Glutamate versus GABA in neuron–oligodendroglia communication
Funding information Agence Nationale de la Recherche, Grant/Award Number: ANR-14-CE13-0023-02; Fondation pour la Recherche Médicale, Grant/Award Number: Equipe FRM DEQ20150331681; Fondation pour l'Aide à la Recherche sur la Sclérose en Plaques; National Multiple Sclerosis Society, Grant/Award Number: RG-1612-26501
Abstract
In the central nervous system (CNS), myelin sheaths around axons are formed by glial cells named oligodendrocytes (OLs). In turn, OLs are generated by oligodendrocyte precursor cells (OPCs) during postnatal development and in adults, according to a process that depends on the proliferation and differentiation of these progenitors. The maturation of OL lineage cells as well as myelination by OLs are complex and highly regulated processes in the CNS. OPCs and OLs express an array of receptors for neurotransmitters, in particular for the two main CNS neurotransmitters glutamate and GABA, and are therefore endowed with the capacity to respond to neuronal activity. Initial studies in cell cultures demonstrated that both glutamate and GABA signaling mechanisms play important roles in OL lineage cell development and function. However, much remains to be learned about the communication of glutamatergic and GABAergic neurons with oligodendroglia in vivo. This review focuses on recent major advances in our understanding of the neuron–oligodendroglia communication mediated by glutamate and GABA in the CNS, and highlights the present controversies in the field. We discuss the expression, activation modes and potential roles of synaptic and extrasynaptic receptors along OL lineage progression. We review the properties of OPC synaptic connectivity with presynaptic glutamatergic and GABAergic neurons in the brain and consider the implication of glutamate and GABA signaling in activity-driven adaptive myelination.
1 INTRODUCTION
Oligodendrocyte precursor cells (OPCs), also named NG2 cells, are the progenitors of oligodendrocytes (OLs), the major myelin-forming cells of the central nervous system (CNS). Regulation of OL cell number is a key process allowing for the dynamic myelination of the CNS that highly depends on OPC proliferation and differentiation. OPCs are abundant during early development, but also persist in the adult brain where they represent the major proliferative cell population (Nishiyama, Komitova, Suzuki, & Zhu, 2009). These progenitors as well as OLs sense their environment through the functional expression of multiple types of receptors responding to neurotransmitters and other external factors released by surrounding cells. These receptors constitute the basis for multiple modes of neuron–oligodendroglia interactions that influence OPC and OL functions during postnatal development (Maldonado & Angulo, 2015). The importance of the partnership between neurons and oligodendroglia is well illustrated by the fact that OPCs are the only glial cell type receiving synaptic inputs from neurons (Bergles, Roberts, Somogyi, & Jahr, 2000), even though the role of these synapses remains controversial. As for neuron–neuron communication, nonsynaptic modes of neuron–oligodendroglia communication like ectopic or extrasynaptic transmission also exist and probably play different roles both in the normal and pathological CNS (Maldonado & Angulo, 2015; Maldonado, Velez-Fort, & Angulo, 2011). Given the close association of adjacent membranes of axons and oligodendroglia, other modes of interactions based on cell adhesion molecules or gap-junction communication may influence oligodendroglia development and myelination (Kleopa, Orthmann-Murphy, & Sargiannidou, 2010; Piaton, Gould, & Lubetzki, 2010).
The communication between neurons and oligodendroglia has mainly been studied at postnatal stages, a period crucial for the process of CNS myelination. Although neuronal activity is not a mandatory requirement for myelination (Bechler, Swire, & Ffrench-Constant, 2018), it influences the homeostasis of OPCs and OLs, impacts their fate and affects myelin architecture and function. Despite the intense investigation of the effect of neuronal activity on these glia cell types, the signals linking neuronal activity and OL lineage cells are still poorly understood in vivo. A better comprehension of these roles is central to improve our knowledge of the impact of oligodendroglia and myelin dynamics on shaping brain circuit maturation and function. Moreover, alterations of neuron–oligodendroglia interactions represent an important candidate mechanism in demyelinating disorders and other CNS pathologies. In this context, glutamate and γ-aminobutyrique acid (GABA), the two major neurotransmitters of the CNS, are ideal molecules to establish a link between neurons and oligodendroglia as evidenced by the diversity of ionotropic and metabotropic receptors for these neurotransmitters expressed by OPCs and OLs in different CNS regions. In this review, we will recall some previous findings on glutamate and GABA receptor expression and function in OPCs and OLs. However, we will mainly highlight recent advances in our understanding of the communication of glutamatergic and GABAergic neurons with oligodendroglia in the CNS. We will attempt to identify the main glutamatergic and GABAergic neuronal subtypes engaged in neuron–oligodendroglia communication and discuss whether the activity of these neurons is required for oligodendroglia migration, survival, proliferation, and/or differentiation as well as for driving a general or a specific myelination.
2 GLUTAMATE IN NEURON–OLIGODENDROGLIA COMMUNICATION
2.1 Glutamate receptors expressed by OPCs and OLs
Among the large array of neurotransmitter receptors present in oligodendroglia, glutamate receptors seem to occupy a key position since they are highly diverse and differentially expressed by progenitors and mature OLs. In particular, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) have aroused great interest since the discovery of AMPAR-mediated neuron–OPC synapses (Figure 1a; Bergles et al., 2000). The description of functional AMPARs in OPCs was first provided in vitro by whole-cell patch-clamp experiments and pharmacological approaches (Barres, Koroshetz, Swartz, Chun, & Corey, 1990; Gallo et al., 1996) and further confirmed by RT-PCR analyses (Itoh, 2002). AMPARs are heterotetramers formed by the combination of GluA1-4 subunits. By analyzing gene expression profiling data obtained from oligodendroglia during OL lineage progression (Cahoy et al., 2008), De Biase, Nishiyama, and Bergles (2010) found that mRNAs of GluA subunits are highly expressed in OPCs compare to both premyelinating and myelinating oligodendrocytes. This downregulation of AMPARs once OPCs start to differentiate into OLs is concomitant with the loss of glutamatergic synapses during the differentiation process (Figure 1b; De Biase et al., 2010; Etxeberria, Mangin, Aguirre, & Gallo, 2010; Kukley, Nishiyama, & Dietrich, 2010). A more complete description of the molecular identity of AMPARs of OPCs in the mouse brain has been established by RNA-seq transcriptomics of cortical NG2-positive cells at P17 (Zhang et al., 2014), and more recently, by in situ hybridization in corpus callosum (Kougioumtzidou et al., 2017). It seems that GluA2-4 subunits are by far the main subunits expressed by mouse OPCs. Despite the presence of GluA2 in most OPCs, a key determinant for AMPAR Ca2+ impermeability, both Ca2+-permeable and Ca2+-impermeable AMPARs are co-expressed in these cells (Ge et al., 2006). Noteworthy, the proportion of Ca2+-permeable receptors differently evolves with time according to the brain region. In the hippocampus, they are more abundant in OPCs of younger animals (Ge et al., 2006) while, in corpus callosum, their expression increases with age (Ziskin, Nishiyama, Rubio, Fukaya, & Bergles, 2007). Moreover, the balance between Ca2+-permeable and Ca2+-impermeable AMPARs can also be modulated by environmental or intrinsic factors. Indeed, the deletion of the obligatory subunit GluN1 of N-methyl-d-aspartate receptors (NMDARs) in OPCs or the activation of their group 1 metabotropic glutamate receptors also increases the proportion of Ca2+-permeable AMPARs (De Biase et al., 2011; Zonouzi, Renzi, Farrant, & Cull-Candy, 2011). Although the expression of GluA1 seems very low in mouse oligodendroglia (Kougioumtzidou et al., 2017), immunostainings performed on postmortem tissue from patients suffering of multiple sclerosis (MS) revealed an upregulation of GluA1 in OLs at active MS lesion borders (Newcombe et al., 2008), suggesting that differences of AMPAR subunit expression on OL lineage cells may exist between normal and pathological conditions, CNS regions and species.
