Antioxidant and antimicrobial activity of two Costa Rican cultivars of ber (Ziziphus mauritiana): An underexploited crop in the American tropic
Abstract
Ber is an antioxidant-rich fruit from Asia and has recently been cultured in Central America. The antioxidant capacity and antimicrobial activity of Z. mauritiana cultured of bers from Guanacaste, Costa Rica, were evaluated. Two farm locations and two cultivars were evaluated. Total polyphenolic compounds (TPC), proanthocyanidin compounds (PAC), and ascorbic acid were spectrophotometrically quantified. Antioxidant activity has been analyzed using the DPPH method. Antimicrobial susceptibility was determined using the Kirby-Bauer disk diffusion method. Ber samples contained 11–44 mg GAE/g TPC. Green fruits and leaves had the highest concentrations. The ascorbic acid concentration in ber fruits was determined between 251 and 466 mg/100 g. Ber vitamin C content is higher than most common fruits. Proanthocyanidin compounds were determined between 1.8 and 9.9 mg 4-MCG/g, and the highest concentration was observed in the leaves. Our samples showed the antioxidant activity of 90–387 μmol TE/g, which was moderate activity. The nutritional quality of ber fruits was related to maturity conditions. The ber fruits, a crop from Asia previously adapted to live in Costa Rica, are rich in vitamin C and TPC, and the concentration of those metabolites was even higher than the concentration reported in bers grown in other countries. The TPC and PACs had an interestingly wide antimicrobial spectrum. Cultivars and farm locations have a significant effect on metabolite production.
1 INTRODUCTION
Ziziphus mauritiana is a fruit native to the subcontinent of India and distributed in some regions of Africa, Oceania, and Asia, such as India, Afghanistan, Algeria, Egypt, Kenya, Pakistan, Malaysia, southern Africa, Japan, Nepal, Australia, Philippines, and Pacific Islands (Prakash et al., 2021). It is called Ber, Indian jujube, Desert apple, Indian plum, Malay apple, Chinese apple, dunks, and Chinese date. Ber is a bush or small tree with round or elliptical sheet-shaped leaves, green-yellow-red flowers, and armed branches with two thorns per node (Zamora et al., 2010).
Several bioactive compounds have been found in Z. mauritiana, such as the alkaloids protopine and berberine; flavonoids such as quercetin and kaempferol; sterols that are sitosterol, stigmasterol, lanosterol, and diosgenin; a complex mixture of flavonoids, polyphenolic hydrolyzable tannins; oses and holosides; mucilage, sterol, triterpenoids, cardiac glucosides, and leucoanthocyanidins; terpenes such as some saponins; and others (Prakash et al., 2021). Secondary metabolites from Z. mauritiana have been found active as antidiarrheal (Dahiru et al., 2006), antibacterial (Abalaka et al., 2010), poison antidote for carbon tetrachloride damage in the liver (Dahiru et al., 2005), antioxidant (Zozio, Servent, Cazal, et al., 2014), antihemorrhagic, anticancer, antidiabetic, and sleep booster (Zhang et al., 2020).
China holds 90% of the world's production, mostly for domestic consumption, and its market represented 16.5 million tons in 2019 (Zhang et al., 2020). Besides its popularity in Asia and Africa, this crop is not very well known in Central America and the American tropics. Although it is considered a crop from temperate regions, Z. mauritana has been introduced in Costa Rica during the late stage of the twentieth century. In 1993, around 20 ha were cultivated (MAG-INTA, 2022); most of them were in Nandayure, Guanacaste, a place located in the dry corridor of Central America. The National Institute for Rural Development (INDER, by its Spanish acronym, formerly IDA) promoted the farming of Z. mauritiana and Psidium guajava in Paquera, Nicoya Peninsula during the early ‘00 s (INDER, 2014). Graft plants from Costa Rica and Taiwan were used to establish the cultures. Currently, there are an estimated 400 trees producing 50 kg/year each, approximately, according to local producers. Z. mauritiana can grow in tropical dry climate conditions, growing well adapted in the tropics, and it is an excellent alternative for agricultural production.
This study is the first phytochemical evaluation of Z. mauritiana in Central America, and it provides valuable insights into the potential of this fruit in terms of antioxidant and antibiotic activity, as well as the content of polyphenolic metabolites and vitamin C, when grows in the American tropic. In this work, the objective was to evaluate the antioxidant and antimicrobial activity of Z. mauritiana cultured in Guanacaste, Costa Rica.
