Transcription factor MafB contributes to the activation of spinal microglia underlying neuropathic pain development
Abstract
Microglia, which are pathological effectors and amplifiers in the central nervous system, undergo various forms of activation. A well-studied microglial-induced pathological paradigm, spinal microglial activation following peripheral nerve injury (PNI), is a key event for the development of neuropathic pain but the transcription factors contributing to microglial activation are less understood. Herein, we demonstrate that MafB, a dominant transcriptional regulator of mature microglia, is involved in the pathology of a mouse model of neuropathic pain. PNI caused a rapid and marked increase of MafB expression selectively in spinal microglia but not in neurons. We also found that the microRNA mir-152 in the spinal cord which targets MafB expression decreased after PNI, and intrathecal administration of mir-152 mimic suppressed the development of neuropathic pain. Reduced MafB expression using heterozygous Mafb deficient mice and by intrathecal administration of siRNA alleviated the development of PNI-induced mechanical hypersensitivity. Furthermore, we found that intrathecal transfer of Mafb deficient microglia did not induce mechanical hypersensitivity and that conditional Mafb knockout mice did not develop neuropathic pain after PNI. We propose that MafB is a key mediator of the PNI-induced phenotypic alteration of spinal microglia and neuropathic pain development.
1 INTRODUCTION
Microglia, one of the glial cell types that form the first line of immune defense in the central nervous system (CNS), constantly survey their territory using their surrounding highly motile processes. When a damage occurs in the CNS, they rapidly respond to the damage and transform into an activated form. In peripheral nerve injury (PNI) models, a damage in the peripheral neurons affects spinal cord microglia that alters their phenotype from a surveillance mode to a reactive mode characterized by hypertrophic morphology (Haynes et al., 2006), proliferation and upregulation of inflammatory genes (Inoue & Tsuda, 2009), and resulting in neuropathic pain (Tsuda, Beggs, Salter, & Inoue, 2013). Pharmacological, molecular, and genetic manipulations of spinal microglia have been shown to modulate PNI-induced pain hypersensitivity. Thus, there is strong evidence that microglia are important players in neuropathic pain (Inoue & Tsuda, 2018). However, the key molecules regulating microglial phenotypic change in the activation step are not fully understood.
Recent accumulating evidence has revealed key transcription factors for microglial development and maintenance: Transcription factor PU.1 is a critical factor for the emergence of microglia (Scott, Simon, Anastasi, & Singh, 1994), Runt-related transcription factor 1 regulates the microglial cell cycle and cell propagation in the perinatal brain (Zusso et al., 2012), Sal-like protein 1 was recently identified as the predominant regulator of adult microglial genes (Buttgereit et al., 2016; Matcovitch-Natan et al., 2016), and interferon regulatory factor 8 (IRF8) is essential for the development of neonatal microglia (Kierdorf et al., 2013). Of the above factors, IRF8 is induced in mature microglia to exert proinflammatory roles and in spinal cord microglia in neuropathic pain models (Akagi et al., 2014; Masuda et al., 2012); however, IRF8 does not fully explain the microglial phenotypic change in neuropathic pain. From the hypothesis that transcription factors regulating microglial development share some mechanisms with the phenotypic changes in a pathological event during adulthood, we focused on a member of the large Maf family of transcription factors, MafB, which has basic leucine-zipper DNA-binding motifs and N-terminal transactivation domains (Eychene, Rocques, & Pouponnot, 2008; Nishizawa, Kataoka, Goto, Fujiwara, & Kawai, 1989). It is recognized as a key regulator of tissue-specific gene expression and cell differentiation in, for example, macrophages, podocytes, and pancreatic α and β cells (Eychene et al., 2008; Hang & Stein, 2011; Kataoka, 2007). In the hematopoietic cell lineage, MafB commits monocytes to differentiate into macrophages rather than dendritic cells, suggesting MafB has the potential to shape the macrophage phenotype (Bakri et al., 2005; Friedman, 2007; Kelly, Englmeier, Lafon, Sieweke, & Graf, 2000). Recent studies indicate that microglia and tissue-resident macrophages share primitive myeloid progenitor cells as an origin (Ginhoux et al., 2010; Ransohoff & Cardona, 2010; Saijo, Collier, Li, Katzenellenbogen, & Glass, 2011). MafB is expressed predominantly in adult microglia and regulates gene sets related to immune activation (Matcovitch-Natan et al., 2016; Silvin & Ginhoux, 2018). Thus, we assessed MafB expression and function in spinal microglia and its involvement in pathogenesis using a well-established mouse model of neuropathic pain that demonstrated upregulation of MafB in spinal microglia, and crucial role of MafB in microglia for development of PNI-induced mechanical hypersensitivity.