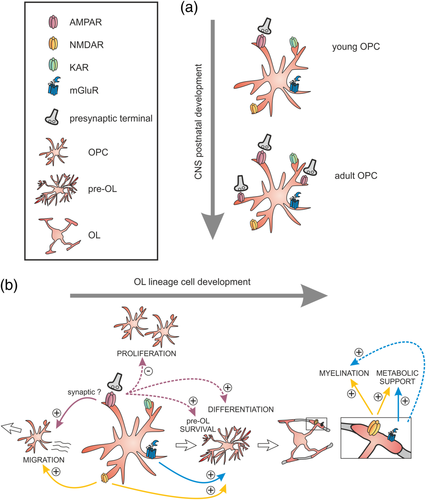
Unlike AMPARs, kainate (KAR) and N-methyl-d-aspartate receptors (NMDARs) have never been detected in OPC synapses. Although the presence of functional kainate receptors has been reported in OPCs (Kukley & Dietrich, 2009) and OLs (García-Barcina & Matute, 1996), their function has not yet been explored further in vivo. The functional expression of NMDARs in cells of the oligodendroglia lineage was first reported by Karadottir, Cavelier, Bergersen, and Attwell (2005) and Salter and Fern (2005). Functional heterotetramers formed by the obligatory subunit GluN1 associated with GluN2A-D and GluN3A are expressed at low densities throughout the OL lineage compared to neurons (Zhang et al., 2014), but their expression probably increases upon cell differentiation. NMDAR-mediated currents in oligodendroglia are less sensitive to extracellular Mg2+ than those in neurons due to the predominant expression of GluN3 and GluN2C-D subunits (Burzomato, Frugier, Pérez-Otaño, Kittler, & Attwell, 2010). Interestingly, NMDARs are preferentially located at the level of cell processes and myelin sheaths of OLs (Saab et al., 2016; Salter & Fern, 2005) which makes their detection difficult with whole-cell patch-clamp recordings due to an inadequate space-clamp. This enrichment of NMDARs on myelin sheaths has led to the concept of “axo-myelinic synapse” which designates a synaptic-like communication occurring between the adjacent membranes of axons and myelin (Micu, Plemel, Caprariello, Nave, & Stys, 2018).
In addition to ionotropic glutamate receptors, oligodendroglia also express metabotropic receptors of group I (mGluR1 and mGluR5), II (mGluR3) and III (mGluR4) in vitro and in vivo (Deng, Wang, Rosenberg, Volpe, & Jensen, 2004; Haberlandt et al., 2011; Luyt, Váradi, Durant, & Molnár, 2006; Spampinato, Merlo, Chisari, Nicoletti, & Sortino, 2015). While no changes in the expression level of Group II mGluRs have been observed during differentiation (Luyt et al., 2006), Groups I and III mGluRs are highly expressed in OPCs and downregulated in mature OLs (Deng et al., 2004; Spampinato et al., 2015).
2.2 Identity of presynaptic glutamatergic neurons of OPCs
The proper functioning of the CNS depends on the coordinated activity of many different types of neurons organized in complex networks. In the last decades, an important research field in neurophysiology has been to elucidate the mechanisms by which elementary neuronal circuits orchestrate neuronal network dynamics in time and space. In this context, the identification of the pre- and postsynaptic elements of elementary circuits has been of paramount importance to determine how the information flows in neuronal networks. The firing properties, the strength of the connections and other features such as synaptic plasticity or specific postsynaptic receptors subtypes are decisive to establish the rules governing brain information processing. In the case of neuron–OPC synapses, however, very little information exists about the identity of presynaptic neurons. It is also completely unknown how (and if) distinct presynaptic neurons differentially affect OPC function.
Although little is known about the identity of glutamatergic neurons monosynaptically connected to OPCs, it is possible to deduce some information from reports performed in acute brain slices. Initial experiments on OPC synapses showed that Schaffer-collateral stimulation in the hippocampus induces synaptic currents in OPCs of the CA1 region, strongly suggesting that these progenitors receive direct inputs from CA3 pyramidal neurons (Bergles et al., 2000; Jabs et al., 2005). Similarly, OPC synaptic responses evoked by extracellular stimulation have been observed in different brain regions, suggesting that these progenitors are directly innervated in the cerebellar molecular layer by glutamatergic projections from the inferior olive nucleus (Lin et al., 2005), in the dentate gyrus of the hippocampus by granule cells (Mangin, Kunze, Chittajallu, & Gallo, 2008), in the medial nucleus of the trapezoid body (MNTB) by globular bushy cells of the anterior ventral cochlear nucleus (Muller et al., 2009) and in cortical layer IV by glutamatergic projections from the thalamic ventrobasal nucleus (Mangin, Li, Scafidi, & Gallo, 2012). Axons crossing the corpus callosum also contact OPCs in this white matter region (Kukley, Capetillo-Zarate, & Dietrich, 2007; Ziskin et al., 2007). These axons most probably belong to layer II/III and/or V pyramidal neurons that constitute the main projections crossing the corpus callosum to their contralateral cortex (Wise, 1975). However, the precise identity of presynaptic glutamatergic neurons impinging onto OPCs and the specific properties of unitary synaptic connections remain uncertain in the experiments mentioned above. Other techniques like rabies viruses for monosynaptic tracing, paired recordings, optical tools (photolysis or optogenetics) could be of interest to precisely define the OPC connectivity in the brain. Given that the position and organization of axons is a precise dynamic process during development and of crucial importance for brain function in the adult, a better understanding of neuron–OPC circuits could help to understand why certain neuronal subtypes—and not others—innervate these progenitors. It should be noted that most of the synapses mentioned above originate from neurons and OPCs located at distal sites or even in different remote structures, indicating that axons travel long distances before reaching their OPC target. Local connections between glutamatergic neurons and OPCs seem to be rare. In fact, when we performed paired recordings between putative pyramidal neurons and OPCs in layer V of the somatosensory cortex at P9-P13 in NG2-DsRed mice, we never observed a connected pair (more than 100 pairs, unpublished observations). In contrast, it is known that layer V pyramidal cells are synaptically connected among them in young rodent animals (Markram, Lübke, Frotscher, Roth, & Sakmann, 1997). Similarly, CA1 pyramidal cells of the hippocampus are synaptically connected, although they receive more monosynaptic inputs from CA3 pyramidal neurons (Debanne, Guérineau, Gähwiler, & Thompson, 1995). It is therefore intriguing why distal sites—but not local sites—should regulate OPC functions through a synaptic mechanism. We can also wonder why glutamatergic synaptic inputs of OPCs increase during postnatal development when most of the proliferation, differentiation, and myelination processes have taken place in the brain (Figure 1a; De Biase et al., 2010; Ziskin et al., 2007).
