Linking the relationship between dietary folic acid intake and risk of osteoporosis among middle-aged and older people: A nationwide population-based study
Yuan-Wei Zhang, Yan Hu and Si-Cheng Wang contributed equally to this work.
Abstract
Among middle-aged and older people, balanced and nutritious diets are the foundation for maintaining bone health and preventing osteoporosis. This study is aimed at investigating the link between dietary folic acid intake and the risk of osteoporosis among middle-aged and older people. A total of 20,686 people from the National Health and Nutritional Examination Survey (NHANES) 2007–2010 are screened and included, and 5312 people aged ≥45 years with integral data are ultimately enrolled in evaluation. Demographics and dietary intake-related data are gathered and analyzed, and the odds ratio (OR) and 95% confidence interval (CI) of each tertile category of dietary folic acid intake and each unit increase in folic acid are assessed via multivariate logistic regression models. On this basis, the receiver operating characteristic (ROC) curve is used to identify the optimal cutoff value of dietary folic acid intake for indicating the risk of osteoporosis. Of 5312 people with a mean age of 62.4 ± 11.0 years old, a total of 513 people with osteoporosis are screened, and the dietary folic acid intake amount of the osteoporosis group is significantly lower than that of the non-osteoporosis group (p < .001). The lowest tertile category is then used to act as a reference category, and a higher dietary folic acid intake amount is observed to be positively related to lower odds for risk of osteoporosis. This trend is also not changed in adjustments for combinations of different covariates (p all < .05). Based on this, a dietary folic acid intake of 475.5 μg/day is identified as an optimal cutoff value for revealing osteoporosis. Collectively, this nationwide population-based study reveals that a higher daily dietary folic acid intake has potential protective effects on osteoporosis in middle-aged and older people.
1 INTRODUCTION
Osteoporosis is the most universal systemic metabolic bone disease among middle-aged and older people, which is characterized by persistent reduction of bone mass and degradation of bone microstructure, leading to enhanced bone fragility and the occurrence of fractures (Miller et al., 2022; Zhang, Cao, Li, Dai, Lu, Zhang, Bai, Chen, Zhang, et al., 2022; Zhou et al., 2023). Of note, osteoporotic fracture means a low-energy or non-violent fracture that occurs without apparent external force, which is also a major and non-negligible complication of osteoporosis (Pinto et al., 2022; Wang et al., 2021). Currently, with the ceaseless progression of the global population aging, the number of patients with fractures caused by osteoporosis is as high as 8.9 million annually (Zhang, Song, et al., 2024), which means that there is one osteoporotic patient complicated with fractures every 3 s (Mills et al., 2022). Besides, it is expected that by 2050, China will also become the country with the highest incidence of osteoporotic fractures in Asia (Wang et al., 2021), which might not only affect the quality of life of middle-aged and older people but also enhance the burden of the social medical service system to certain extents (Chen et al., 2021; Guo et al., 2023; Hu, Li, Zhang, et al., 2021; Hu, Li, Zhi, et al., 2021; Liu et al., 2022, 2023).
Additionally, a healthy, reasonable, balanced, and nutritious diet is the foundation for maintaining the bone and whole health of middle-aged and older people (Farshbaf-Khalili et al., 2023; Kheiridoost et al., 2022; Li et al., 2024), and the prevention and treatment of osteoporosis also requires cooperative management from multiple perspectives (Warensjö et al., 2011; Zhang, Cao, Li, Dai, Lu, Zhang, Bai, Chen, Shi, et al., 2022). In addition to actively seeking medical treatment, it is necessary to pay attention to rational diets in daily life to supplement the necessary nutrients for the bones (Zhang, Lu, et al., 2021). Regarding this, in addition to supplementing common nutrients such as calcium (Ca), vitamin D (Vit D), phosphorus (P), protein, and other nutrients that are beneficial to bone, the regular and quantitative supplementation of folic acid for middle-aged and older individuals is also of vital significance (Schisterman et al., 2020; Zhang, Li, et al., 2021). Folic acid, as a member of the B vitamin family, participates in the synthesis and metabolism of purines, thymine, amino acids, hemoglobin, and methyl compounds in the host (Rydlewicz et al., 2002), while the deficiency of folic acid may result in diseases such as megaloblastic anemia, leukopenia, and hyperhomocysteinemia, among which hyperhomocysteinemia has also been verified to be a crucial factor contributing to the development of atherosclerosis and osteoporosis (Stone et al., 2017). Of note, several studies have reported a negative link between homocysteine level and vitamin B12 (Vit B12) and folic acid levels in the host (Mehrpour et al., 2021; Wu et al., 2022), and the enhancement of homocysteine level in middle-aged and older people has also been widely recognized as one of the risk factors for osteoporosis (Clements et al., 2022; Enneman et al., 2015). Taken together with the above findings, supplementing folic acid in daily diets is expected to prevent osteoporosis and subsequent osteoporotic fractures in middle-aged and older people by improving the homocysteine level in the host.
