The Effect of Non-Nutritive Sweeteners' Consumption on Body Weight: A Randomized-Controlled Trial
Funding: This study was partially funded by the Holy Spirit University of Kaslik (USEK). USEK was not involved at any stage of the study and had no role in the study design, data collection, data analysis and interpretation, writing of the report, or the decision to submit the article for publication. Our research was conducted independently, ensuring that all findings are unbiased and solely reflect the outcomes of our investigation.
ABSTRACT
For several decades, non-nutritive sweeteners (NNS) have been used as alternatives to sugar for caloric control, but their metabolic effects remain unclear. In the literature, they were blamed to contribute to several conditions, including obesity. While their effect on body weight has been investigated in numerous studies, the results have been inconsistent. Our study aims to investigate the effect of NNS consumption on body weight, and whether the results vary among different types of sweeteners. A randomized-controlled trial was conducted on a final number of 20 healthy participants over a period of 6 weeks, with an initial screening session and two follow-up sessions. Anthropometric measurements were taken at each visit. While NNS consumers were randomly assigned to the sucralose (0.507 mg/kg; n = 7) or stevia group (0.375 mg/kg; n = 6), non-consumers were allocated to the control group (no sweeteners; n = 7); all were given an isocaloric diet and were counseled to follow a healthy lifestyle. Our results showed that weight and body mass index significantly decreased among controls (pweight = 0.026; pbody mass index = 0.02) and sucralose (pweight = 0.028; pbody mass index = 0.017), but not among stevia consumers (pweight = 0.183; pbody mass index = 0.138). No significant difference was found between groups where no group had a benefit over the other in terms of weight loss. Moreover, no favorable effect of one sweetener was reported over the other. In our population, sucralose and stevia consumption contributed to weight loss that reached significance among sucralose consumers, and could therefore be considered safe for weight management when used as a part of a healthy lifestyle.
1 Introduction
Obesity, a chronic complex disease, has become an epidemic, with the number of individuals affected estimated to exceed one billion worldwide by 2030 (World Health Organization 2023). It is the result of many factors, and causes several medical problems including cardiovascular diseases, cancers, glucose intolerance and type 2 diabetes mellitus (T2D) to cite a few (Nettleton et al. 2016; World Health Organization 2023). Weight management and weight loss are the fundamentals in the treatment of obesity (Cornier 2022) or its related diseases (Shankar et al. 2013), where non-nutritive sweeteners (NNS) are unintentionally consumed, and have become a popular sugar substitute (Nettleton et al. 2016). Because of their sweet taste and their low caloric contribution, the consumption of that “magical alternative to white sugar” has increased in the past few years; in the United States, the consumption of NNS-containing beverages has increased from 18.7% to 24.4% among adults, and 33% of women consume food and beverages containing low-calorie sweeteners (Sylvetsky et al. 2012). In Lebanon, studies addressing the consumption of NNS-containing items are limited, and show that consumption of such products is prevalent. According to Mousawi et al. (2020), 30% of the population was consuming pills and powders (pp), or products containing NNS. Another study conducted in 2022 revealed that consumption of NNS is highly prevalent among the Lebanese population under study, where 94.4% reported using an item containing NNS at least once in the last 6 months. In the same population, 20.5% were using pp while 94.1% were consuming a NNS-containing food and beverage (Daher et al. 2022).
Until recently, NNS were thought to be healthy sugar substitutes (Pepino 2015), but it is uncertain whether they can improve metabolic health (Swithers 2013). Their effect on body weight (BW) and obesity has long been investigated, and results were contradictory. While some studies demonstrated that weight loss was equivalent with NNS-containing beverages and water (Harrold et al. 2023), others have reported an unfavorable effect of NNS (Azad et al. 2017; Bouchard et al. 2010; Pearlman et al. 2017) or NNS-containing beverages (Fowler et al. 2015; Madjd et al. 2018; Nettleton et al. 2009; Ruanpeng et al. 2017) on BW and obesity. In contrast, some studies found that NNS-containing beverages do not promote weight gain (Schulze et al. 2004) or had no effect on BW (Bonnet et al. 2018). In addition, other findings reported that NNS consumption decreased BW (Ebbeling et al. 2020; Laviada-Molina et al. 2020; Rogers and Appleton 2021) and was associated with a lower risk of overweight in women, suggesting it as a potential protective factor against obesity (Duran Aguero et al. 2015).