2.3 Roles of glutamatergic signaling in oligodendroglia development and migration
Since AMPAR expression and synaptic inputs are restricted to the progenitor state of oligodendroglia and various other receptor subtypes are expressed throughout the whole lineage, synaptic and nonsynaptic glutamate-mediated mechanisms of communication coexist and should play different roles (Figure 1b). The source of glutamate is also probably variable: this neurotransmitter can arise from spontaneous or evoked synaptic vesicular release (Bergles et al., 2000), activity-dependent nonsynaptic vesicular release (Wake et al., 2015), vesicular release prior to synaptogenesis (Andreae & Burrone, 2015), or nonsynaptic release from surrounding sources such as astrocytes (Le Meur, Galante, Angulo, & Audinat, 2007), blood stream or reversal glutamate transporters (Spitzer, Volbracht, Lundgaard, & Káradóttir, 2016). Depending on the concentration of glutamate and the location and identity of the receptors, the intracellular signaling pathway may induce different responses. Interestingly, the activation of glutamate receptors expressed by oligodendroglia often induces intracellular Ca2+ increases (Maldonado & Angulo, 2015; Pitman & Young, 2016). Ca2+ signals have been implicated in OPC proliferation, differentiation, and migration during normal and pathological myelination and could therefore represent the intracellular transducer of receptor activation regulating all these fundamental functions (Cheli et al., 2016, 2018; Maldonado & Angulo, 2015; Pitman & Young, 2016). Furthermore, two recent reports also demonstrated that short-duration local Ca2+ signals regulate myelin sheath elongation while long-duration Ca2+ elevations promote myelin retraction, highlighting the importance of distinct forms of Ca2+ activity at mature OL stages (Baraban, Koudelka, & Lyons, 2018; Krasnow, Ford, Valdivia, Wilson, & Attwell, 2017).
Initial in vitro evidences showed that glutamate inhibits OPC proliferation through the activation of AMPARs (Gallo et al., 1996; Liu & Almazan, 1995). With the surprising discovery of AMPAR-mediated synapses of OPCs in different brain regions, the effect of these receptors on cultures led to the speculation about a potential role of these synapses in inhibiting OPC proliferation in vivo. In line with this, a first correlative study combining patch-clamp recordings in acute slices with immunostainings in the mouse barrel cortex showed that a decrease in the strength of AMPAR-mediated synapses of OPCs is associated to an increase in OPC proliferation (Mangin et al., 2012). More recently, Fannon, Tarmier, and Fulton (2015) showed that the application of AMPAR antagonists, but not NMDAR antagonists, also enhances OPC proliferation in organotypic cerebellar slices. However, Hamilton et al. (2017) did not reproduce this effect in organotypic cortical slices. In fact, pharmacological treatments are not specific and do not allow to discern the effects on oligodendroglia from those on neurons. To search for a causal link between glutamatergic synapses and OPC development, two recent complementary studies manipulated AMPAR signaling directly in OPCs using different genetic approaches. In one case, all AMPAR subunits were deleted in a knockout mouse (Kougioumtzidou et al., 2017) and, in the second case, the biophysical properties of the receptors were changed by genetically modifying the GluA2 subunit (Chen et al., 2018). The specific deletion of all AMPAR subunits in OPCs completely abolishes glutamatergic synaptic currents but, contrary to what was expected, it does not cause modifications of OPC proliferation and differentiation. Instead, AMPAR deletion compromises the survival of pre-OLs and thus decreases the population of mature OLs (Figure 1b; Kougioumtzidou et al., 2017). On the contrary, different GluA2 mutations change the properties of AMPAR-mediated currents (Chen et al., 2018), but probably do not affect the frequency of synaptic currents. In this study, the expression of either the unedited Ca2+-permeable or pore-dead GluA2 subunit promotes OPC proliferation, reducing the differentiation of recombinant cells. However, a mutation affecting the interaction between GluA2 and intracellular AMPAR-binding proteins decreases OPC differentiation without affecting proliferation (Chen et al., 2018). While the absence of synaptic activity seems to affect the survival of the pre-OLs but not OPC development, modifications of AMPAR-mediated synaptic currents reduces OPC differentiation after increasing or not cell proliferation (Figure 1b). Of note, sensory experience also promotes the survival of newly differentiating OLs since whisker deprivation induces an increase in the proportion of dying oligodendroglia in the barrel cortex (Hill, Patel, Goncalves, Grutzendler, & Nishiyama, 2014). Interestingly, the increased death observed in deprived conditions is accompanied by an increased OPC proliferation, indicating that both proliferation and death could be two linked phenomena. Indeed, it is known that OPCs tend to homeostatically regulate their own population (Hughes, Kang, Fukaya, & Bergles, 2013). Therefore, one interesting possibility could be that AMPAR-mediated mechanisms driven by experience participate to the balance between proliferation and death.
Interestingly, the deletion of GluN1 of NMDARs (De Biase et al., 2011) or the activation of group I mGluRs increases the proportion of Ca2+-permeable AMPARs in OPCs while ATP has the opposite effect (Zonouzi et al., 2011). This dynamic regulation of AMPARs represents a form of plasticity that may adjust the OPC response in different conditions. Whether this dual effect could explain part of the paradoxical results mentioned above remains an open question. It is also puzzling that the specific stimulation of glutamatergic neurons in vivo promotes rather than inhibits OPC proliferation (Gibson et al., 2014), an effect that is consistently reproduced using other less specific stimulation protocols (Mitew et al., 2018; Nagy, Hovhannisyan, Barzan, Chen, & Kukley, 2017). It is not excluded that the neuronal stimulation paradigms used to increase the activity in vivo induces the release of different molecules (i.e., potassium) and has a number of direct and indirect effects on neuronal networks and glial cells. Nevertheless, they should also induce an extensive vesicular release of glutamate at neuron–neuron and neuron–OPC synapses. Therefore, either neuronal activity has as primary function of enhancing OPC proliferation and differentiation in a synaptic-independent fashion and, in this case, neuron–OPC synapses are only ancillary components for OPC development, or OPC synapses play other major roles. For the moment, it is difficult to reconcile all these recent results in a comprehensive picture and more studies are needed to clarify the role of AMPAR-mediated OPC synapses in vivo.