2 METHODOLOGY
2.1 Plant tissues
Stems, leaves, and fruits, at two maturity conditions of Ziziphus mauritiana, were collected from two local farms (referred to as F1, and F2) at Cangelito, Nandayure (10°0′8′′, 85°10′48′′), Nicoya Peninsula, Costa Rica. The farms are located 2 km apart. The samples were freeze-dried and ground to 0.5 mm. The maturity stages are defined as follows: the green fruits are full size strong green color, and ripe fruits are “bleached” green turning yellow. According to Zozio's scale, green fruit is stage 1, and ripe fruit stage 2 (Zozio, Servent, Hubert, et al., 2014). Additional information about the crops is in Figure S1. Cultivars are defined according to the fruit shape: ovoid or round shape. Composed samples were taken from random places on each farm during the dry season (December–March), containing around 30 fruits per sample. Three repetitions of each sample were used for the following analysis.
2.2 Polyphenol extraction
We preliminary tested four solvent mixtures: acetone:methanol:water (4:4:2), acetone:ethanol:water (4:5:1), acetone: water (7:3), and ethanol 95%. Seventy-five milligrams of plant material was extracted three times with 3 mL of each solvent for 10 min in an ultrasonic bath. Then, the mixture was centrifuged at 1760 × g for 5 min. The three extraction supernatants were collected together and adjusted to 10 mL. Then, the samples were analyzed using the Folin-ciocalteau method, as described hereunder, in order to determine which solvent offers better extraction rate acetone: water (7:3) yields the highest polyphenol concentration (Results shown in Figures S2 and S3). Similarly, the number of extractions were evaluated using from 1 to 5 steps of 2 mL, instead of 3 mL, as the only change to the method described above. The maximum concentration was observed with three extractions. Samples extracted at optimum conditions were used for the next steps.
2.3 Preparation of Ziziphus mauritiana's phenolic standard (zm-PS)
We purified the phenolic fraction from the same fruit to obtain a standard representative of the veritable mass of the sample. This approach has been previously validated with other proanthocyanidin extracts (Feliciano et al., 2012). Ten grams from Z. mauritiana. previously freeze-dried and grounded, were extracted with 70 mL of acetone: water (7:3). Then, the extract was concentrated in a rotatory evaporator at 40°C and reduced pressure, and purified through column chromatography, utilizing C-18 and distilled water, as stationary phase and eluent, respectively. The fraction containing the highest number of polyphenols was identified using Folin-Ciocalteau's method. Then, it was freeze-dried and used to quantify total phenolics and proanthocyanidins.
2.4 Total polyphenol compounds (TPC) quantification
75 mg of plant tissue, extracted as mentioned hereabove, were analyzed utilizing the Folin-Ciocalteau method, according to our previously published protocol (Syedd-León et al., 2020). Two standard curves of 0–0.200 g/L zm-PS and 0–0.12 mg/L gallic acid were used.
2.5 Preparation of Ziziphus mauritiana's proanthocyanidin standard (zm-PAC)
We prepared zm-PAC using a modification of the previously published protocol (Birmingham et al., 2021). Ten grams of each Ziziphus mauritiana sample, previously freeze-dried and grounded, were individually extracted in acetone: water (7:3). The solid was dumped, and the extracts were concentrated by rotavaporation at 40°C and reduced pressure. Then, the concentrated extract was purified using a glass chromatographic column filled with Sephadex LH-20 as a stationary phase. First, the column was eluted with ethanol: methanol (1:1) to eliminate impurities. Then, the mobile phase was changed to acetone: water (7:3), and the proanthocyanidin fraction was collected. Finally, the proanthocyanidin solution was dried by rotavaporation followed by lyophilization and used as Ziziphus mauritiana's proanthocyanidin standard (zm-PA).
2.6 Total proanthocyanidins (PAC) determination
We followed our previously published protocol (Mesén-Mora et al., 2019) with minor modifications. Two microliters of a 0.1 mg/mL solution of 4-(Dimethylamino) cinnamaldehyde (DMAC, obtained from Merck KGaA, Darmstadt, Germany), and 70 μL of each plant extract (as described above) were mixed into a microplate well. Absorbance was recorded at 640 nm against a 0–0.03 mg/L 4'-O-methyl-gallocatechin standard curve. Additionally, a second standard curve of 0–0.16 g/L zm-PS was utilized.
2.7 Ascorbic acid determination
0.4 g of fruit was extracted three times with distilled water, filtrated, and adjusted to 50 mL. Then, ascorbic acid was determined by iodine redox titration, as reported before (Satpathy et al., 2021) with minor modifications. Five milliliters of the previously prepared solution, 1 mL of 15% HCl, and 1 mL of 1% starch solution were titrated with 0.05 mol/L iodine solution, previously standardized, using sodium thiosulphate.