Dietary folic acid supplementation might contribute to maintaining bone mass in the middle-aged and elderly populations and prevent the occurrence and progression of osteoporosis (Stone et al., 2017). However, there are still few studies focusing on the significance of dietary intake and osteoporosis at the current stage, and there is also a lack of large-scale population-based studies to investigate the link between dietary folic acid intake and the risk of osteoporosis in middle-aged and older people. Moreover, the dietary intake of folic acid is still relatively scarce in some countries and regions where diets are mainly characterized by cereals, and the digestive and absorption functions of middle-aged and older people gradually deteriorate with the growth of age, which poses certain obstacles to the intake of dietary nutrients (Monteagudo et al., 2013; Steluti et al., 2019). Thus, it is crucial to analyze and recognize the link between dietary folic acid intake and the risk of osteoporosis among middle-aged and older people. Herein, based on NHANES 2007–2010, this study aims to investigate the above correlation and further determine the cutoff value of daily dietary folic acid intake, thereby providing certain references for the prevention and treatment of osteoporosis in middle-aged and older people from the perspective of dietary intake.
2 METHODS
2.1 Data provenance and research population
As a nationwide population-based survey research program established by Centers for Disease Control and Prevention (CDC), NHANES is committed to noticing the health and nutritional status of non-institutionalized adults and children in the US (Zhang et al., 2023). This research program has surveyed a nationally representative sample of approximately 5000 people annually, with the interviews covering demographic, socio-economic, dietary, laboratory, and other health-related matters. The examination section includes medical, dental, and physiological measurements, as well as laboratory tests managed by trained medical personnel (Hong et al., 2022). The survey findings might be applied to ascertain the prevalence and risk factors of the main diseases and then provide extensive information support for the formulation of nutrition and health policies (Nigra et al., 2017). NHANES conducts surveys every 2 years, and 2 years are defined as a study period, and the overall data are utterly de-identified and publicly obtainable to researchers on the page of www.cdc.gov/nchs/nhanes. More significantly, this current study is performed according to the principles of the Declaration of Helsinki and is also a simply exempt study involving secondary processing data, which is approved by the Research Ethics Review Board of the National Center for Health Statistics (NCHS). The written informed consent of participants included in the NHANES is also obtained (Chen et al., 2019).
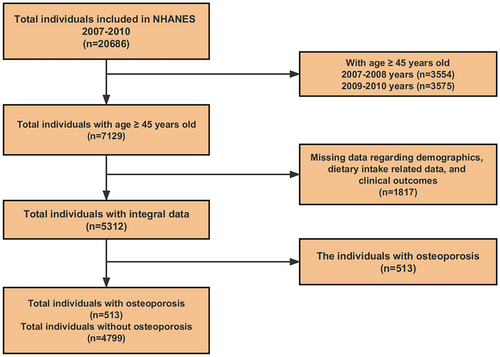
As exhibited in Figure 1, a total of 20,686 people from NHANES 2007–2010 (2007–2008 and 2009–2010 years) are screened and included in this current study, and the research object is defined as the individuals aged greater than or equal to 45 years old (n = 7129). Subsequently, a total of 1817 people with absent data regarding demographic characteristics, dietary intake related data, and clinical consequences are eliminated, and a total of 5312 people with integral data are ultimately enrolled in the next evaluation.
2.2 Demographics
As a continuous cross-sectional survey conducted over the years by the CDC, NHANES gathers data on the prevalence of chronic diseases and related indicators in the population. It is also able to understand the risk factors of various diseases, that is, the individual's lifestyle, constitution, genetics, and environmental factors that might enhance the risk of diseases, including smoking, alcohol consumption, sexual behavior, exercise, dietary intake, and other parameters (Jayanama et al., 2022). Moreover, the demographically relevant variables of NHANES 2007–2010, including age, race, gender, education level, body mass index (BMI), smoking, ratio of family income to poverty, drinking alcohol, and common comorbidities (such as hypertension and diabetes), are included in the subsequent analysis.