The role of NMDARs in OPC development and myelination has also been controversial (Maldonado & Angulo, 2015). In cell cultures, the activation of these receptors has a pro-differentiation effect via the mTOR pathway (Li et al., 2013) or the PKC/NADPH oxidase (NOX)-dependent ROS generation (Figure 1b; Cavaliere, Benito-Muñoz, Panicker, & Matute, 2013). Recent works, however, have opened other novel possibilities. Saab et al. (2016) showed that the stimulation of NMDARs of OLs mobilizes the glucose transporter GLUT1 into the myelin compartment, leading to a subsequent glucose uptake from the extracellular space. Then, this glucose is probably transformed into lactate which is further locally released from OLs toward axons through the monocarboxylate transporter MCT1 expressed at the inner tongue of myelin sheaths (Saab et al., 2016). Finally, lactate fuels axons (probably expressing MCT2) in contact with myelin sheaths. Therefore, by releasing glutamate close to NMDARs of myelin sheaths, axons may finely regulate their own energy supply (Figure 1b). The source of glutamate activating these myelinic NMDARs is unknown, but it probably arises from direct axonal vesicular release onto myelin rather than from glial or neuronal remote sources as recently observed in white matter ischemic conditions (Doyle et al., 2018). In addition, the movement of mitochondria within the myelin sheath compartment is increased by activating NMDARs, a process that might contribute with the OL-to-axon energy supply (Rinholm et al., 2016). Altogether, these results may indicate a higher expression of NMDARs at mature OL lineage stages and strengthen the importance of OLs in providing a metabolic support to axons (Philips & Rothstein, 2017).
Glutamate has been shown to act as an important long-range signal for OPC migration (Figure 1b). The implication of AMPARs in OPC migration has been demonstrated in vitro (Gudz, Komuro, & Macklin, 2006) and ex vivo in cerebellar slices of P4 mice (Harlow, Saul, Komuro, & Macklin, 2015). AMPAR activation induces a binding of the αv integrin/PLP complex to GluA2, resulting in an internalization of this AMPAR subunit and an increase of the intracellular Ca2+ signaling. In turn, this effect enhances OPC migration probably by reducing the integrin binding to the extracellular matrix. On the other hand, results obtained in cell cultures and embryonic brain slices showed that NMDAR stimulation induces intracellular Ca2+ elevations and activates the Tiam1/Rac1/ERK signaling pathway that promotes OPC migration (Figure 1b; Xiao et al., 2013). Since AMPARs of OPCs are considered as synaptic, whereas NMDARs as extrasynaptic (Maldonado & Angulo, 2015), these results suggest that either modes of communication converge to a same function. Alternatively, AMPARs can also be expressed extrasynaptically, at least at perinatal stages. In any case, in vivo data showing glutamate-dependent oligodendroglia migration during brain development or in a pathological context are still missing. In vivo two-photon microscopy experiments have revealed that while cortical OPCs survey their local environment with motile filopodia and continuously migrate (Hill et al., 2014; Hughes et al., 2013), mature OLs are relatively stable (Hill, Li, & Grutzendler, 2018; Hughes, Orthmann-Murphy, Langseth, & Bergles, 2018). Whether these effects are mediated or modulated by glutamate release is unknown. If there are accumulated evidences that all the steps of oligodendrogenesis, that is, migration, proliferation, and differentiation, are affected by glutamate (Figure 1b), the role of this major neurotransmitter will need to be more finely dissected in vivo in the future.
2.4 Glutamate in adaptive myelination
Despite a large bulk of evidences showing that neuronal activity impacts the pattern of myelination around axons (Baraban, Mensch, & Lyons, 2016; Monje, 2018), OLs have also the capacity to enwrap non-neuronal fibers and produce myelin in the absence of neuronal signals (Bechler et al., 2018). Collective data from different laboratories have led to a general view in which an intrinsic encoded program instructs initial myelin formation; then, neuronal activity reinforces active pathways in response to experience and sculpts myelin structure around axons (Bechler et al., 2018). Myelination is therefore a very plastic process throughout life. This activity-dependent plasticity of myelin is at the origin of the concept of “adaptive myelination.”
Although not the only candidate, glutamate is a suitable signaling molecule capable of linking neuronal activity with myelin plasticity. In DRG-oligodendroglia cocultures, the activity-dependent vesicular release of glutamate from axons increases myelin production. It stimulates the formation of cholesterol-rich microdomains where local translation of the myelin basic protein (MBP) is promoted via a Fyn kinase pathway (Wake, Lee, & Fields, 2011). In this case, glutamate release from neurons acts through NMDARs and mGluRs located at functional nonsynaptic axo-oligodendroglia junctions (Figure 1b; Wake et al., 2015). In a different coculture system, neuregulin-1 highly increases NMDAR activation in OPCs and OLs which also drives an elevation and acceleration of myelination, suggesting that the mechanisms governing this process can switch from activity-independent to activity-dependent in order to enhance myelin formation (Figure 1b; Lundgaard et al., 2013).
However, the role of glutamate in activity-driven adaptive myelination in vivo is still unclear. Recent reports using different in vivo preparations have shown that either neuronal activity (Gibson et al., 2014; Mitew et al., 2018; Ortiz et al., 2019) or the abrogation of vesicular release (Etxeberria et al., 2016; Hines, Ravanelli, Schwindt, Scott, & Appel, 2015; Mensch et al., 2015) alters the myelination of neuronal fibers in different ways. It has been found in normal conditions that neuronal activity increases the myelin thickness (Gibson et al., 2014) with a strong bias toward activated axons (Mitew et al., 2018). However, it is unknown if this effect is glutamate-dependent. In fact, the attenuation of synaptic glutamate release in the retinogeculate pathway by ablating the vesicular glutamate transporter vGluT2 only reduces the internode length without modifying the myelin thickness or the number of myelinated axons (Etxeberria et al., 2016). Moreover, while the myelination of glutamatergic reticulospinal neurons in zebrafish is decreased after blocking the release of vesicules, presumably containing glutamate (although a corelease with others molecules is not excluded), that of glutamatergic commissural primary ascending neurons remains normal (Koudelka et al., 2016). Therefore, the preferential myelination of CNS active axons probably exists, but cannot be generalized to all glutamatergic fibers or specifically attributed to glutamate. If activity-driven myelin remodelling is cell-type specific, and in addition, (i) the pattern of myelin along a unique axon is not uniform (Tomassy et al., 2014), (ii) there is a large heterogeneous OL populations with potential different myelinating capacities (Marques et al., 2016), and (iii) striking intrinsic differences exist between gray and white matter OLs (Vigano, Mobius, Gotz, & Dimou, 2013), it will take time to understand the logic of myelination in the CNS and it is better to start now working on it!