2 MATERIALS AND METHODS
2.1 Animals
Male WT C57BL/6J mice were purchased from CLEA Japan, Inc. Male plt mice (Mori et al., 2001; Nakano & Gunn, 2001) were kindly provided by Prof. Terutaka Kakiuchi (Department of Immunology, Toho University School of Medicine). Mafb+/GFP mice (Moriguchi et al., 2006) were kindly provided by Prof. Satoru Takahashi (Department of Anatomy and Embryology, University of Tsukuba). Since Mafb deficient mice (MafbGFP/GFP) are lethal in neonatal stage (Moriguchi et al., 2006), the mice were maintained heterozygously. Mafb-flox mice (Yu et al., 2013) were kindly provided by Prof. Lisa Goodrich (Department of Neurobiology, Harvard Medical School). Cx3cr1-CreERT2 mice were purchased from Jackson Laboratory. For induction of Cre recombinase and generation of microglia specific knockout, 5-week-old Cx3cr1-CreERT2 mice were injected s.c. with 2 mg of tamoxifen (Sigma, St. Louis, MO) dissolved in 100 μl corn oil (Wako, Osaka, Japan), twice with a 48 hr interval (Wieghofer, Knobeloch, & Prinz, 2015). Mice were housed at a constant temperature of 22 ± 1°C with a 12 hr light–dark cycle (light on 8:00 to 20:00), and were fed food and water ad libitum. All animal experiments were conducted in accordance with the guidelines of Kyushu University.
2.2 Neuropathic pain model
For the neuropathic pain model, we used the spinal PNI model (Kim & Chung, 1992) with some modifications (Rigaud et al., 2008; Tsuda, Kuboyama, et al., 2009). In brief, while mice were under 2% isoflurane anesthesia, a small left-side incision was made at L3–S1. Paraspinal muscle and fat were separated from the L5 traverse process, and part of this traverse process was removed to expose the parallel-lying L3 and L4 spinal nerves. The L4 nerve was isolated and amputated. The wound and the surrounding skin were sutured with 5-0 silk and a surgical stapler. We treated the result from contralateral side of injury as an experimental control instead of using sham-treated animals.
2.3 Real-time quantitative RT-PCR analysis
Total RNA from L4 spinal cords of mice were extracted using Trisure (Bioline, Humber Road, London) according to the manufacturer's protocol. The amount of total RNA was quantified by measuring optical density at 260 nm (OD260) using a Nanodrop spectrophotometer (Nanodrop, Wilmington, DE). Total RNA from sorted cells was extracted and purified with the NucleoSpin RNA XS kit (Takara, Otsu, Japan). Prime Script reverse transcriptase (Takara) was used for reverse transcription with random 6-mer primers. Quantitative PCR for the obtained cDNA was performed with Premix Ex Taq (Takara) using a 7500 real-time PCR system (Applied Biosystems, Foster City, CA) according to the protocol of the manufacturer, and the data were analyzed with 7500 System SDS Software 1.3.1 (Applied Biosystems) using the standard curve method. All values were normalized to 18S ribosomal RNA expression except for values from sorted cells that were normalized to the total cell number in the sample (3,000 cells). Sequences of the forward primer, reverse primer, and TaqMan probe are listed in Table 1. MicroRNA was quantified with TaqMan MicroRNA Cells-to-CT Kit following manufacturer's protocol. Probe; TaqMan® MicroRNA Assays (Assay ID: 000475) was purchased from Applied Biosystems.
| Gene | 5′ to 3′ sequence | |
|---|---|---|
| Mafb | Forward primer | GCCTTCTTCTCCCAGCTTCA |
| Reverse primer | TCGGGATTCATCTGCTGGTAGT | |
| Taqman probe | TCCGACTGAACAGAAGACCCATCTCGA | |
| Aif1(iba1) | Forward primer | GATTTGCAGGGAGGAAAAGCT |
| Reverse primer | AACCCCAAGTTTCTCCAGCAT | |
| Taqman probe | CAGGAAGAGAGGCTGGAGGGGATCAA | |
| Irf8 | Forward primer | TGGTGACTGGGTATACTGCCTATG |
| Reverse primer | TGCCCCCGTAGTAAAAGTTGA | |
| Taqman probe | CGCACACCATTCAGCCTTATCCCAG | |
| Irf5 | Forward primer | CCTCAGCCGTACAAGATCTACGA |
| Reverse primer | GTAGCATTCTCTGGAGCTCTTCCT | |
| Taqman probe | CCAACGGCCCTGCTCCCACA | |
| P2rx4 | Forward primer | ACAACGTGTCTCCTGGCTACAAT |
| Reverse primer | GTCAAACTTGCCAGCCTTTCC | |
| Taqman probe | CAATGAGCAACGCACACTCACCAAGG | |
| P2rx7 | Forward primer | TGCAGCTGGAACGATGTCTT |
| Reverse primer | CCAAAGCAAAGCTCTAATGTAGGAA | |
| Taqman probe | TATGAGACAAACAAAGTCACCCGGATCCA | |
| P2ry12 | Forward primer | TGAAGACCACCAGGCCATTT |
| Reverse primer | AGGCCCAGATGACAACAGAAA | |
| Taqman probe | AAACGTCCAGCCCCAGCAATCTCTTG |
- All probes were conjugated with FAM at the 5′ end and TAMRA at the 3′ end.