In Lebanon, studies on the effect of NNS consumption on BW are limited. In addition, human studies specifically assessing the effects of sucralose and stevia are scarce, as most of the existing research focuses on NNS consumption in general without distinguishing between the different types of sweeteners. For this reason, we conducted this study to investigate the effect of NNS consumption, specifically sucralose and stevia, on BW among Lebanese adults residing in Beirut and Mount-Lebanon, and to investigate if the results vary between different types (natural or artificial) of NNS available in the Lebanese market.
2 Materials and Methods
2.1 Study Design and Procedure
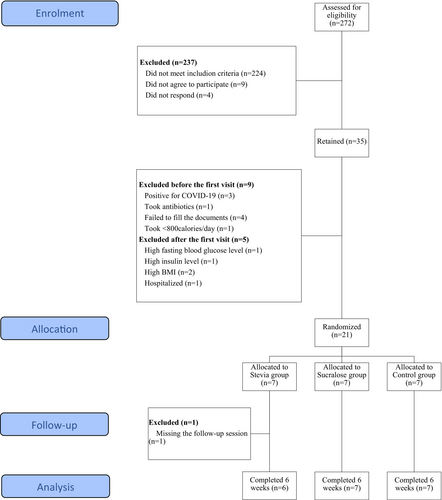
The present study was conducted on a final number of 20 healthy Lebanese participants, recruited from Beirut and Mount-Lebanon, over a period of 6 weeks from May to July 2021. Announcements were posted on social media from January to May 2021, to which 272 participants responded and filled an online screening survey. Participants who met inclusion criteria (Table S1) were contacted and screened to be assigned to one of our three experimental groups; a final number of 35 participants was retained, and individual virtual meetings were conducted, during which the study was explained in detail, individual artificial sweeteners' intake was assessed by filling a validated food frequency questionnaire (FFQ) (Myers et al. 2018), and participants were asked to sign the consent form and to perform a food record over 3 days (two weekdays and one weekend). At the end of this phase, nine subjects were excluded either because they tested positive for coronavirus disease (COVID-19) (n = 3), took antibiotics (n = 1), did not fill the requested documents (n = 4) or were taking less than 800 cal per day (n = 1); 26 participants made it to the first visit. After the first visit, five participants were excluded either because they were found to have a high fasting blood glucose level (n = 1), a high insulin level (n = 1), a high body mass index (BMI) (n = 2), or because they were hospitalized (n = 1); 21 participants started the experimental part. At the end of Week 3, one participant was excluded for missing the scheduled follow-up session. Twenty participants finished the study (Figure 1).
2.2 Experimental Protocol
In this study, NNS consumers were randomly assigned to one of the two experimental groups (Group 1: Stevia, Group 2: Sucralose), while non-consumers were allocated to the control group. All participants underwent a washout period of 2 weeks. A document containing the majority of NNS-containing products was provided, and all subjects were educated to avoid any product containing NNS except the one prescribed for participants in the experimental groups. An isocaloric diet that matches individual caloric needs was prescribed for each subject.
2.3 Procedure
All participants were asked to attend three sessions consisting of one screening session and two follow-up sessions. During the screening session, anthropometric measurements were taken, and the BMI was calculated. Blood tests and other measurements were conducted to study factors other than obesity, not addressed in this article. A questionnaire was filled out by all participants by a trained dietitian after being tested and verified. NNS consumers were randomly assigned to one of the sweetener groups (n = 7 per group) at a dose equivalent to 0.507 mg/kg for sucralose, 0.375 mg/kg for stevia, as obtained from a previous study (Daher et al. 2022). Non-consumers, defined as individuals consuming less than one food or beverage containing low-calorie sweeteners per month (Sylvetsky et al. 2017) were assigned to the control group, as many individuals either prefer not to consume sweeteners or dislike their taste, which could affect compliance and consequently the overall results of the study (Peters et al. 2016). Commercially available sucralose and stevia (Steviol glycosides), which were previously found to be commonly used in the population under study (Daher et al. 2022) were used. This allows for a comparison between artificial and natural sweeteners. Participants were instructed about the amount of sweeteners to be consumed per day, and the isocaloric diet was prescribed. They were informed that sweeteners could be taken anytime throughout the day. In addition, they were asked to complete a food record consisting of two weekdays and one weekend and to bring any unused sweetener for follow-up. Follow-up sessions were scheduled once every 3 weeks.