2.3 Identification of individuals with osteoporosis
In NHANES 2007–2010, bone mineral density (BMD) of the spine and femur among the included population was analyzed by means of dual-energy X-ray absorptiometry (DXA), with a diagnostic standard of osteoporosis as a T-score lower than −2.5 standard deviation (SD) (Narayanasamy et al., 2022). Based on this diagnostic standard, the above enrolled individuals (n = 5312) are then separated into the osteoporosis group (n = 513) and the non-osteoporosis group (n = 4799).
2.4 Evaluation of dietary intake-related data
As a branch of data collection in NHANES 2007–2010, a research survey of “What We Eat in America” (WWEIA) is promoted by collaboration between two departments, including the US Department of Agriculture (USDA) and the US Department of Health and Human Services (USDHHS). Specifically, USDA is mainly committed to screening, collecting, and identifying dietary intake-related data, and USDHHS is mainly committed to performing survey overviews and gathering baseline information (Archer et al., 2015). Therein, during a whole 24-h period, the dietary intake-related data of enrolled individuals is recorded in two recall interviews by the trained dietitians in the mobile examination centers (MECs) (Kirkpatrick et al., 2022), and the specific gathered dietary intake-related indicators include total energy, carbohydrates, folic acid, total fat, P, zinc, choline, protein, Ca, iron, Vit B6, fiber, Vit B12, cholesterol, and so on. The Food and Nutrient Database for Dietary Studies 2.0 (FNDD 2.0) of the USDA is then applied to analyze the calculations of dietary intake-related data (Shelton et al., 2021). Moreover, considering the potential impact of total energy intake on various kinds of dietary nutrients, we have also adjusted for the total energy intake by means of the residual method and further standardized it to 2000 kcal (El Kinany et al., 2018).
2.5 Statistical data analysis
Demographics, dietary intake-related data, and clinical outcomes information in the NHANES 2007–2010 are extracted by R 4.0.3 software (R Foundation, Vienna, Austria) and statistically analyzed using SPSS 23.0 software (SPSS Inc., Chicago, IL, USA). The data extraction and analysis processes follow the definition in the NHANES. Then, in accordance with the NHANES analysis guide, we consider NHANES-related sample weights, and a p value less than .05 is regarded as statistically significant.
Next, the continuous variables are presented in the form of mean ± SD, median (interquartile range), or count (percentage), and categorical variables are presented in the form of percentage or frequency. Continuous variables are compared based on the Mann–Whitney U test for non-normally distributed variables and Student's t-test for normally distributed variables. Categorical variables are analyzed and compared by means of Chi-square test.
Subsequently, multivariate logistic regression models are constructed to investigate the link between the risk of osteoporosis and dietary folic acid intake among middle-aged and older people, which is expressed in the form of odds ratio (OR) and 95% confidence interval (CI). Based on this, we further apply the receiver operating characteristic (ROC) curve to identify the optimal cutoff value of daily dietary folic acid intake for indicating the risk of osteoporosis among middle-aged and older people. In accordance with the daily dietary folic acid intake amount below or equal to and above optimal cutoff value, the research object is then divided into two independent groups, and the OR and 95% CI of the higher dietary folic acid intake group in contrast with the lower group are further analyzed. The criteria for selection of potential confounding variables mainly refers to risk factors related to osteoporosis in middle-aged and elderly people, including age, gender, BMI, smoking, drinking alcohol, as well as dietary nutrients related to bone health, such as calcium, vitamin D, phosphorus, protein, and so on (Levis & Lagari, 2012; Zhang, Song, et al., 2024).
3 RESULTS
3.1 Demographics
The demographics of individuals with or without osteoporosis based on NHANES 2007–2010 are displayed in Table 1. Specifically, a total of 5312 people with a mean age of 62.4 ± 11.0 years old are involved in the ultimate assessment, and it is also observed that the individuals in the osteoporosis group (68.2 ± 9.9 years old) are older than those in the non-osteoporosis group (61.8 ± 10.9 years old, p < .001). Besides, significant differences are identified in terms of gender (p < .001), race (p < .001), smoking (p = .029), drinking alcohol (p < .001), combined with hypertension (p = .001), and ratio of family income to poverty (p = .002), except for the terms of education level, combined with diabetes, and BMI (p all > .05).