2.4 Immunohistochemistry
Mice deeply anesthetized by intraperitoneal injection of pentobarbital were perfused transcardially with ice-cold phosphate buffered saline (PBS) followed by 4% (w/v) paraformaldehyde/PBS. The L4 segments of the spinal cord were removed, postfixed in the same fixative for 3 hr at 4°C, and placed in 30% (w/v) sucrose solution for 24 hr at 4°C. A subset of transverse spinal cord sections (30 μm) was cut from the center region of L4 segments and incubated in blocking solution (3% v/v normal goat serum) for 2 hr at room temperature. The sections were then incubated for 48 hr at 4°C with the primary antibodies for MafB (rabbit polyclonal, 1:3,000, Bethyl, Montgomery, TX, Supporting Information Figure S1), CD11b (rat monoclonal 5C6, 1:1,000; Serotec, Raleigh, NC), IBA1 (rabbit polyclonal, 1:2000; Wako), GFAP (rat monoclonal 2.2B10, 1:1000; Zymed Laboratories, San Francisco, CA), NeuN (mouse monoclonal A60, 1:200; Chemicon, Temecula, CA) and APC (mouse monoclonal CC1, 1:200; Calbiochem, San Diego, CA). Following incubation, the sections were washed and then incubated for 3 hr at room temperature with the appropriate secondary antibodies (Alexa Fluor™ 488 and/or 546 conjugated with goat anti-mouse/rat/rabbit IgG, 1:1000; Molecular Probes, Eugene, OR). The processed tissue sections were washed, slide-mounted, and coverslipped with Vectashield hard mount with 4′,6′-diamidino-2-phenylindole (DAPI; a cellular nuclear marker, 1.5 μg/ml; Vector Laboratories, West Grove, PA). Fluorescence images were obtained with a confocal microscope (LSM 5 Pascal; Carl Zeiss, Jena, Germany) and analyzed with the Zeiss LSM Image Browser (Carl Zeiss, Dunlin, CA).
For quantitative assessment of the immunofluorescence intensity of MafB in ipsilateral and contralateral spinal dorsal horn sections, all MafB-positive signals that were co-labeled with CD11b and with an intensity over 10-fold of background signal were thresholded and outlined, the pixel intensities within each region were measured by ImageJ (https://imagej.nih.gov/ij/; NIH, Bethesda, MD). The average value was normalized to that of the contralateral side.
2.5 Isolation of spinal microglia
Spinal microglia were sorted by a cell sorter to isolate CD11b-positive cells. The L4 spinal cord was digested with Liberase DL enzyme mix (2 WU/ml in Hank's balanced salt solution [HBSS], Roche, Basel, Switzerland) for 25 min at 37°C. Myelin debris was removed by magnetic separation using Myelin Removal Beads II and a MACS LS column (Miltenyi Biotec, Bergisch-Gladbach, Germany) according to the manufacturer's protocol. After cells were washed and resuspended in PBS with 0.5% (v/v) fetal bovine serum, cells were incubated with anti-rat CD11b/c antibody conjugated to allophycocyanin (1:100, BioLegend, San Diego, CA) on ice. Three thousand CD11b/c-positive and propidium iodide-negative single cells, which were 70–100% of the total CD11b/c-positive cells isolatable from L4 spinal segment with our procedure, were counted and isolated from each sample by FACSAria III (BD Biosciences, San Jose, CA).
2.6 Intrathecal catheter placement
For intrathecal injection in mice, we used intrathecal catheter placement (Tsuda, Masuda, et al., 2009; Yaksh, Jessell, Gamse, Mudge, & Leeman, 1980) with some modifications. While mice were under 2% isoflurane anesthesia, a 32-gauge intrathecal catheter (ReCathCo, Allison Park, PA) was inserted through the atlanto-occipital membrane into the lumbar enlargement (close to the L4 segment) and externalized through the skin. The catheter placement was verified by the observation of hind limb paralysis induced by intrathecal administration of lidocaine (2%, 5 μl). Mice that failed to exhibit paralysis were excluded from the experiments.
2.7 Behavioral tests
To assess mechanical hypersensitivity, mice were placed individually in an opaque plastic cylinder, which was placed on wire mesh, and habituated for 1 hr to allow acclimation to the measuring environment. After that, calibrated von Frey filaments (0.02–2.0 g, Stoelting) were applied to the plantar surface of the hindpaw from below the mesh floor, and the 50% paw withdrawal threshold (PWT) was determined using the up–down method (Chaplan, Bach, Pogrel, Chung, & Yaksh, 1994). The PWT was measured before and after L4 spinal PNI in siRNA-treated mice. To assess inflammatory pain, CFA (0.01 mg per 20 μl, Sigma) was injected subcutaneously in the plantar surface of left hindpaw under transient restraint, and the 50% PWT was determined using the up–down method (Chaplan et al., 1994).