2.4 Data Tools Collection and Techniques
In this study, the baseline questionnaire covered personal status (age, gender, marital status, educational level and living district), economic status (work and income), daily habits (smoking, alcohol consumption, physical activity levels, and water intake), NNS consumption (current and previous consumption of NNS), medical data, and nutrition data in addition to a 24-h dietary recall and a FFQ. Moreover, a food record was requested prior to each visit (Fink and Mikesky 2013). During follow-up visits, a questionnaire covering health and nutrition status was filled out, unused sweeteners were counted, and anthropometrics were taken, in addition to a 24-h dietary recall.
2.5 Dietary Habits
The Mifflin-St Jeor equation (Mifflin et al. 1990) and the revised Harris-Benedict equation (Roza and Shizgal 1984) were used to calculate the basal metabolic rate. The mean was calculated, and an isocaloric diet that matches the caloric needs of each participant was prescribed accordingly. The diet was designed following a macronutrient distribution of 50% carbohydrates, 20% proteins, and 30% fats. To make sure that subjects were taking their sweeteners and were following the diet, a 3-day food record was requested once every 10 days. In addition, unused sweeteners were checked, and a 24-h dietary recall was conducted at each visit.
2.6 Anthropometric Measurements
Anthropometric measurements including weight, height, and BMI were taken. Weight was measured using a medical scale with participants wearing light clothing; they were asked to remove their footwear and stand still (WHO 2005). Measurements were taken in duplicate, and the mean value was considered, to the nearest 0.01 kg. Height was measured using a stadiometer, to the nearest 0.01 cm. Participants were asked to stand and look straight with their feet held together, their heels against the measuring board, and their knees being straight (WHO 2005). The BMI was calculated as the ratio of weight (kg) to the square of height (m). A healthy weight is defined as 18.5 ≤ BMI ≤ 24.9 kg/m2, while overweight and obesity are defined as a BMI ≥ 25 kg/m2 and ≥ 30 kg/m2 respectively (CDC 2021).
2.7 Statistical Analysis
The Statistical Package for Social Sciences (SPSS) version 22.0 was used for data entry and analysis. The confidence interval (CI) was set at 95% and significance was considered at p-value < 0.05. Descriptive statistics were performed on data covering personal status (gender, age, marital status, educational level and place of living), economic status (work and income), daily habits (smoking, alcohol consumption, physical activity levels, water intake), BW, and BMI. Results were presented as percentages for qualitative variables and as minimum, maximum, mean, and standard deviation (SD) for quantitative variables. After performing normality and homogeneity tests, paired samples T-test was used to assess the effect of NNS consumption on BW and BMI within groups (within the control group, the sucralose group, the stevia group and all sweeteners group) when the data was normally distributed. When the data was not normally distributed, the non-parametric Wilcoxon test was used for symmetric data, whereas the bootstrap test was used for non-symmetric data. Independent samples T-test was used to assess the effect of NNS on those variables between groups (between control and stevia groups, control and sucralose groups, sucralose and stevia groups, as well as between the control and all sweeteners groups) when the data was normally distributed. For non-normally distributed data, bootstrap was used.
3 Results
3.1 General Characteristics of the Study Population
Our study included a total of 20 participants (7 males and 13 females), with a mean age of 25.95 ± 4.99 years. Among our participants, 55% (n = 11) were living in Mount-Lebanon, while 45% (n = 9) were living in Beirut. The mean value of BW at baseline was 60.59 ± 8.42 kg among controls, 61.74 ± 6.32 kg among the sucralose group, and 62.97 ± 11.51 kg among the stevia group. Participants in the three groups started the study with a normal BMI: controls had a BMI of 22.75 ± 2.18 kg/m2, and those in the sucralose and stevia groups had a BMI of 23.27 ± 0.87 and 23.39 ± 2.15 kg/m2 respectively. Other characteristics of our population can be found in Table S2.
3.2 Effect of NNS Consumption on BW and BMI Within Groups
In this part, we assessed if NNS consumption affected the mean values of BW and BMI within the control group, the sucralose group, the stevia group, in addition to the all sweeteners group, each group apart, over the 6 weeks. It is important to note that the “all sweeteners” group is formed of all participants that consumed either sucralose or stevia over the course of the study (Table 1).
