signaling can be modeled using computational and mathematical models, as we have done here. Informed by both animal and human data, biologically inspired computational and mathematical models can be developed and tested in silico to determine whether timing of neural interactions—that are simulated to reflect changes in white matter—constrains the emergence of coherent oscillations within large-scale neural networks and cortical microcircuits. Computational and modeling approaches—which are able to generate thousands of trials and scan large intervals of parameters' settings in matters of seconds—represent invaluable tools to generate new hypotheses and explore a large variety of different scenarios in which perturbations of timing—reflecting myelin plasticity—interfere with synchrony across distances that vary widely between species and across development.
5.3 Harnessing white matter plasticity for cognitive recovery
Finally, with increasing understanding of myelin plasticity, we are now on the cusp of harnessing neuroplastic processes to repair the injured brain and foster cognitive recovery. Indeed, multiple targets for therapy to repair myelin following pediatric injury have been identified (Chakrabarti, Scafidi, Gallo, & Haydar, 2011; Dadwal et al., 2015; Scafidi et al., 2014). As noted previously, there is promising evidence in children with white matter damage from curative brain tumor treatment that activity-dependent and pharmacological treatments can foster white matter growth and improved cognition (Riggs et al., 2017; Ayoub et al., submitted). Determining the mechanisms of how white matter plasticity may control and coordinate neural communication and cognitive maturation has the potential to profoundly alter our fundamental understanding of neuroplasticity and guide future interventions targeting brain repair.
ACKNOWLEDGMENTS
The authors thank Stephanie Grover, Cynthia De Medeiros, Tara Berenbaum, Danusha Nandamalavan, and Austin Noguera for their assistance with participant recruitment and Jovanka Skocic, Tammy Rayner, and Ruth Weiss for image acquisition. We would also like to thank Els Firemans and Benjamin Ades-Aron at NYU for their invaluable help in applying the DESIGNER pipeline. This research was supported by an operating grant from by the Ontario Institute of Regenerative Medicine.