For the hot-plate test, mice were placed on a metal surface maintained at 45, 49, or 52°C within a 25-cm-high Plexiglass box (25 × 25 cm). The latency to either lick the hindpaw or jump was recorded as a nocifensive endpoint (Cao et al., 1998). Noxious heat-evoked tail-flick responses were measured following the application of radiant heat (Ugo Basile, Italy) to the tail. The intensity of the heat stimulus was adjusted to 50 V, and the latency of the tail withdrawal response (in s) was measured (Masuda et al., 2014).
In the tests of formalin-induced pain, mice were housed in individual boxes and allowed to habituate to the testing environment for 15 min. Then, mice were injected intraplantarly with formalin (5%, 10 μl, The duration of the licking and biting of the injected hindpaw was recorded at 5 min intervals for 60 min after the injection (Tsuda et al., 2007). Durations between 0–10 min and 30–60 min after injection were separated as the first and second phases of formalin-induced pain.
2.8 Cell culture
Mouse primary cultured microglia from MafbGFP/GFP mice were prepared in accordance with a method described previously (Biber et al., 2011; Nakajima et al., 1992) with some modifications. Briefly, pairs of Mafb+/GFP mice were mated overnight, and pregnant mice were used to obtain E18–19 embryos. A mixed glial culture was prepared for individual embryos whose genotypes were determined for each culture. Embryos were not divided by sex. The cultures were maintained for 9–15 days in Dulbecco's modified Eagle's medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS). Immediately before experiments, microglia were collected by gentle shaking, as the cells floated over the mixed glial culture, and were transferred to the appropriate culture dish for subsequent experiments.
2.9 Microglia transfer
Primary microglia were prepared as described above. Cells were plated in 60 mm UpCell® culture dishes (CellSeed Inc, Tokyo, Japan), which have low cell attachment property at room temperature. After a 1 hr incubation at 37°C, ATP (50 μM) or PBS was added and cells were incubated for 1 hr. The medium was replaced by HBSS(−) and the cells detached at room temperature. Microglia were collected by pipetting, centrifuged, and resuspended to adjust cell concentration to 2 × 103 cells/μl in HBSS(−), then 104 cells were injected through the implanted catheter.
2.10 Treatment with siRNA and microRNA mimic
To transfect siRNA, Stealth RNAi with the Lipofectamine™ RNAiMAX transfection system (Invitrogen, Japan) was used. Stealth RNA targeting Mafb mRNA and control Stealth RNA were designed by and purchased from Invitrogen, and the transfection procedure was performed generally according to the manufacturer's guidelines. The sequences are listed in Table 2. For in vivo siRNA treatment, we treated mice with Stealth RNA (20 pmol, 4 μl, 1:1 mixture with Lipofectamine™ RNAiMAX) through the implanted intrathecal catheter twice per day for 3 days before spinal PNI.
| siCont | Sense | CAGUGGAGGCGUCUUUACUCGAUCA |
| Antisense | UGAUCGAGUAAAGACGCCUCCACUG | |
| siMafB | Sense | GAGAAACUCGCCAACUCCGGCUUCA |
| Antisense | UGAAGCCGGAGUUGGCGAGUUUCUC |
For microRNA mimic and negative control, we chose hsa-miR-152 mirVana miRNA mimic (Assay ID: MC12269) and mirVanaTM miRNA mimic Negative Control #1(Ambion). Reagents (25 pmol) were premixed with Lipofectamine™ RNAiMAX (Invitrogen) and intrathecally administered through the implanted catheter at the volume 5 μl.
2.11 Statistical analysis
All data are presented as mean ± SEM. Statistical analyses of the results were evaluated by SigmaPlot version 11.0 (Systat Software, Inc., San Jose, CA) using the Student's t test (for results comparing two groups), one-way analysis of variance (ANOVA) with Dunn's post hoc tests (for results comparing multiple groups) or two-way repeated measures ANOVA with Bonferroni post hoc tests (for behavioral analysis of paw withdrawal threshold).