| Control | Sucralose | Stevia | All sweeteners | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | N | p | Mean ± SD | N | p | Mean ± SD | N | p | Mean ± SD | N | p | |
| WeightW0 (kg) | 60.59 ± 8.42 | 7 | 0.096 | 61.74 ± 6.32 | 7 | 0.087 | 62.97 ± 11.51 | 6 | 0.055 | 62.31 ± 8.70 | 13 | 0.014* |
| WeightW3 (kg) | 59.77 ± 7.92 | 60.67 ± 6.86 | 61.82 ± 11.40 | 61.20 ± 8.84 | ||||||||
| WeightW3 (kg) | 59.77 ± 7.92 | 7 | 0.093 | 60.67 ± 6.86 | 7 | 0.011* | 61.82 ± 11.40 | 6 | 0.916 | 61.20 ± 8.84 | 13 | 0.194 |
| WeightW6 (kg) | 58.40 ± 7.26 | 60.21 ± 6.83 | 61.73 ± 11.59 | 60.92 ± 8.94 | ||||||||
| WeightW0 (kg) | 60.59 ± 8.42 | 7 | 0.026* | 61.74 ± 6.32 | 7 | 0.028* | 62.97 ± 11.51 | 6 | 0.183 | 62.31 ± 8.70 | 13 | 0.02* |
| WeightW6 (kg) | 58.40 ± 7.26 | 60.21 ± 6.83 | 61.73 ± 11.59 | 60.92 ± 8.94 | ||||||||
| BMIW0 (kg/m2) | 22.75 ± 2.18 | 7 | 0.09 | 23.27 ± 0.87 | 7 | 0.049* | 23.39 ± 2.15 | 6 | 0.039* | 23.33 ± 1.52 | 13 | 0.009* |
| BMIW3 (kg/m2) | 22.44 ± 1.95 | 22.85 ± 0.71 | 22.94 ± 1.86 | 22.89 ± 1.30 | ||||||||
| BMIW3 (kg/m2) | 22.44 ± 1.95 | 7 | 0.013* | 22.85 ± 0.71 | 7 | 0.01* | 22.94 ± 1.86 | 6 | 0.772 | 22.89 ± 1.30 | 13 | 0.165 |
| BMIW6 (kg/m2) | 21.93 ± 1.77 | 22.67 ± 0.76 | 22.89 ± 1.81 | 22.77 ± 1.29 | ||||||||
| BMIW0 (kg/m2) | 22.75 ± 2.18 | 7 | 0.02* | 23.27 ± 0.87 | 7 | 0.017* | 23.39 ± 2.15 | 6 | 0.138 | 23.33 ± 1.52 | 13 | 0.010* |
| BMIW6 (kg/m2) | 21.93 ± 1.77 | 22.67 ± 0.76 | 22.89 ± 1.81 | 22.77 ± 1.29 | ||||||||
- Abbreviations: BMI, body mass index; N, sample size; SD, standard deviation; W, week.
- * Significant difference.
Data analysis indicates that weight loss occurred among all three groups, where the mean value of BW and that of BMI decreased after 6 weeks of intervention. The decrease in BW was found to be significant among controls and the sucralose group at the end of Week 6, where the mean value of BW significantly decreased from 60.59 ± 8.42 to 58.40 ± 7.26 kg (p = 0.026), and from 61.74 ± 6.32 to 60.21 ± 6.83 kg (p = 0.028) among the controls and sucralose groups respectively. Among the stevia group, values failed to reach significance (p = 0.183).
The same was reported for BMI that started to decrease significantly after 3 weeks of intervention, especially among sucralose consumers (p = 0.049). When it comes to the stevia group, BMI significantly decreased from 23.39 ± 2.15 kg/m2 at baseline to 22.94 ± 1.86 kg/m2 (p = 0.039) at Week 3.
Surprisingly, the significant decrease in the mean value of BMI among the stevia group was not recorded for BW in the first 3 weeks, but it was near significance where p = 0.055. Despite the fact that the mean values of BW and BMI decreased at the end of Week 6 from 62.97 ± 11.51 kg (BMI 23.39 ± 2.15 kg/m2) to 61.73 ± 11.59 kg (BMI 22.89 ± 1.81 kg/m2), values failed to reach significance among stevia consumers. When we combined stevia and sucralose consumers in one group, the “all sweeteners” group, we found a significant decrease in the mean value of BW and BMI where the mean value of BW significantly decreased from 62.31 ± 8.70 to 60.92 ± 8.94 kg (p = 0.02) and that of BMI significantly decreased from 23.33 ± 1.52 to 22.77 ± 1.29 kg/m2 (p = 0.010).
Those results highlight the fact that weight loss was significant among controls, sucralose, and the all sweeteners group, but not among the stevia group.