| Total (n = 5312) | Osteoporosis group (n = 513) | Non-osteoporosis group (n = 4799) | p value | |
|---|---|---|---|---|
| Age, years | 62.4 ± 11.0 | 68.2 ± 9.9 | 61.8 ± 10.9 | <.001 |
| Gender (n, %) | <.001 | |||
| Male | 2657 (50.0) | 78 (15.2) | 2579 (53.7) | |
| Female | 2655 (50.0) | 435 (84.8) | 2220 (46.3) | |
| Race, n (%) | <.001 | |||
| Mexican American | 793 (14.9) | 73 (14.2) | 720 (15.0) | |
| Other Hispanic | 488 (9.2) | 46 (9.0) | 442 (9.2) | |
| Non-Hispanic White | 2836 (53.4) | 325 (63.4) | 2511 (52.3) | |
| Non-Hispanic Black | 1013 (19.1) | 52 (10.1) | 961 (20.0) | |
| Other race | 182 (3.4) | 17 (3.3) | 165 (3.5) | |
| Education level, n (%) | .167 | |||
| Less than 9th grade | 783 (14.7) | 81 (15.8) | 702 (14.6) | |
| 9–11th grade | 854 (16.1) | 83 (16.2) | 771 (16.1) | |
| High school grad or equivalent | 1255 (23.6) | 137 (26.7) | 1118 (23.3) | |
| Some college | 1348 (25.4) | 127 (24.8) | 1221 (25.4) | |
| College graduate or above | 1072 (20.2) | 85 (16.5) | 987 (20.6) | |
| Smoking (n, %) | .029 | |||
| Yes | 2729 (51.4) | 240 (46.8) | 2489 (51.9) | |
| No | 2583 (48.6) | 273 (53.2) | 2310 (48.1) | |
| Drinking alcohol (n, %) | <.001 | |||
| Yes | 3651 (68.7) | 283 (55.2) | 3368 (70.2) | |
| No | 1661 (31.3) | 230 (44.8) | 1431 (29.8) | |
| Combined with hypertension (n, %) | .001 | |||
| Yes | 2716 (51.1) | 297 (57.9) | 2419 (50.4) | |
| No | 2596 (48.9) | 216 (42.1) | 2380 (49.6) | |
| Combined with diabetes (n, %) | .527 | |||
| Yes | 1001 (18.8) | 102 (19.9) | 899 (18.7) | |
| No | 4311 (81.2) | 411 (80.1) | 3900 (81.3) | |
| Ratio of family income to poverty | 2.7 ± 1.6 | 2.5 ± 1.6 | 2.7 ± 1.6 | .002 |
| BMI, kg/m2 | 29.4 ± 6.5 | 28.3 ± 6.4 | 29.5 ± 6.4 | .525 |
- Abbreviation: BMI, body mass index.
3.2 Dietary intake-related data
The dietary intake-related data of individuals with or without osteoporosis based on NHANES 2007–2010 is exhibited in Table 2. Specifically, in order to minimize the potential influences of total energy intake on various kinds of dietary nutrients, we have adjusted for the total energy intake by means of the residual method. It is observed that the energy-adjusted dietary nutrients (such as protein, carbohydrates, total fat, fiber, Ca, P, zinc, Vit B6, iron, Vit B12, and cholesterol) of the individuals in the osteoporosis group are generally lower than those in the non-osteoporosis group (p all < .05). It is also noticed that the daily dietary folic acid intake of the individuals in the osteoporosis group (294.1 ± 247.4 μg/day) is significantly less than that of the non-osteoporosis group (341.6 ± 313.1 μg/day, p < .001), which is worthy of further attention and analysis.