In disease, glutamate dysregulation alters myelin structure and compromises neuronal survival. The glutamatergic system represents a potential therapeutic target in some pathological conditions in which remyelination fails such as in MS (Macrez, Stys, Vivien, Lipton, & Docagne, 2016). In MS patients, an excessive extracellular glutamate concentration is accumulated in the extracellular space by an ineffective glutamate uptake and an aberrant release of this neurotransmitter from neuronal and non-neuronal sources (Macrez et al., 2016). However, given the complexity of the glutamatergic system, a variety of potential cellular and molecular targets are put into play and thus, glutamate could have both negative and positive effects on myelin disorders. In fact, reports on rodent models have shown beneficial effects of glutamate on remyelination. In different demyelination/remyelination in vivo models, the systemic administration (Li et al., 2013) or the intracerebral infusion (Lundgaard et al., 2013) of NMDAR antagonists delayed remyelination. However, it is not clear how this pharmacological effect is specific of oligodendroglia. Indeed, Dąbrowska-Bouta, Strużyńska, Chalimoniuk, Frontczak-Baniewicz, and Sulkowski (2015) found on the contrary that the intraperitoneal injection of NMDAR antagonists improves the condition and increase mRNA expression levels of selected myelin proteins in the experimental autoimmune encephalomyelitis (EAE) model. In any case, the deletion of GluN1 in OLs induces neuroinflammation and neurodegeneration in 10 month-old mice, indicating that NMDAR activation is at least needed to preserve in the long term both myelin and axon integrities (Saab et al., 2016). Concerning AMPAR signaling in acute demyelinated lesions, newly generated OPCs do not receive synaptic inputs at the active proliferation phase but gain synapses during the remyelination process, indicating that AMPAR-mediated synapses in oligodendroglia are highly regulated in pathological conditions (Sahel et al., 2015). Moreover, the intracerebral infusion of AMPAR antagonists or the Na+ channel blocker tetrodotoxin (TTX) inside acute lesions reduces remyelination, suggesting that the activity-dependent release of glutamate may promote OPC differentiation and myelin repair (Gautier et al., 2015). Finally, group I mGluR activation has been found to be cytoprotective for oligodendroglia of the optic nerve at all stages of differentiation and development in excitotoxicity and ischemic conditions (Butt, Vanzulli, Papanikolaou, De La Rocha, & Hawkins, 2017). Even if glutamate is considered a harmful molecule in many pathological contexts, the possible meaning of these results is that the maintenance of the axon–oligodendroglia communication is important to improve pathological conditions (Ortiz et al., 2019). Future envisioned therapies should consider this important point.
3 GABA IN NEURON–OLIGODENDROGLIA COMMUNICATION
3.1 GABA receptors expressed by OPCs and OLs
As for glutamate receptors, a wide diversity of ionotropic and metabotropic GABA receptors are expressed in oligodendroglia at different stages of cell maturation. An early description of GABAA receptor (GABAAR) in OLs was provided using intracellular electrophysiological recordings and pharmacological approaches in explant cultures of spinal cord (Gilbert, Kettenmann, & Schachner, 1984). Their functional expression was later confirmed in other CNS regions such as the hippocampus, neocortex, and cerebellum (Velez-Fort, Audinat, & Angulo, 2012). Although the first evidences of GABA receptor expression in OL lineage cells were obtained in culture more than three decades ago, their precise molecular composition and properties has been less explored than for glutamate.
Several reports have revealed that the sensitivity to GABA of mature OLs is greatly diminished compared to OPCs, suggesting a specific regulation of GABAAR expression during OL lineage cell development (Figure 2b; Arellano et al., 2016; Berger, Walz, Schnitzer, & Kettenmann, 1992; Von Blankenfeld, Trotter, & Kettenmann, 1991). Concerning the OL mature state, Arellano et al. (2016) recently observed that GABAAR-mediated responses also drastically decreases with time in isolated OL cultures, while the presence of neurons in cocultures allows for maintaining their functional expression. A direct interaction between neurons and OLs seems therefore necessary to stabilize GABAARs in differentiated cells. However, the neuron-to-OL signals involved in this process remain unknown. Blockade of neuronal activity with the Na+ channel blocker TTX does not change OLs sensitivity to GABA, suggesting that a basal neuronal activity is not required to keep GABAAR functional expression (Arellano et al., 2016).
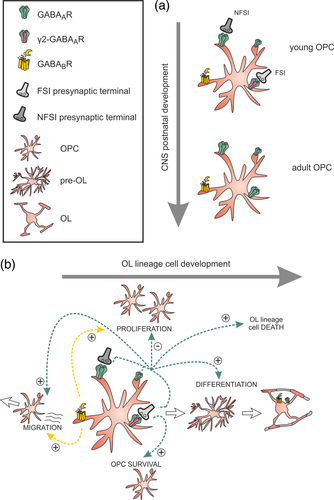
After the discovery of glutamatergic synapses, Lin and Bergles (2004) described the existence of GABAAR-mediated synaptic currents in hippocampal OPCs. Since then, the GABAergic innervation of OPCs has been described in different CNS regions (Maldonado & Angulo, 2015). Interestingly, GABAergic synaptic inputs of OPCs are lost during postnatal development in the somatosensory cortex (Vélez-Fort, Maldonado, Butt, Audinat, & Angulo, 2010) and in the white matter cerebellum (Figure 2a; Zonouzi et al., 2015). In this latter, migrating interneurons transiently contact OPCs during their journey in white matter. After the loss of GABAergic synapses, however, the stimulation of neuronal fibers still elicits GABAAR-mediated extrasynaptic responses solely relying on local GABA spillover from nearby interneurons (Balia et al., 2015; Vélez-Fort et al., 2010). Activity-mediated GABAergic currents in these progenitors can therefore be induced via the activation of synaptic and extrasynaptic GABAARs (Figure 2a).
GABAARs are heteropentamer complexes composed from a family of 19 subunits (α, β, γ, δ, ε, θ, π, and ρ). In the CNS, they are mostly formed by the association of αβγ subunits with a stoichiometry of two α, two β, and one γ subunits. The transient connectivity and the postnatal switch of GABAergic transmission on OPCs, from synaptic to extrasynaptic, suggest modifications of GABAAR subunit composition during postnatal development. By combining single cell RT-PCR and specific pharmacology for GABAAR subunits, we evaluated a possible developmental change in the subunit composition of GABAAR in OPCs (Balia et al., 2015). We found that postsynaptic GABAARs of OPCs in the somatosensory cortex are mainly formed by α1, α5, and γ2 subunits, although they may also contain γ1 and γ3. Interestingly, the expression of γ2, a crucial molecular subunit contributing to the clustering of GABAARs at postsynaptic sites in neurons, is downregulated in OPCs during the switch from synaptic to extrasynaptic GABAergic transmission mode after the second postnatal week (Figure 2a; Balia et al., 2015). In line with this, Passlick et al. (2013) demonstrated by RT-PCR and pharmacological analyses an abundant expression of α1 and γ2 subunits at postsynaptic GABAARs of OPCs in the juvenile mouse hippocampus. Moreover, they observed the existence of a persistent (extrasynaptic) tonic current mediated by GABAARs lacking γ2 (Passlick et al., 2013). While synaptic GABAARs containing or not γ2 subunits co-exist in OPCs (Orduz et al., 2015), extrasynaptic GABAARs never express this subunit (Figure 2a).