3 RESULTS
3.1 PNI upregulates MafB expression in spinal microglia
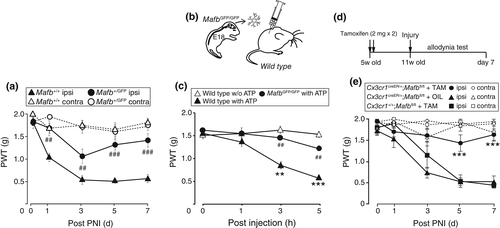
To investigate MafB expression in a PNI model of neuropathic pain (4th lumbar spinal nerve transection), we used MafbGFP/+ mice (Moriguchi et al., 2006) that have a Mafb allele replaced with cDNA encoding GFP and that express GFP in a manner that depends on Mafb expression. In this mouse line, we found an increasing number of GFP and Iba1-positive cell in the dorsal horn ipsilateral to PNI (Figure 1a). A predominant expression of GFP was found in Iba1-positive microglia (73 ± 2% of GFP-positive cell in dorsal horn), and some neuronal populations (13 ± 3%) in the dorsal spinal cord also expressed GFP, but no co-localization in astrocytes (GFAP) or oligodendrocytes (CC1) was detected in the all sample from four animals (Figure 1b,c). MafB-expressing neurons were seen in the outer layer of lamina III and in part of the ventral horn. However, at day 3 after PNI, the number of GFP+ neurons was not changed in contralateral and ipsilateral side (Figure 1d), which was in stark contrast to that of GFP+ microglia (Figure 1e).
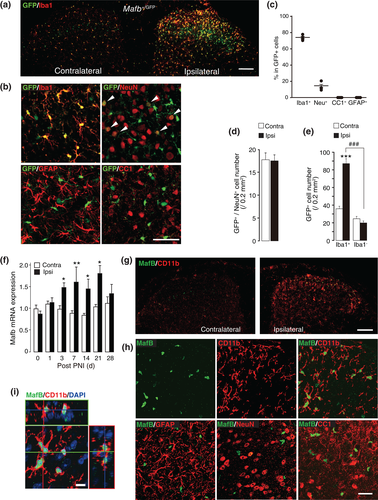
To see the intrinsic MafB expression, we used wild-type C57BL6J mice and found that the levels of Mafb mRNA in the L4 spinal cord after PNI were significantly increased in the ipsilateral side from day 3 to at least day 21 (Figure 1f). We next performed immunohistochemical analysis to evaluate the expression of MafB protein. Because of host incompatibility of anti-iba1 antibody we used anti-CD11b for detection of microglia with anti-MafB antibody. In the spinal dorsal horn 3 days after PNI, a marked microglial activation was reflected by the upregulation of CD11b in the ipsilateral side to PNI, and also an increase in MafB immunofluorescence (MafB-IF) was observed in the similar region (Figure 1g): the number of MafB-positive cells and the intensity of MafB-IF in individual cells were significantly increased. By double-immunostaining with cell-type specific markers, we found that the most MafB-positive cells were co-labeled with the microglial marker CD11b (93 ± 2% of MafB-positive cells in the ipsilateral side to PNI) and much smaller number of NeuN-positive cells (3 ± 2%), but no signal was observed in the cell possessing the astrocytic marker glial fibrillary acidic protein (GFAP) or the oligodendrocyte marker CC1 (Figure 1h). The orthogonal view of z-stacked images of MafB+ microglia shows that MafB-IF co-localized with the nuclear marker 4′, 6-diamidino-2-phenylindole (DAPI; Figure 1i). These observations demonstrate that the PNI-induced MafB upregulation selectively occurs in microglia and not in neurons in the spinal dorsal horn.
3.2 Microglial MafB expression is elevated early after PNI
We considered that MafB could have a role in induction of activated microglia in response to PNI. We therefore examined its expression at earlier time points following PNI; a PNI-induced increase in MafB-IF in spinal microglia with activated morphology was already observed as early as day 1 (Figure 2a). In histogram analysis of MafB-IF intensity per individual microglial cell, the distribution of MafB-IF intensities was skewed to the right at day 1 and gradually reduced its expression level by day 7 while MafB-positive cell number increased (Figure 2b). The number of MafB-positive microglia were significantly increased from day 1 and maintained after day 3 to day 7 after PNI (Figure 2c). The mean intensity of MafB-IF per microglial cell was 2.8-fold higher on average in the ipsilateral compared with the contralateral dorsal horn, and its increased expression gradually decreased after day 1 (Figure 2d). Interestingly, in microglia isolated from the spinal cord by fluorescence-activated cell sorting (FACS), the levels of Mafb mRNA from an equal number of CD11b+ microglial cells were not changed by PNI (Figure 2e). In contrast, Aif1 (Iba1) mRNA was increased in the same samples at day 3 post-PNI (Figure 2f). These findings suggest that mRNA upregulation observed from day 3 post-PNI (Figure 1f) was a result from the increasing population of microglia by its proliferation in the spinal cord after PNI (Supporting information Figure S2), and thus suggest a possible posttranscriptional upregulation of MafB protein.