3.3 Effect of NNS Consumption on BW and BMI Between Groups
In this part, we investigated if there is a significant difference in the mean value of BW and BMI between different groups; we compared the effect of NNS consumption on the mean value of BW and BMI between the control and stevia groups, between the control and sucralose groups, between the sucralose and stevia groups, and between the control and all sweeteners groups at Week 0 (baseline), Week 3, and Week 6.
3.3.1 Difference in BW and BMI Between Control and Stevia Groups
Our results showed that there is a non-significant difference in the mean value of BW between the control and stevia groups at Week 0 (p = 0.670), Week 3 (p = 0.717), and Week 6 (p = 0.540). The same was reported for the mean value of BMI that did not differ significantly between the controls and stevia consumers at Week 0 (p = 0.604), Week 3 (p = 0.648), and Week 6 (p = 0.356) (Table 2).
| Indicator | Groups | N | Mean ± SD | p |
|---|---|---|---|---|
| WeightW0 (kg) | Control | 7 | 60.59 ± 8.42 | 0.670 |
| Stevia | 6 | 62.97 ± 11.51 | ||
| WeightW3 (kg) | Control | 7 | 59.77 ± 7.92 | 0.717 |
| Stevia | 6 | 61.82 ± 11.40 | ||
| WeightW6 (kg) | Control | 7 | 58.40 ± 7.26 | 0.540 |
| Stevia | 6 | 61.73 ± 11.59 | ||
| BMIW0 (kg/m2) | Control | 7 | 22.75 ± 2.18 | 0.604 |
| Stevia | 6 | 23.39 ± 2.15 | ||
| BMIW3 (kg/m2) | Control | 7 | 22.44 ± 1.95 | 0.648 |
| Stevia | 6 | 22.94 ± 1.86 | ||
| BMIW6 (kg/m2) | Control | 7 | 21.93 ± 1.77 | 0.356 |
| Stevia | 6 | 22.89 ± 1.81 |
- Abbreviations: BMI, body mass index; N, sample size; SD, standard deviation; W, week.
3.3.2 Difference in Weight and BMI Between Control and Sucralose Groups
The same was reported while comparing the mean value of BW between the control and sucralose consumers at Week 0 (p = 0.798), Week 3 (p = 0.821), and Week 6 (p = 0.650), and that of BMI which did not differ significantly between the control and sucralose consumers at Week 0 (p = 0.569), Week 3 (p = 0.618), and Week 6 (p = 0.336) (Table 3).
| Indicator | Groups | N | Mean ± SD | p |
|---|---|---|---|---|
| WeightW0 (kg) | Control | 7 | 60.59 ± 8.42 | 0.798 |
| Sucralose | 7 | 61.74 ± 6.32 | ||
| WeightW3 (kg) | Control | 7 | 59.77 ± 7.92 | 0.821 |
| Sucralose | 7 | 60.67 ± 6.86 | ||
| WeightW6 (kg) | Control | 7 | 58.40 ± 7.26 | 0.650 |
| Sucralose | 7 | 60.21 ± 6.83 | ||
| BMIW0 (kg/m2) | Control | 7 | 22.75 ± 2.18 | 0.569 |
| Sucralose | 7 | 23.27 ± 0.87 | ||
| BMIW3 (kg/m2) | Control | 7 | 22.44 ± 1.95 | 0.618 |
| Sucralose | 7 | 22.85 ± 0.71 | ||
| BMIW6 (kg/m2) | Control | 7 | 21.93 ± 1.77 | 0.336 |
| Sucralose | 7 | 22.67 ± 0.76 |
- Abbreviations: BMI, body mass index; N, sample size; SD, standard deviation; W, week.
3.3.3 Difference in BW and BMI Between Sucralose and Stevia Groups
After comparing the difference between control and sweetener groups, we tried to assess if there is a significant difference between different types of sweeteners where sucralose is considered an artificial sweetener while stevia is considered a natural one. Data analysis shows that there is a non-significant difference in the mean value of BW between sucralose and stevia consumers at Week 0 (p = 0.823), Week 3 (p = 0.840), and Week 6 (p = 0.774). The same was reported for the mean value of BMI that did not differ significantly between the sucralose and stevia consumers at Week 0 (p = 0.897), Week 3 (p = 0.906), and Week 6 (p = 0.792) (Table 4).