In mature OLs, functional genomic analyses showed a high expression of α1, α3, and γ1, along with low levels of γ2 subunits (Cahoy et al., 2008; Zhang et al., 2014). Pharmacological analyses of GABAARs in cultured OLs determined that the response to GABA is sensitive to benzodiazepines and Zn2+ but insensitive to indiplon, suggesting that GABAARs in OLs contain γ1 and/or γ3 subunits, but not γ2 subunits (Arellano et al., 2016). Furthermore, immunofluorescence studies also revealed the expression of α1 and α3 subunits only when OLs are cocultured with neurons. The GABAAR composition of OLs also displays a specific composition characterized at least by the contribution of α1 and α3 subunits associated with γ1 or γ3 subunits.
Finally, oligodendroglia expresses metabotropic GABABRs and, similarly to GABAARs, they are downregulated during the differentiation process (Figure 2b; Charles, Deuchars, Davies, & Pangalos, 2003; Luyt et al., 2007). The presence of both GABAB1 and GABAB2 isoforms has been identified in OPCs from young mouse brain periventricular white matter (Luyt et al., 2007).
3.2 Identity of presynaptic GABAergic neurons of OPCs
Despite of efforts done to characterize the properties and roles of neuron–OPC synapses, many questions still remain unanswered. In particular, the identity of presynaptic GABAergic neurons synaptically connected to OPCs has been poorly explored. Most studies describing GABAergic synaptic currents in OPCs have analyzed spontaneous or evoked responses that result from the activity of unidentified neurons. Since GABAergic neurons are the most heterogeneous neuronal population in the CNS (Petilla Interneuron Nomenclature Group, 2008), they cannot be differentiated using these techniques. However, the characterization of the identity of presynaptic GABAergic neurons would be determinant to understand the dynamics and roles of GABAergic OPC synapses in the network.
Two distinct groups of presynaptic GABAergic neurons could target OPCs in the CNS: (1) short-range GABAergic interneurons which, in the cerebral cortex, have been regrouped in three large subsets of neurons according to the expression of parvalbumin (PV), somatostatin (SST), or the ionotropic serotonin receptor 5HT3A (Petilla Interneuron Nomenclature Group, 2008; Rudy, Fishell, Lee, & Hjerling-Leffler, 2011), and (2) long-range projecting GABAergic neurons such as Purkinje cells of the cerebellum, spiny neurons of the striatum, neocortical-cortical or hippocampo-entorhinal neurons, among others (Caputi, Melzer, Michael, & Monyer, 2013). Only short-range GABAergic interneurons have been identified as presynaptic inputs of these progenitors in the somatosensory cortex and the cerebellum (Orduz et al., 2015; Zonouzi et al., 2015). Immunostaining experiments provided a first evidence of a putative synaptic contact between a PV+ interneuron and an OPC (Tanaka et al., 2009). However, it took some years before getting the functional demonstration of a connectivity between specific short-range GABAergic interneurons and OPCs (Orduz et al., 2015). Although a complete description of the identity of presynaptic interneuron subtypes is not available yet, paired recordings allowed us to determine that fast-spiking PV-expressing interneurons (FSI) are highly connected to OPCs and target proximal subcellular domains containing GABAARs with γ2 subunits (Figure 2a). In contrast, non-fast spiking interneurons (NFSI) are poorly connected and target distal sites lacking GABAARs with γ2 subunits (Figure 2a). OPCs thus compartmentalize input regions according to the identity of the presynaptic interneuron. In addition, each interneuron contacts an OPC through single or double release sites independently of its identity and thus establishes a restricted point-to-point communication in a specific anatomical postsynaptic domain (Orduz et al., 2015). Although some advances have been done, it remains to clarify which subtypes of non-fast spiking interneurons are connected to OPCs. Given that long-range projecting GABAergic neurons are myelinated, it would also be of interest to determine if these neurons innervate these progenitors. As previously mentioned for glutamate, paired recordings, rabies viruses for monosynaptic tracing, optical tools (photolysis or optogenetics) in transgenic mice could help to understand the details of the connectivity between the complex populations of GABAergic neurons and OPCs in the CNS.
Most cortical interneurons derive from the medial ganglionic eminence (MGE) in the embryo and give rise to PV+ and SST+ interneurons (Fogarty et al., 2007; Miyoshi, Butt, Takebayashi, & Fishell, 2007; Xu, Cobos, De La Cruz, Rubenstein, & Anderson, 2004). The other major interneuron population derives from the caudal ganglionic eminence (CGE) and constitutes the group of 5HT3A+ interneurons (Lee, Hjerling-Leffler, Zagha, Fishell, & Rudy, 2010). More recently, it was also showed that the embryonic POA is responsible for the production of a small interneuron population including PV+, SST+, and Reelin+ interneurons (Marin, 2013). Interestingly, these regions are also the source of ventral OPCs in the cortex (Kessaris et al., 2006). It would thus be possible that the common origin of interneurons and ventral OPCs influence their connectivity within cortical inhibitory networks at postnatal stages. Indeed, a close relationship between MGE-derived interneurons and OPCs is already known since these interneurons secrete fractalkine and other factors that promote cortical OPC differentiation (Voronova et al., 2017).
3.3 Roles of GABAergic signaling in oligodendroglia development and migration
A diversity of synaptic and extrasynaptic GABA receptors are expressed by oligodendroglia and, as for glutamate, the source of GABA could be neuronal and non-neuronal (Velez-Fort et al., 2012). However, the functions and pathways implicated have been less explored than for glutamate receptors.
Unlike the hyperpolarizing effect of GABAAR activity on mature neurons, these receptors have a depolarizing effect on OPCs (Lin & Bergles, 2004; Tanaka et al., 2009) and their stimulation leads to an intracellular Ca2+ increase via the activation of voltage-gated calcium channels (Tanaka et al., 2009; Vélez-Fort et al., 2010). We recall that intracellular Ca2+ signals are important regulators of OPC proliferation, differentiation, migration, and myelin stabilization (Baraban et al., 2018; Cheli et al., 2016, 2018; Krasnow et al., 2017; Maldonado & Angulo, 2015; Pitman & Young, 2016). However, whether Ca2+ increases induced by GABAAR activation occur in physiological conditions and affect OPC function is unclear. Two-photon Ca2+ imaging of OPCs recently revealed a high level of spontaneous intracellular Ca2+ activity that increases with neuronal stimulation in acute slices of the immature somatosensory cortex (Balia, Benamer, & Angulo, 2017). However, these signals persist in the absence of GABAAR-mediated synapses containing γ2 subunits, suggesting that they do not involve synaptic mechanisms (Balia et al., 2017). Moreover, the presence of type A potassium channels in OPCs limits possible synaptic Ca2+ responses in these cells (Sun, Matthews, Nicolas, Schoch, & Dietrich, 2016). Whether GABAAR-mediated intracellular Ca2+ changes of OPCs may occur in physiological conditions remain unknown. Ideally, this point could be addressed by combining in vivo imaging experiments and targeting of GABAergic-specific pathways in OPCs. However, alternatively to Ca2+ activity, changes on GABAAR-mediated intracellular Cl− concentration during neuronal activity may affect OPC function. Interestingly, the activation of GABAARs in Cajal Retzius neurons, for which GABA is also depolarizing, causes their programmed cell death through dynamic changes of intracellular Cl− concentrations involving the Cl− transporter NKCC1 and p75NTR signaling (Blanquie, Liebmann, Hübner, Luhmann, & Sinning, 2017; Kolbaev, Luhmann, & Kilb, 2011). Programmed cell death is also an important mechanism of oligodendroglia to ensure OL lineage cell balance and region-specific myelination (Kessaris et al., 2006; Sun et al., 2018). It would thus be interesting to test if a mechanism similar to that described for Cajal Retzius neurons, whether synaptic or extrasynaptic, promotes either the death or the survival of oligodendroglia.