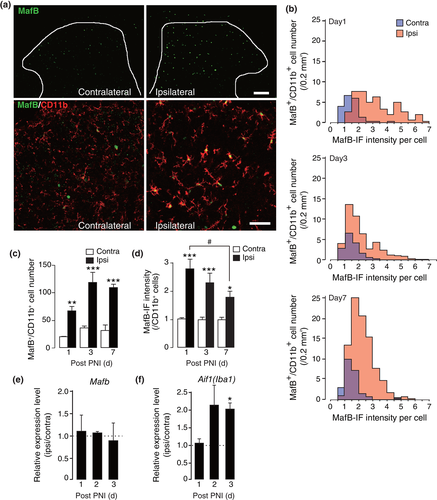
3.3 Reduced expression of mir-152 correlated with MafB expression in the spinal cord after PNI
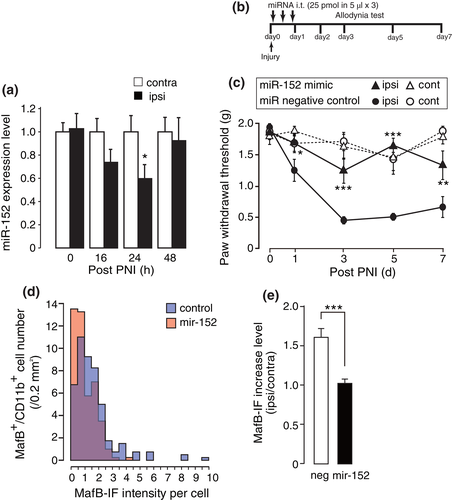
Regarding posttranscriptional regulation mechanism of MafB, we assessed an involvement of microRNA-mediated translational repression. Since previous reports suggested that mir-152 directly targets MafB expression (Li et al., 2017; Rio-Machin et al., 2013), first we quantified a mir-152 expression in the spinal cord. We found a transient but significant reduction of mir-152 at 24 hr after PNI. The decrease was returned to baseline by the end of 48 hr after PNI (Figure 3a). Thus, for the purpose of compensation of the decrease, we injected mir-152 mimic intrathecally after PNI (Figure 3b). This treatment significantly suppressed the development of mechanical allodynia (Figure 3c), but no abnormality in motor function was observed in uninjured hind limb. Immunohistochemical analysis indicated a reduction of MafB-IF level in the spinal microglia at day 1 after PNI (Figure 3d,e). Although microRNA commonly targets multiple gene expression, the results suggest that down-regulation of mir-152 which are reported to regulate MafB expression was one of the factors responsible for the upregulation of MafB after PNI.
3.4 Microglial MafB contributes to mechanical hypersensitivity
To investigate the role of MafB in neuropathic pain, we again utilized Mafb+/GFP mice. Because of the lethality of Mafb knockout mice (Mafb GFP /GFP), we used a Mafb+/GFP. After PNI, wild-type mice were hypersensitive to light mechanical stimuli applied to the hindpaw but Mafb+/GFP mice showed reduced hypersensitivity without affecting mechanical sensitivity in the contralateral hindpaw (Figure 4a). In contrast to this phenotype, mechanical hypersensitivity in a model of peripheral tissue inflammation by intraplantar injection of complete Freund's adjuvant was indistinguishable between wild-type and Mafb+/GFP mice (Supporting information Figure S3a). In addition, intraplantar formalin-induced pain behaviors, responses to tail flick test and hot plate tests were identical between the two genotypes (Supporting Information Figure S3b–d). Since MafB reporter GFP expression also observed in spinal dorsal horn neurons, to assess the microglial MafB contribution to pain hypersensitivity, we carried out microglial transfer experiments in which delivery of ATP-stimulated primary cortical microglia into the lumbar spinal subarachnoid space induces mechanical hypersensitivity (Figure 4b). As previously reported (Tsuda et al., 2003), ATP-stimulated primary cortical microglia induced hypersensitivity to mechanical stimulation for at least 5 hr, whereas unstimulated microglia did not (Figure 4c). In contrast, mechanical hypersensitivity was suppressed by ATP-stimulated primary cultured microglia possessing MafbGFP/GFP alleles that lack MafB protein, suggesting the ability of microglial MafB to produce mechanical hypersensitivity. Since MafB has been implicated in microglial maturation (Matcovitch-Natan et al., 2016), to determine its role in adult spinal cord, we crossed floxed-Mafb (Mafbfl/fl) mice with mice possessing a tamoxifen-inducible Cre recombinase under the control of Cx3cr1 (Cx3cr1-CreERT2 mice) to obtain Cx3cr1+/creER;Mafbfl/fl mice. Their 5-week-old offspring were treated with tamoxifen (Figure 4d), as controlled Mafbfl/fl mice, to obtain microglia specific MafB knockout mice (Supporting Information Figure S4) and Cx3cr1+/creER;Mafbfl/fl mice with corn oil vehicle as same genotype control. We subjected these groups of mice to PNI, and found that microglia specific MafB knockout resulted in a significant reduction of allodynic behavior (Figure 4e). These results strongly suggest that microglial MafB contributes to neuropathic pain development.