| Indicator | Groups | N | Mean ± SD | p |
|---|---|---|---|---|
| WeightW0 (kg) | Sucralose | 7 | 61.74 ± 6.32 | 0.823 |
| Stevia | 6 | 62.97 ± 11.51 | ||
| WeightW3 (kg) | Sucralose | 7 | 60.67 ± 6.86 | 0.840 |
| Stevia | 6 | 61.82 ± 11.40 | ||
| WeightW6 (kg) | Sucralose | 7 | 60.21 ± 6.83 | 0.774 |
| Stevia | 6 | 61.73 ± 11.59 | ||
| BMIW0 (kg/m2) | Sucralose | 7 | 23.27 ± 0.87 | 0.897 |
| Stevia | 6 | 23.39 ± 2.15 | ||
| BMIW3 (kg/m2) | Sucralose | 7 | 22.85 ± 0.71 | 0.906 |
| Stevia | 6 | 22.94 ± 1.86 | ||
| BMIW6 (kg/m2) | Sucralose | 7 | 22.67 ± 0.76 | 0.792 |
| Stevia | 6 | 22.89 ± 1.81 |
- Abbreviations: BMI, body mass index; N, sample size; SD, standard deviation; W, week.
3.3.4 Difference in BW and BMI Between Control and All Sweeteners Groups
At the end, we investigated if there is a significant difference in the mean values of BW and BMI between control and all sweeteners groups (sucralose and stevia combined). Data analysis shows that there is a non-significant difference in the mean values of BW between the control and all sweeteners groups at Week 0 (p = 0.672), Week 3 (p = 0.723) and Week 6 (p = 0.482). The same was reported for the mean value of BMI that did not differ significantly between the control and sweeteners groups at Week 0 (p = 0.514), Week 3 (p = 0.595) and Week 6 (p = 0.274) (Table 5).
| Indicator | Groups | N | Mean ± SD | p |
|---|---|---|---|---|
| WeightW0 (kg) | Control | 7 | 60.59 ± 8.42 | 0.672 |
| Sweeteners | 13 | 62.31 ± 8.70 | ||
| WeightW3 (kg) | Control | 7 | 59.77 ± 7.92 | 0.723 |
| Sweeteners | 13 | 61.20 ± 8.84 | ||
| WeightW6 (kg) | Control | 7 | 58.40 ± 7.26 | 0.482 |
| Sweeteners | 13 | 60.92 ± 8.94 | ||
| BMIW0 (kg/m2) | Control | 7 | 22.75 ± 2.18 | 0.514 |
| Sweeteners | 13 | 23.33 ± 1.52 | ||
| BMIW3 (kg/m2) | Control | 7 | 22.44 ± 1.95 | 0.595 |
| Sweeteners | 13 | 22.89 ± 1.30 | ||
| BMIW6 (kg/m2) | Control | 7 | 21.93 ± 1.77 | 0.274 |
| Sweeteners | 13 | 22.77 ± 1.29 |
- Abbreviations: BMI, body mass index; N, sample size; SD, standard deviation; W, week.
4 Discussion
NNS have long been used for caloric control but the literature investigating their effect on BW is inconsistent. To assess the effect of NNS consumption on weight, we investigated if BW and BMI changed significantly over 6 weeks of intervention among the control, sucralose, stevia, and all sweeteners groups. Moreover, in order to investigate if a group has an advantage over the other, we compared those parameters between the “control and stevia,” “control and sucralose,” “sucralose and stevia,” and “control and all sweeteners” groups.