One of the leading hypotheses concerning the function of the GABAergic signaling in oligodendroglia is that GABA plays a central role in controlling OPC proliferation and differentiation. However, a number of contradictory results have been reported when considering either a specific role of GABAergic synaptic signaling or a more general role of GABA in OPC function (Balia et al., 2017; Hamilton et al., 2017; Zonouzi et al., 2015). In a model of perinatal hypoxia, Zonouzi et al. (2015) demonstrated an important dysregulation of GABA signaling in both neurons and OPCs during postnatal development. Specially, they observed that a decrease in the synaptic activity of both migrating short-range interneurons and OPCs of the immature white matter cerebellum is correlated with an increased OPC proliferation, a decreased OL differentiation (Figure 2b) and a delayed myelination, resulting in a severe cerebellar dysmyelination. The progression from OPCs into mature OLs is therefore highly dependent on GABA. Similar effects were reproduced by the specific deletion of the Cl− transporter NKCC1 in OPCs or by the intraperitoneal injection of the GABAAR antagonist biccuculine (Zonouzi et al., 2015). The GABAergic signaling thus regulates OL lineage cell development. These results also suggest that the activity of GABAergic OPC synapses plays a role in inhibiting OPC proliferation and promoting lineage progression (Figure 2b). In a more recent study, however, the pharmacological blockade of GABAARs in organotypic cortical slice cultures increases the number of both OPC and OLs, by promoting OPC proliferation and OL lineage cell survival (Hamilton et al., 2017). While these results also imply that GABA signaling inhibits proliferation, they suggest that GABAAR activation increases OL lineage cell death (Figure 2b). The differences observed in these studies could be explained by a distinct role of GABA in different brain regions, but also by an unspecific action of pharmacological treatments on both neurons and glia. In addition, although the pharmacological or genetic tools used in these studies affect GABA signaling, they are not exclusive for synaptic GABAARs and could affect OL lineage cells at any maturation stage. To assess a more specific role of GABAAR-mediated synapses of OPCs, we recently inactivated γ2-GABAAR synapses by deleting the γ2 subunit in these progenitors (Balia et al., 2017). We observed a robust decrease in the frequency of postsynaptic currents suggesting that, among the two major interneuron–OPC synapses described so far (Orduz et al., 2015), those containing γ2-GABAARs are specifically and completely inactivated in these mice. Interestingly, the inactivation of γ2-GABAAR synapses in OPCs did not modify either their glutamatergic synaptic inputs (Balia et al., 2017) or the synaptic nature of the remaining evoked GABAergic currents—the rise time was not slowed down as in extrasynaptic responses (see Vélez-Fort et al., 2010). This suggests that the lack of γ2-GABAAR synapses is not compensated by glutamatergic synaptic mechanisms or extrasynaptic transmission. Unlike expected, the inactivation of these synapses does not impact OPC proliferation and differentiation, but induces a moderate decrease in OPC density, suggesting a role of these synapses in OPC survival rather than in OL lineage cell development (Figure 2b; Balia et al., 2017). In line with this, the in vivo optogenetic stimulation of MGE-derived interneurons in mouse pups does not modify the proliferation rate of OPCs (Ortolani, Manot-Saillet, Orduz, Ortiz, & Angulo, 2018). Although these experiments are not specific of GABAergic synapses and are not necessarily correlated with the previous study, a stimulation of glutamatergic fibers does induce rapid changes in OPC prolifaration in normal (Gibson et al., 2014) and demyelinated conditions (Ortiz et al., 2019), suggesting that the activity of glutamatergic and GABAergic neurons may exert different effects or effects that can be modulated according to the receptors activated or the conditions. Finally, Tong et al. (2009) proposed that GABAARs may play a role in OPC migration via the activation of Na+/Ca2+ exchangers type 1 (Figure 2b), but this result has not been confirmed yet in vivo.
Further studies will be needed to resolve the present discrepancies about the role of GABA signaling in oligodendroglia and to reconcile contradictory results. As for glutamate, we are at the beginning of the understanding of the role of OPC synapses and neuron–oligodendroglia communication in the CNS. Considering the multiple types and roles of GABAergic interneurons in the brain, it would be very exciting to determine whether different interneuron–OPC synapses, that are known to target different OPC domains and display different properties (Orduz et al., 2015), also play distinct roles in regulating OPC functions. Moreover, it would be interesting to analyze the interplay between synaptic and extrasynaptic GABAARs expressed by OPCs, and to study extrasynaptic GABAARs in OLs in vivo. Very little is known about the functional expression and roles of extrasynaptic GABAARs in oligodendroglia. For instance, it is unknown whether they are expressed at the level of myelin sheaths of OLs wrapping GABAergic neurons. A metabolic or pro-myelinating role of these receptors could be considered.
Concerning our knowledge of the role of GABABRs in oligodendroglia, it is almost nonexistent. A study performed in cell cultures showed that a treatment with the agonist of GABABR baclofen induces a decrease of cAMP levels in OPCs—probably through a negative coupling to adenylate cyclase—causing an increase in OPC proliferation and migration (Figure 2b; Luyt et al., 2007). Another study using stereology and histological analysis of the dorsal neocortex of the adult mouse revealed that 40% of cortical OPCs form anatomical pairs with neurons, mainly with GABAergic interneurons expressing PV, calbindin, or calretinin (Boulanger & Messier, 2017). Interestingly, Boulanger and Messier (2017) observed that the systemic application of baclofen or the GABAAR antagonist picrotoxin significantly increases the number of anatomical neuron–OPC pairs in vivo while reference memory training has no effect. A positive role of GABABRs in OPC proliferation and migration as well as in maintaining interneuron–OPC physical association is therefore possible, but need to be confirmed and deepened.
3.4 GABA in adaptive myelination
Myelination by OLs represents a central process in brain development and is determinant to allow for a normal axonal conduction and metabolic support. Long-range GABAergic neurons, which axons can travel very long distances, are myelinated (Jinno et al., 2007). In contrast, although first described in 1980s (Stedehouder & Kushner, 2017), the myelination of short-range interneurons was overlooked for several decades until recent studies revealed that a large fraction of the myelin present in the neocortex and hippocampus—sometimes more than 50%—surrounds the axons of PV+ interneurons (Micheva et al., 2016; Stedehouder et al., 2017). In fact, we and other two groups recently demonstrated that virtually all PV+ interneurons are myelinated in the cortex (Balia et al., 2017; Micheva et al., 2016; Stedehouder et al., 2017). Interestingly, compared to glutamatergic axons which are myelinated over long distances, the myelination of PV+ axons is biased toward their proximal part in rodents and humans (Stedehouder et al., 2017) and has a different myelin composition (Micheva et al., 2016). Moreover, non-fast spiking SST+ interneurons, the other major interneuron subtype, are barely or not myelinated (Micheva et al., 2016; Stedehouder et al., 2017). Interneuron myelination is therefore highly regulated, specific of PV+ interneurons and much more significant than suspected before. It is also possible that differences in node of Ranvier assembly and axon myelination exist since prenodes are detected before myelin deposition in GABAergic axons, but not in glutamatergic axons (Freeman et al., 2015).