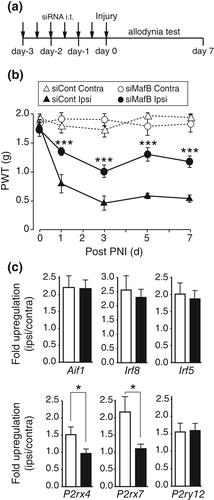
Finally, we tested the effect of MafB-targeted short interfering RNA (siMafB) administered intrathecally to knock down spinal MafB to achieve more shorter duration of MafB knock down before PNI. In mice treated intrathecally with siMafB twice per day for 3 days before PNI (Figure 5a), the PNI-induced increase in MafB-IF intensity per spinal microglial cell was reduced in siMafB-treated groups (49% reduction, Supporting Information Figure S5a,b). The number of MafB-positive microglia was not changed (Supporting Information Figure S5c). In von Frey testing, the PNI-induced mechanical hypersensitivity was significantly alleviated in siMafB-treated mice (Figure 5b). The threshold was unchanged for the hindpaw contralateral to the PNI and no abnormal motor dysfunction was observed. At day 7 in the spinal cord, we found that P2rx4 and P2rx7 mRNA upregulation was significantly suppressed in the siMafB-treated mice (Figure 5c). Iba1 (Aif1), Irf8, Irf5, and P2ry12 which related to microglial activation and neuropathic pain were not affected by siMafB treatment. Together, these results suggest that MafB expressed in spinal microglia contributes to the development of PNI-induced mechanical hypersensitivity through a regulation of P2rx4 and P2rx7 gene expression.
4 DISCUSSION
Following PNI, spinal microglia are activated and play a crucial role in neuropathic pain development and maintenance through a functional shift that leads to aberrant excitability of pain transmission neurons (Inoue & Tsuda, 2009). Because such a pathological shift is considered a reflection of differential gene expression, transcription factors could have a significant contribution to the shift. MafB is recognized as a lineage-specific transcription factor involved in the differentiation of cells such as macrophages, podocytes, pancreatic α and β cells, and spiral ganglion neurons (Artner et al., 2007; Bakri et al., 2005; Sadl et al., 2002; Sieweke, Tekotte, Frampton, & Graf, 1996; Stam et al., 2012; Yu et al., 2013). It was also known that MafB is one of the predominant transcription factors that are highly elevated upon the shift from pre- to adult microglia (Matcovitch-Natan et al., 2016), and the report revealed a set of genes regulated by MafB with Mafbfl/fl;Csf1rcre/+ mice which include immune and antiviral gene pathways. Although we did not perform detail assessed about target genes of MafB, our findings showed some candidate genes regulated by MafB that were known to be involved in the pathology of neuropathic pain. Our observations in this study illustrate the substantial importance of MafB in adult microglia, especially in the pathology of neuropathic pain.
The present study demonstrated the upregulation of MafB in spinal microglia after PNI but the increase in MafB-IF requires some assumptions for interpreting its regulatory mechanisms. We noticed that (a) while Mafb mRNA upregulation in the spinal cord tissue was not detected on day 1 post-PNI (Figure 1e), MafB-IF upregulation observed from day 1 after PNI (Figure 2a), and (b) when Mafb mRNA upregulation was detected in the spinal cord at day 3 post-PNI, isolated spinal microglia from injured and noninjured side of spinal cord had same level of Mafb mRNA expression (Figure 2e). Therefore, we considered that PNI-induced Mafb mRNA increase in spinal cord may be due to the increase in the number of MafB+ microglia since microglial proliferation is usually observed after day 2 after PNI (Echeverry, Shi, & Zhang, 2008; Suter, Berta, Gao, Decosterd, & Ji, 2009; Tsuda et al., 2011; Supporting Information Figure S2), and that the data from isolated microglia indicate little alteration in Mafb mRNA levels in individual spinal microglial cells. Then we thought posttranscriptional regulation of MafB. A possible explanation for MafB-IF upregulation may be a microRNA-mediated translational regulation (Ponomarev, Veremeyko, & Weiner, 2013). We revealed that transient reduction of mir-152 in the spinal cord after PNI and supplementation of this microRNA clearly suppressed the development of neuropathic pain. MicroRNA usually target set of genes, thus the behavioral effect observed here may not be caused by merely a MafB downregulation; however, the fact that mir-152 directly regulates MafB expression has been proved by 3′ UTR reporter assay (Li et al., 2017). Thus, it was suggested that the effect of mir-152 supplementation was at least in part due to reactivation of translational repression activity of mir-152 on MafB. Other factors may also be involved in the MafB expression, indeed we found that intrathecal CCL21 increased MafB expression in spinal microglia (Supporting Information Figure S7). This chemokine is produced in damaged primary afferent neurons immediately after PNI and transported to their central terminals in the dorsal horn, where it contributes to pain hypersensitivity (Biber et al., 2011), implying a direct induction of MafB in microglia by the injured-neuron-derived factor. However, reduced expression of MafB in CCL21 deficient mice (plt) after PNI was partial, and CCL21 administration did not suppress mir-152 expression in the spinal cord thus there may be several parallel pathways regulating MafB expression. Another possibility may involve an increase in stability of the MafB protein. MafB is subjected to negative regulation through the ubiquitin-proteasome pathway (Tanahashi, Kito, Ito, & Yoshioka, 2010) or ncRNA mediated-translational regulation (Zhang, Zhang, & Yu, 2012), and also a conformational change in the transcriptional complex which exposes epitopes in the MafB protein can also be involved in the MafB-IF upregulation.