A significant weight loss occurred, especially among the control and the sucralose groups, where their BW and BMI significantly decreased after 6 weeks. In the stevia group, values decreased after 6 weeks, without reaching significance. When we combined the stevia and the sucralose groups under the “all sweeteners” group, the mean value of BW and BMI decreased significantly, where NNS consumers lost 1.39 kg after 6 weeks (p = 0.02), and their BMI decreased significantly (from 23.33 ± 1.52 to 22.77 ± 1.29 kg/m2; p = 0.010). Those results show that controls, sucralose, and all sweeteners consumers lost weight except for stevia. Moreover, when we performed a comparison between groups, none of the parameters differed significantly between control and sweeteners. Therefore, NNS consumption did not prevent weight loss nor cause weight gain as claimed in observational studies in particular, and did not have any beneficial nor detrimental effect on BW over controls. In the literature, several authors agree that the beneficial or non-detrimental effect of NNS on BW was shown in systematic reviews drawing conclusions from clinical trials or intervention studies (Clifton and Fayet-Moore 2016; Laviada-Molina et al. 2020; Miller and Perez 2014; Rogers and Appleton 2021), whereas the detrimental effect of NNS on BW was reported in observational studies (Lee et al. 2021; Normand et al. 2021). From our extensive literature review, we support this conclusion, especially that observational studies are known for having the weakest level of evidence where causality cannot be determined, and reverse causality cannot be ruled out (World Health Organization et al. 2022). As for NNS and BW, reverse causality is possible where the weight gain attributed to NNS consumption can be reported to the fact that overweight or obese people tend to consume NNS for caloric control. Therefore, the best level of evidence comes from randomized controlled trials and meta-analyses performed on those trials. Such studies have reported beneficial (Miller and Perez 2014; Peters et al. 2016; Tate et al. 2012) or neutral effects (Higgins and Mattes 2019; Laviada-Molina et al. 2020; Bueno-Hernández et al. 2020; Thomson et al. 2019) on BW which is consistent with our results where a significant weight loss was reported among controls, sucralose, and all sweeteners consumers but not among stevia after 6 weeks. The weight loss reported in our study among controls, sucralose, and all sweeteners consumers was also reported in Tate et al. (2012), where all groups including controls lost a significant weight after 3 and 6 months. Consistently, Miller and Perez (2014) found that NNS consumption decreases BW and BMI without specifying the type of sweeteners studied. Analyzing the effect of NNS consumption on BW by type of sweetener was challenging because the majority of the studies were conducted on low-calorie sweetened beverages without specifying the type of sweetener used (Miller and Perez 2014), and systematic reviews and meta-analyses investigating the effect of sucralose or stevia on BW are rare, with the majority conducted on NNS in general or on low-calorie sweetened beverages.
From the rare studies that investigated the effect of sucralose in particular on weight and BMI, results varied between neutral to no effect; those showed that the decrease in BW among sucralose consumers after 12 weeks was insignificant (Higgins and Mattes 2019), and that BW of sucralose and control groups remained stable across the study (Bueno-Hernández et al. 2020; Thomson et al. 2019).
When it comes to stevia, only the mean value of BMI decreased significantly in the first 3 weeks without any significant change in BW. After 6 weeks, no significant changes in BW and BMI were reported despite that all values were tending towards decrease, which was consistent with the literature (Higgins and Mattes 2019; Maki et al. 2008). However, the small sample size in the stevia group might have affected the results because when we combined the sweeteners under “all sweeteners” group, the p-value that compared the mean value of BW at baseline to that at Week 6 tended towards more significance and exerted a mild decrease from 0.028 in the sucralose group to 0.020 in the “all sweeteners” group. The same was reported for BMI, where the p-value decreased from 0.017 to 0.010.
In our study, NNS in general and sucralose in particular did not prevent weight loss nor caused weight gain. The reported weight loss can be attributed to the behavioral changes where all participants were asked to follow a healthy lifestyle, and all participants including controls lost weight, which proves that NNS can still lead to weight loss if integrated as part of a behavioral change program. Our results are consistent with the findings of Laviada-Molina et al. (2020) who found no evidence that NNS consumption promotes weight gain, and with Duran Aguero et al. (2015) who reported that sucralose consumption was associated with a lower risk of overweight in women, making it a protective factor against obesity. However, our findings contradict studies that have linked NNS consumption to obesity through various mechanisms, including overestimation of caloric savings, compensation (Fowler et al. 2008), disruption of energy balance (Peters et al. 2016), making NNS, in our study, a non-culprit in BW increase.
Interestingly, NNS consumption did not have any favorable effect on BW and BMI over controls; when we compared BW and BMI between control and “stevia,” “sucralose,” and “all sweeteners,” the mean value of those parameters did not differ significantly neither between groups nor between the types of sweeteners. This was consistent with several studies which concluded that there are no significant differences in BW between NNS consumers compared to placebo (Harrold et al. 2023; Maersk et al. 2012; Tate et al. 2012; Toews et al. 2019). Conversely, Stamataki et al. (2020) had different results where controls had a significant increase in BW and BMI when compared to stevia consumers after 6 weeks. However, in their study, no isocaloric diet was given and participants were not required to change their usual diet, which might have affected the results. Moreover, our results removed any role of the type of sweetener in weight loss, consistent with the findings of Higgins and Mattes (2019) at Week 6. However, in their study, sucralose consumers had a significantly lower BW than stevia consumers after 12 weeks, which puts the long-term difference in the effect of those sweeteners under question.
Our results as a whole indicate that NNS consumption can be an effective method for weight management, but has no advantage over avoiding consumption. The opposite is true, where those who decide to avoid NNS consumption will not have better results than those who use them in their daily life. Moreover, no beneficial effect of one type of sweetener over the other was recorded in our study where BW and BMI did not differ significantly between sucralose and stevia consumers.