| Total (n = 5312) | Osteoporosis group (n = 513) | Non-osteoporosis group (n = 4799) | p value | |
|---|---|---|---|---|
| Total energy, kcal | 1938.2 ± 910.0 | 1666.5 ± 698.7 | 1967.2 ± 925.1 | <.001 |
| Energy-adjusted protein, g/day | 75.8 ± 39.0 | 64.6 ± 29.0 | 77.0 ± 39.8 | <.001 |
| Energy-adjusted carbohydrates, g/day | 234.0 ± 111.8 | 210.1 ± 92.4 | 236.5 ± 113.3 | <.001 |
| Energy-adjusted total fat, g/day | 73.7 ± 43.7 | 62.6 ± 33.7 | 74.9 ± 44.5 | <.001 |
| Energy-adjusted fiber, g/day | 16.1 ± 9.9 | 14.8 ± 8.3 | 16.3 ± 10.0 | <.001 |
| Energy-adjusted choline, mg/day | 322.2 ± 195.1 | 273.1 ± 146.2 | 327.4 ± 198.9 | <.001 |
| Energy-adjusted Ca, mg/day | 867.9 ± 542.7 | 813.0 ± 499.6 | 873.7 ± 546.8 | .008 |
| Energy-adjusted P, mg/day | 1255.4 ± 618.0 | 1106.4 ± 497.0 | 1271.3 ± 627.5 | <.001 |
| Energy-adjusted iron, mg/day | 14.3 ± 8.2 | 13.0 ± 6.6 | 14.5 ± 8.3 | <.001 |
| Energy-adjusted zinc, mg/day | 11.1 ± 9.2 | 9.6 ± 5.3 | 11.3 ± 9.6 | <.001 |
| Energy-adjusted Vit B6, mg/day | 1.9 ± 1.2 | 1.6 ± 1.0 | 1.9 ± 1.3 | .001 |
| Energy-adjusted Vit B12, μg/day | 5.1 ± 3.1 | 4.4 ± 2.8 | 5.2 ± 3.3 | .027 |
| Energy-adjusted cholesterol, mg/day | 280.1 ± 237.5 | 231.2 ± 180.3 | 285.3 ± 242.3 | <.001 |
| Energy-adjusted folic acid, μg/day | 337.0 ± 307.7 | 294.1 ± 247.4 | 341.6 ± 313.1 | <.001 |
- Abbreviations: Ca, calcium; P, phosphorus; Vit B6, Vitamin B6; Vit B12, Vitamin B12.
3.3 Link between the risk of osteoporosis and dietary folic acid intake
By means of constructing the multivariate logistic regression models, we then assess the link between the risk of osteoporosis and dietary folic acid intake. In this regard, Table 3 exhibits the prevalence and exact sample of osteoporosis in each tertile category of dietary folic acid intake amount, as well as the OR and 95% CI for osteoporosis according to different dietary folic acid intake levels. The prevalence of osteoporosis in each tertile category of dietary folic acid intake amount exhibits a gradual descending tendency (10.3%, 8.7%, and 6.5%, respectively, p for trend < .001). Based on this, the lowest tertile category is used to act as a reference category, and a higher dietary folic acid intake amount is observed to be positively correlated with lower odds for the risk of osteoporosis in middle-aged and older people. More significantly, it is also noticed that the above-mentioned trend is still not changed in the detached univariate model (p = .007), as well as adjustments for parameters of BMI, education level, ratio of family income to poverty, and combined chronic diseases (Model 1, p = .018), covariates of age and gender based on Model 1 (Model 2, p = .020), covariates of drinking alcohol and smoking based on Model 2 (Model 3, p = .027), and covariates of daily dietary intake data based on Model 3 (Model 4, p = .033).
| Dietary folic acid intake levels | Osteoporosis/n | Prevalence/% | Univariate model | Model 1a | Model 2b | Model 3c | Model 4d |
|---|---|---|---|---|---|---|---|
| Total (n = 5312) | |||||||
| Tertile 1 (≤400 μg/day) | 375/3627 | 10.3 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Tertile 2 (>400, ≤800 μg/day) | 112/1285 | 8.7 | 0.715 (0.459–1.113) | 0.714 (0.459–1.112) | 0.731 (0.470–1.138) | 0.730 (0.469–1.137) | 0.733 (0.471–1.141) |
| Tertile 3 (>800 μg/day) | 26/400 | 6.5 | 0.570 (0.377–0.862) | 0.592 (0.392–0.894) | 0.603 (0.399–0.910) | 0.610 (0.404–0.921) | 0.616 (0.408–0.931) |
| p for trend | <.001 | .007 | .018 | .020 | .027 | .033 | |
- a Model 1 adjusted for the covariates of education level, BMI, ratio of family income to poverty, and combined chronic diseases.
- b Model 2 adjusted for the covariates of age and gender based on Model 1.
- c Model 3 adjusted for the covariates of smoking and drinking alcohol based on Model 2.
- d Model 4 adjusted for the covariates of daily dietary intake data (including nutrients related to bone health such as calcium, vitamin D, phosphorus, and protein) based on Model 3.