To talk about the role of myelin in PV+ interneurons is premature. However, we should notice that these neurons provide a robust perisomatic inhibitory control of excitatory neurons, regulate the excitation/inhibition balance and the synchronization of cortical networks. Hence, it may not be trivial that they are myelinated. The preferential myelination of this interneuron subtype may ensure the integrity of their precisely timed action potentials and, therefore, support their role in local neuronal network synchronization. Another possibility is that myelin plays a metabolic and supportive role for these interneurons since myelinated GABAergic axons contains more mitochondria than glutamatergic axons (Micheva et al., 2018). Finally, since a dysfunction of these neurons represents a major feature in neurodevelopmental disorders such as schizophrenia (Lewis, Curley, Glausier, & Volk, 2012; Spellman & Gordon, 2015), defects on interneuron myelination could be at the origin of different psychiatric disorders (Stedehouder & Kushner, 2017).
It is unknown whether GABAARs or GABABRs of oligodendroglia play a role in myelin formation and remodeling. Given that myelination is a plastic process modulated by neuronal activity, an interesting possibility is that myelination of PV+ interneurons is driven by their own activity and relies on the stimulation of synaptic and/or extrasynaptic GABA receptors in oligodendroglia. While the implication of PV+ interneuron activity on their own myelination has been confirmed (Stedehouder, Brizee, Shpak, & Kushner, 2018), the role of GABA receptors is uncertain. In a recent study, Stedehouder et al. (2018) showed that the specific chemogenetic stimulation of PV+ interneurons modifies the morphology and myelination of PV+ axons without changing the total myelin content or the OL cell density. The activity of PV+ interneurons seems therefore to specifically control the myelination of PV+ axons. We could also expect that PV interneuron–OPC synapses—known to be mediated by γ2-GABAARs (Orduz et al., 2015)—participate to this process. However, the inactivation of γ2-GABAAR-mediated synapses of OPCs does not affect the proportion of myelinated PV+ axons or the amount of total myelin (Balia et al., 2017). Nevertheless, since PV+ axons are myelinated at their proximal part (Stedehouder et al., 2017) and this axonal region probably innervates OPCs (Orduz et al., 2015), the PV interneuron–OPC synapses could participate to the organization or pattern of myelination rather than to the amount of myelin. In any case, it must be considered that OPCs receive GABAergic synapses from other interneurons (Orduz et al., 2015) that are not myelinated (Micheva et al., 2016; Stedehouder et al., 2017). Furthermore, the myelin remodeling observed on PV+ axons after activity seems to mainly depend on changes in axon morphology rather than a released molecule. Indeed, axonal morphology predicts the myelin changes induced by activity (Stedehouder et al., 2018). This observation is in agreement with recent experiments showing that increasing the caliber of normally unmyelinated axons induces their myelination (Goebbels et al., 2017; Mayoral, Etxeberria, Shen, & Chan, 2018). However, it disagrees with another recent report showing that neuronal activity does not change the axon diameter of newly myelinated axons (probably glutamatergic; Mitew et al., 2018). On the basis of these new results, it will be probably necessary to study in details the mechanisms governing the activity-driven adaptive myelination of specific neuronal subtypes.
4 GLUTAMATE VERSUS GABA IN NEURON–OLIGODENDROGLIA COMMUNICATION: CONCLUDING REMARKS
As depicted in this review, numerous evidences suggest that glutamate and GABA signaling between neurons and OL lineage cells constitute major mechanisms during development and in brain function. Convergent characteristics of these signaling mechanisms in oligodendroglia suggest that these cells represent an important partner of both glutamatergic and GABAergic neurons in the CNS. It is established that the receptors for these neurotransmitters are preferentially expressed at the OPC state (probably except for NMDARs), but they also play crucial roles in OLs. The neuron–oligodendroglia communication mediated by glutamate and GABA during lineage progression occurs through different transmission modes including synaptic for OPCs and extrasynaptic for the entire cell lineage. In another hand, neuronal stimulation increases the myelination of both glutamatergic and GABAergic axons following the concept of adaptive myelination. It would be interesting to test whether changes in myelin structures occurring during learning processes (McKenzie et al., 2014) depends specifically on glutamatergic and GABAergic neurons belonging to neuronal networks engaged in a behavioral task.
However, from the information collected today, differences also exist between the two systems. Axons from glutamatergic neurons travel long distances before innervating an OPC, a process that increases with age. On the contrary, GABAergic interneurons transiently contact OPCs with synapses early in postnatal development and form very local circuits with these progenitors. Interestingly, glutamatergic neurons generally need to be myelinated along their axons to efficiently conduct the information from one region to another, while short-range GABAergic interneurons display a local myelination that is mainly distributed at the proximal part of their axon. It will be thus interesting to know if myelin distribution is related to the spatial distribution of neuron–OPC circuits in the brain.
Despite several studies over the last years allowing for a better understanding of neuronal signaling on OPCs and OLs, the comprehension of the physiopathological role of glutamate and GABA in OL lineage development and CNS myelination is still controversial in many points and needs further investigation. Our knowledge of the diversity and distribution of the receptors expressed at each step of oligodendroglia maturation in the CNS (for instance in pre-OLs), the transmission modes allowing for their activation in vivo and their implication in oligodendroglia function and myelination is still insufficient. In particular, the roles of glutamatergic and GABAergic synapses of OPCs need to be refined and the extrasynaptic receptors to be taken into consideration. Since the signals linking neuronal activity and OL lineage cell function affect myelin and axon dynamics, they constitute a critical building brick for circuit formation and maturation, neuronal information processing and network functioning. To manipulate OL lineage cells and myelin in vivo is probably a less conventional but interesting alternative to understand how sensory, motor and cognitive behaviors take place.
ACKNOWLEDGMENTS
This work was supported by grants from Fondation pour la Recherche Médicale (FRM, «Equipe FRM DEQ20150331681»), Fondation pour l'aide à la recherche sur la sclérose en plaques (ARSEP), Agence Nationale de la Recherche (ANR-14-CE13-0023-02) and from a subaward agreement from the University of Connecticut with funds provided by Grant No. RG-1612-26501 from National Multiple Sclerosis Society (NMSS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of U.Conn or NMSS. C.H. was recipient of ARSEP post-doctoral fellowships. M.C.A. is a CNRS investigator and a team of the ENP-Ile-de-France network.
CONFLICT OF INTEREST
The authors have declared that no conflict of interest exists.