Our behavioral assays in chronic pain models revealed the crucial role of MafB in neuropathic mechanical hypersensitivity. As the data from Mafb+/GFP mice indicated the expression of MafB (reporter GFP expression) in a subpopulation of spinal dorsal horn neurons (NeuN-positive cells) in lamina III and ventral spinal cord that is consistent with a recent report (Bikoff et al., 2016; Del Barrio et al., 2013). Thus, a contribution of neuronal MafB to sensory processing was suspected as a cause of pain reduction. However, there was no difference between Mafb+/GFP and wt mice in chronic inflammatory pain model, intraplantar formalin-induced pain behavior, tail flick test and hot plate tests denying influence of the heterozygous deficiency of MafB on physiological pain processing (Supporting Information Figure S3). Furthermore, transferring MafB knockout (MafbGFP/GFP) primary microglia obtained from fetus possessing a homozygous Mafb reporter gene into naive mice had little, if any, effect on mechanical hypersensitivity, and microglia-selective Mafb-knockout mice (Cx3cr1+/creER;Mafbfl/fl) did not develop allodynia. These results support that MafB expression specifically in microglia is necessary for the development of allodynia. Furthermore, we demonstrated that intrathecal siMafB effectively reduced the MafB expression and suppressed PNI-induced hypersensitivity. Previous studies have reported that neuropathic pain hypersensitivity is reversed by intrathecal siRNA targeting either Irf8 and Irf5, transcription factors whose expression is upregulated selectively in spinal microglia after PNI (Masuda et al., 2014), but in additional assessments we found that siMafB given in the maintenance phase had no effect on the PNI-induced hypersensitivity (Supporting Information Figure S6a) and MafB conditional knockout by tamoxifen administration from 7 days after PNI did not affect established mechanical allodynia (Supporting Information Figure S6b). As our data demonstrated, modulation of MafB expression at early time point after PNI were effective to suppress the development of neuropathic pain, but MafB seems no longer have regulatory role after neuropathic pain established.
MafB in spinal microglia may have a role of triggering P2X4 receptor-positive microglial activation that has a crucial role in the development of neuropathic pain (Beggs, Trang, & Salter, 2012). Our data from qPCR analysis of siMafB-treated mice indicates MafB is involved in P2X4 and P2X7 receptor expression. It has been known that there is sexual dimorphism in microglial P2X4 receptor-evoked pain hypersensitivity (Sorge et al., 2015). Since all our experiment done with male mice and our data suggested an involvement of P2X4 receptor as a target of MafB, MafB contribution to neuropathic pain in female needs to be carefully analyzed in future study.
In summary, our present data provide evidence that PNI increases MafB expression in spinal microglia in the early phase of neuropathic pain, at least in part through mir-152 mediated regulation, and that MafB is a necessary factor to trigger the microglia-mediated development of neuropathic pain.
ACKNOWLEDGMENTS
This work was supported by grants from the Japan Agency for Medical Research and Development (AMED, Research Project on Elucidation of Chronic Pain, Core Research for Evolutional Science and Technology (CREST) program under Grant Number JP18gm0910006 (M.T.)), the Japan Society for the Promotion of Science through the Ministry of Education, Culture, Sports, Science and Technology of Japan (KAKENHI Grant Numbers 25117013, 23229008, 15H02522, 25460721), the Uehara Memorial Foundation (H-T.S), and the Toray Science Foundation (MT). The authors thank Prof. Terutaka Kakiuchi (Department of Immunology, Toho University School of Medicine) for the kind supply of plt mice and Prof. Satoru Takahashi (Department of Anatomy and Embryology, University of Tsukuba) for the kind supply of Mafb+/GFPmice. The authors appreciate Prof. Lisa Goodrich (Department of Neurobiology, Harvard Medical School) and Prof. Yu Wei-Ming (Department of Biology, Loyola University) for kind supply of floxed-Mafb mice. The authors appreciate the technical support from the Research Support Center, Graduate School of Medical Sciences, Kyushu University. The authors thank Ann Turnley, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
HT-S, KI, and MT participated in the research design. The experiments were performed by HT-S, JM, RK, CK, SY, and TM. The data were analyzed by HT-S. HT-S, JM, KI, MT contributed to the writing of the manuscript. All authors have read and approved the final version of the manuscript.