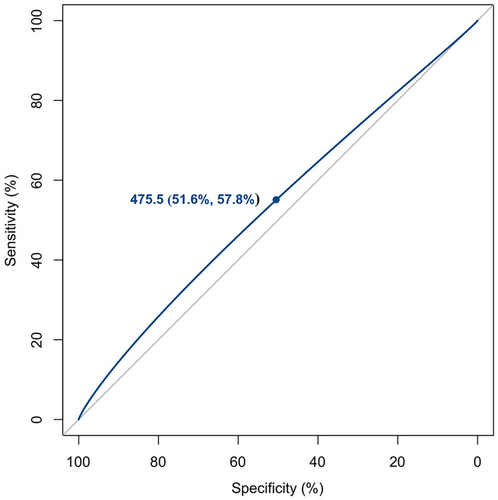
Based on the above-mentioned results, Figure 2 reveals that the ROC curve is further used to investigate the dietary folic acid intake (475.5 μg/day) as an optimal cutoff value for revealing the risk of osteoporosis in middle-aged and older individuals, with 51.6% specificity and 57.8% sensitivity. Furthermore, according to the dietary folic acid intake amount below or equal to and above optimal cutoff value (475.5 μg/day), research object is then divided into two independent groups. Next, the results of multivariate logistic regression models in Table 4 also indicate that individuals with dietary folic acid intake amount ≥475.5 μg/day are equipped with 0.636-fold enhanced odds of osteoporosis in the detached univariate model, 0.645-fold increased odds in Model 1, 0.774-fold enhanced odds in Model 2, 0.663-fold enhanced odds in Model 3, and 0.776-fold enhanced odds in Model 4.
| Daily dietary folic acid intake | Osteoporosis/n | Prevalence/% | Univariate model | Model 1a | Model 2b | Model 3c | Model 4d |
|---|---|---|---|---|---|---|---|
| Optimal cutoff valuee | |||||||
| Below | 423/4019 | 10.5 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Equal and above | 90/1293 | 7.0 | 0.636 (0.502–0.806) | 0.645 (0.509–0.818) | 0.774 (0.607–0.988) | 0.663 (0.523–0.841) | 0.776 (0.603–0.999) |
- a Model 1 adjusted for the covariates of education level, BMI, ratio of family income to poverty, and combined chronic diseases.
- b Model 2 adjusted for the covariates of age and gender based on Model 1.
- c Model 3 adjusted for the covariates of smoking and drinking alcohol based on Model 2.
- d Model 4 adjusted for the covariates of daily dietary intake data (including nutrients related to bone health such as calcium, vitamin D, phosphorus, and protein) based on Model 3.
- e Optimal cutoff value of daily dietary folic acid intake is 475.5 μg.
4 DISCUSSION
With the enhancing aging of the global population, the prevalence of osteoporosis among middle-aged and older people, as well as the combined disability and mortality rates, are also rapidly ascending (Zhang, Cao, Li, Chen, et al., 2022; Zhang, Cao, Li, Zhang, et al., 2022). In this context, healthy and balanced dietary intake is a pivotal cornerstone for maintaining bone and whole health among middle-aged and older people (Fardellone et al., 2010), and it is also necessary to timely screen, identify, and discover the misconceptions and deficiencies in terms of the dietary intake among middle-aged and older people. Herein, based on NHANES 2007–2010, this nationwide population-based study reveals that a higher daily dietary folic acid intake has potential protective effects on osteoporosis among middle-aged and elderly population, and it is also recommended to ensure that the daily dietary folic acid intake amount is greater than or equal to 475.5 μg to prevent the occurrence and development of osteoporosis.
This nationwide population-based study mainly indicates that the individuals in the osteoporosis group are older than those in the non-osteoporosis group (p < .001), and the daily dietary folic acid intake of individuals in the osteoporosis group is lower than that of the non-osteoporosis group (p < .001), which suggests an underlying correlation between high daily dietary folic acid intake and low risk of osteoporosis among middle-aged and older people. Regarding this, the above-mentioned results are also consistent with several previous studies. A former study conducted by Keser et al. (2013) revealed that the application of folic acid and Vit B12 as dietary supplements to enhance the level of homocysteine could be conductive to middle-aged and older women and positively modify the bone turnover markers among the above population. Rejnmark et al. (2008) showed in a population-based cohort (including 1869 perimenopausal women) that a higher dietary folic acid intake, but not Vit B12 or B2, may exert the positive effects on BMD of perimenopausal women. Li et al. (2017) also indicated in a community-based study that the BMD of the hip in middle-aged and older patients with chronic stroke has decreased, and their dietary folic acid intake amount is also significantly lower than the recommended daily intake standard. Hence, this series of results emphasizes the potential advantages and necessity of dietary folic acid supplementation in maintaining bone health among middle-aged and older populations, and deserves further attention and research.