The choice of sucralose and stevia among sweeteners was not due to chance. In fact, aspartame in the form of pills or powder is no longer widely available in the Lebanese market, and acesulfame-K is usually used in combination with other sweeteners in food products (FDA 2025). Therefore, sucralose and stevia were chosen for being commonly used, and because this allows for a comparison between natural and artificial sweeteners. Due to the lack of published data in Lebanon and due to the scarcity of human studies, our study was among the few human studies that investigated the effect of sucralose and stevia separately on BW at a dose representative of the daily dose consumed by individuals living in the area under study. It was the first to compare the different types of sweeteners and the first to be conducted in Lebanon.
However, this study had its limitations, one of which is the sample size. This was addressed by using the appropriate statistical tests and respecting all the assumptions needed to conduct the respective tests, which gives strength to our results. Moreover, compliance of participants made the study harder, but follow-ups were scheduled regularly to ensure that all participants are following the protocol. In addition, our results could not be extrapolated over the Lebanese population because our sample was selected from Beirut and Mount-Lebanon. At the end, studies that analyze changes in body composition and that perform gender-based analysis are needed. Those should be performed over a longer duration and over a larger sample size to investigate the long-term effect of sucralose and stevia on BW and BMI.
5 Conclusion
In conclusion, our study revealed a statistically significant reduction in weight and BMI among participants in the control and sucralose groups, but not in the stevia group. Although the trend within the stevia group was towards decrease, the lack of significance may be attributed to the small sample size within this group because when we combined the sucralose and stevia in one group (all sweeteners group), the p-value approached greater significance. Moreover, no statistically significant differences were observed between the three groups, indicating that neither sucralose nor stevia offered a superior benefit; no group had a benefit over the other in terms of weight loss and BMI decrease, and no favorable effect of one sweetener was reported over the other. Our findings suggest that both sucralose and stevia can be considered to be safe for weight management when used as a part of a healthy lifestyle among the population under study. However, given the small sample size and short duration of this study, further research with a larger sample size is needed to confirm these results and to explore the long-term effects of the sweeteners on BW and BMI.
This article aligns with Sustainable Development Goal 3: Good Health and Well-being, as it aims to assess the effect of sweeteners on body weight, especially as obesity is becoming an epidemic across the globe. By understanding one of the multiple factors affecting body weight, our study contributes to global initiatives to decrease the burden of non-communicable diseases.
Author Contributions
Mira Daher: conceptualization (equal), data curation (equal), formal analysis (equal), funding acquisition (equal), investigation (equal), methodology (equal), project administration (equal), resources (equal), software (equal), validation (equal), visualization (equal), writing – original draft (equal), writing – review and editing (equal). Elham El Darazi: writing – review and editing (equal). Mohammad Kacim: data curation (equal), formal analysis (equal), writing – original draft (equal), writing – review and editing (equal). Maya Hobeika: conceptualization (equal), funding acquisition (equal), methodology (equal), resources (equal), supervision (equal), validation (equal), writing – review and editing (equal). Yonna Sacre: conceptualization (equal), funding acquisition (equal), methodology (equal), project administration (equal), resources (equal), supervision (equal), validation (equal), visualization (equal), writing – review and editing (equal).
Acknowledgments
The authors would like to acknowledge the National Council for Scientific Research of Lebanon (CNRS-L) and the Holy Spirit University of Kaslik (USEK) for granting a doctoral fellowship to M.D., and the Food and Drug Corporation (FDC), Lebanon for providing free sweetener samples. The FDC was not involved at any stage of the study and had no role in the study design, data collection, data analysis and interpretation, writing of the report, or the decision to submit the article for publication. Our research was conducted independently, ensuring that all findings are unbiased and solely reflect the outcomes of our investigation. The authors would like to also acknowledge all those who agreed to participate in the current study.
Ethics Statement
The study was conducted in accordance with the declaration of Helsinki, and approved by the Institutional Review Board of the Centre Hospitalier Universitaire-Notre Dame des Secours (reference number 4/2019, on December 20, 2019).
Consent
Written informed consent was obtained from all study participants. Those gave their consent via the statement “I have read the foregoing information and I consent voluntarily to participate as a participant in this research” where an affirmative reply was required to enter the study. They were able to withdraw from the study at any time without giving a reason. The sweeteners under study were safe for consumption.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The original contributions presented in this study are included in the article and Supporting Information. Further inquiries can be directed to the corresponding author.