Folic acid, as a vital nutrient for the human body, plays a pivotal role in bone growth and preventing bone loss. For one thing, oxidative stress, as an important risk factor for osteoporosis, has raised increasing attention from the research community, and folic acid, as a novel type of antioxidant, has also gradually become a research focus (Zhang et al., 2020). In this regard, several previous studies have suggested that folic acid may have direct impacts on osteoporosis, as it is required to participate in reducing oxidative stress, avoiding intracellular DNA damage, and preventing cell apoptosis (Mohammadi et al., 2012; Saito & Marumo, 2018; Tyagi et al., 2011). Meanwhile, low folic acid levels have also been reported to significantly decrease the thickness and number of trabeculae, and the supplementation of folic acid could rectify the coupling imbalance between the osteoblasts and osteoclasts in the host (Holstein et al., 2009; Kalimeri et al., 2020). Fan et al. (2012) observed through a study that the long-term use of methotrexate (MTX) can damage the growth plate and metaphyseal bone cells of the body, while subsequent supplementation with folic acid might have protective effects. Specifically, the intake of chronic folic acid supplements can prevent the MTX-induced apoptosis of chondrocytes and preserve the columnar arrangement and quantity of chondrocytes. Li et al. (Li et al., 2021) also noticed in a study that supplementation of dietary folic acid might improve the lipid metabolism, regulate the oxidative stress response, and activate the AMPK signaling pathway, thereby mitigating the high-fat diet (HFD)-induced osteoporosis. Thus, it can be recognized that folic acid plays a dominant role in the growth, progression, and homeostasis of bones, and a deficiency of folic acid might result in the descending bone formation and bone loss. Another potentially necessary factor for the occurrence of osteoporosis among middle-aged and elderly populations is the subclinical deficiency of folic acid (Ratajczak et al., 2021). It is universally recognized that with the growth of age, middle-aged and older people might be accompanied by tooth loss, decreased appetite, descending gastrointestinal absorption, and other weakening of body functions to a certain extent, thus resulting in inadequate intake of several daily dietary nutrients (Brazaitis et al., 2017; Sinikumpu et al., 2021). Hence, it is of great clinical significance to investigate the link between the risk of osteoporosis and dietary folic acid intake in the middle-aged and elderly population and further explore relevant intervention measures to promote dietary folic acid intake based on this. Specifically, the following measures could more intuitively integrate dietary recommendations into current medical practices, including (1) Integrating nutritional interventions for dietary folic acid intake with healthcare systems to provide more sources of nutrient intake; (2) enhancing the investment and construction of nutritional departments in hospitals or health care units and further emphasizing the significance of nutritional support for maintaining resident health; and (3) strengthening the formulation and updating of clinical guidelines related to dietary nutrients, thereby guiding clinical practice more effectively (Saunders et al., 2023; Zhang, Wu, et al., 2024).
After identifying the correlation and significance between the risk of osteoporosis and dietary folic acid intake in the middle-aged and elderly populations, we further verified the dietary folic acid intake amount (475.5 μg/day) as an optimal cutoff value to reveal the risk of osteoporosis in the middle-aged and elderly populations. Regarding this, several studies have suggested that the daily dietary folic acid intake for most middle-aged and older people is far below the recommended standards in the dietary nutrition guidelines for residents in multiple countries and regions around the world (Boilson et al., 2012; Olayiwola et al., 2014). Specifically, US dietary and nutritional guidelines have broadly recommended that middle-aged and elderly people over 50 years old need to supplement approximately 400 μg of folic acid per day to maintain physical functions (Bailey et al., 2012). Although this has certain nutritional guidance significance, there is still a lack of specific evidence to indicate the dietary folic acid intake value required to maintain bone mass in middle-aged and elderly people. From this, the value of this study lies in revealing that dietary folic acid intake should not be lower than 475.5 μg/day in middle-aged and elderly people to avoid the occurrence of osteoporosis as much as possible. Meanwhile, the impacts of other potential factors (such as body size, dietary nutrient combinations, family dietary habits, cooking methods, and so on) on dietary folic acid intake in middle-aged and elderly populations should also be closely monitored and focused on (Bailey & van Wijngaarden, 2015).
This nationwide population-based study also has several shortcomings that need to be pointed out. First, different countries and regions around the world are equipped with different dietary characteristics, nutritional structures, lifestyles, and living habits (Fabiani et al., 2019). Based on the NHANES 2007–2010, this study mainly focuses on the middle-aged and elderly population in the US, and the findings may not necessarily be generalizable to other ethnic or racial groups. In subsequent studies, it is still necessary to conduct large-scale surveys of other ethnic groups and other age groups. Second, during the process of conducting studies on the correlation between chronic diseases and dietary nutrients, although we have considered and adjusted for partial potential confounding factors as far as possible, given the relatively complicated relationship between chronic diseases and dietary nutrients, some of the confounding factors are inevitably overlooked and cannot be ruled out. Third, it should be closely noted that the research based on NHANES is constrained by its self-reporting nature. During the process of gathering baseline data and quantifying dietary intake-related data from the individuals, there may inevitably be biases in the individuals' recall and self-report. Fourth, as a cross-sectional design, this current study focuses on a single point in time and cannot be used to analyze relationships over time or establish long-term trends (Timpka et al., 2015). Fifth, precise control of dietary folic acid intake is particularly pivotal; excessive intake of folic acid can mask the symptoms of vitamin B12 deficiency (including anemia, fatigue, pigmentation, diarrhea, and so on), while long-term vitamin B12 deficiency might cause irreversible neurological damage (Derin et al., 2016; Yang et al., 2020). Ultimately, the specificity and sensitivity percentages obtained in this study are not ideally conclusive, which is another major drawback that still needs to be pointed out. Despite this, NHANES contains a robust and nationwide dataset and has a stable and reliable data collection and analysis process. The secondary analysis of available progression data is also worthy of recognition, and the results of the analysis are worthy of confidence (Chen & Han, 2023; Shen et al., 2023).
5 CONCLUSION
Taken together, this nationwide population-based study suggests that a higher daily dietary folic acid intake has potential protective effects on osteoporosis in middle-aged and older people. Based on this, it is also recommended to ensure that their daily dietary folic acid intake amount is greater than or equal to 475.5 μg to avoid the occurrence and development of osteoporosis, although subsequent investigations in other countries and races around the world still need to be performed. Additionally, in response to the existing limitations, further research covering more countries, regions, and races is needed in the future to verify the relevance explored in this study and enhance its credibility.
AUTHOR CONTRIBUTIONS
Yuan-Wei Zhang: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal). Yan Hu: Conceptualization (equal); data curation (equal); formal analysis (equal). Si-Cheng Wang: Conceptualization (equal); data curation (equal); methodology (equal). Zu-Hao Li: Data curation (equal); formal analysis (equal). Gui-Quan Cai: Investigation (equal); methodology (equal). Hao Shen: Data curation (equal); formal analysis (equal). Shi-Hao Sheng: Data curation (equal); investigation (equal). Xiao Chen: Conceptualization (equal); data curation (equal). Wei-Zong Weng: Formal analysis (equal); methodology (equal). Wen-Cai Zhang: Conceptualization (equal); methodology (equal). Yuan Chen: Conceptualization (equal); investigation (equal); supervision (equal); visualization (equal). Jia-Can Su: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal).
ACKNOWLEDGMENTS
The authors thank those who took part in the NHANES survey and the Centers for Disease Control and Prevention for their data sharing.
FUNDING INFORMATION
The work was supported by grants from the General Program of China Postdoctoral Science Foundation (2023M742203), Integrated Project of Major Research Plan of National Natural Science Foundation of China (92249303), National Natural Science Foundation of China (82371603, 82230071), Shanghai Committee of Science and Technology Laboratory Animal Research Project (23141900600), Shanghai Hospital Development Center (SHDC2023CRT013), and Interdisciplinary of Medicine and Engineering Foundation of Shanghai JiaoTong University (YG2024QNA20, YG2024QNA21).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
The data from NHANES did not contain any identifiable or protected health information and was publicly available for public download by researchers. This current study was performed according to the principles of the Declaration of Helsinki and was also a simply exempt study involving secondary processing data, which was approved by the Research Ethics Review Board of NCHS.
INFORMED CONSENT
Written informed consent was provided by the included participants in NHANES.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this current study are available in NHANES at (https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/).