2.8 Antioxidant capacity determination
Antioxidant Capacity was determined by DPPH following our previously published protocol with some modifications (Araya et al., 2017). Thirty microliters of the previously described extracts were individually mixed in microplate wells with 270 μL of 0.042 mg/mL DPPH solution. Solutions were incubated for 20 min and Trolox equivalents (TE) were determined by recording the absorbance at 517 nm in a microplate reader BiotekSynergy HT.
2.9 Antibiotic activity
Antibacterial activity was tested against four bacteria: Staphylococcus aureus ATCC 25923, Bacillus subtilis ATCC 6633, Escherichia coli ATCC 25922, and Pseudomonas aeruginosa ATCC 9027. For this purpose, 250 μL of each bacterial suspension with 0.5 McFarlan turbidity (1.8 × 106 UFC/mL) were individually cultured into Muller Hinton agar plates. Then, three 6 mm glass fiber disks containing 50 μL of extract, 30 μg chloramphenicol (as positive control), and 50 μL acetone: water (7:3, as negative control), respectively, were placed in different positions of each inoculated plate and incubated at 35°C during 24 h. Finally, the inhibition ring was measured.
2.10 Statistical analysis
Results were calculated using mean values and standard deviations (M ± SD). Post hoc Tukey's tests were performed for the analysis of TPC, PAC, vitamin C, antioxidant activity, and antibiotic activity.
3 ANALYSIS AND RESULTS
3.1 Total phenolic compounds (TPC)
Figure 1 summarizes the results for TPC using both Gallic Acid and the Ziziphus mauritiana standard zm-PS. TPC concentration was between 57 and 64% higher for the green spheric cultivar, respecting the ovoid cultivar. The green ovoid cultivar has a TPC of 56%–71% higher than ripe ones. TPC concentration is higher for the spheric cultivar all over the plant. Such difference is bigger in the fruits than in other tissues. The TPC of the spheric cultivar's leaves is 26%–37% higher than the ovoid cultivar and TPC of stems is 16%–22% higher (spheric vs ovoid cultivar).
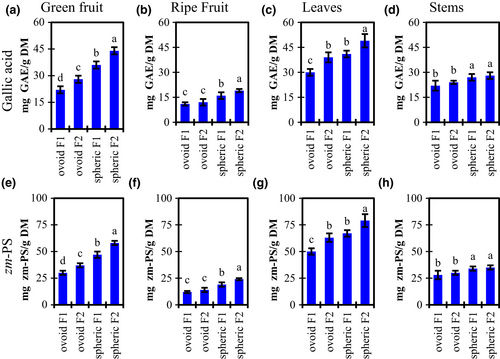
Table 1 shows the TPC reported on the literature for some ber fruits from different locations over the globe. Interestingly, the fruits included in this study had a TPC higher than most reports. For example, in Thailand and Pakistan, ber fruits had a polyphenol content of around 8.5 mg GAE/g (Ashraf et al., 2015; San et al., 2013), but we found samples reaching 44 mg GAE/g (more than 5-fold-up). The polyphenolic content was remarkably high in the fruits harvested in Costa Rica, respecting other latitudes.
| Fruit | Species | Country | TPC (mg GAE/g) | Reference |
|---|---|---|---|---|
| Ber seeds | Z. mauritiana | Thailand | 8.34 ± 0.43 | San et al. (2013) |
| Ber | Zizyphus sp. | India | 1.72–3.29 | (Koley et al. (2016) |
| Ber | Z. mauritiana | Pakistan | 8.469 ± 0.092 | Ashraf et al. (2015) |
| Ber | Z. mauritiana | India | 2.38–4.00 | Koley et al. (2011) |
| Ber | Z. mauritiana | Costa Rica | 11–44 | This work |
- Abbreviations: GAE, Gallic Acid Equivalents; TPC, Total Phenolic Content.
Also, we expressed the results of Figure 1 in terms of two kinds of standards: the gallic acid equivalents and the Ziziphus mauritiana polyphenol standard (zm-PS). Gallic acid equivalents are the most common units utilized to describe TPC. Although, the molecular weight distribution of polyphenolic compounds in the fruits is not often represented by gallic acid. Then, we used the purified polyphenolic standard from the same plant to represent a more realistic mass. This approach had been used by other authors (Feliciano et al., 2012). There was a similar zm-PS/GAE ratio for individual measurements within each part of the plant, but differences between groups. Therefore, zm-PS/GAE ratio is 1.18 ± 0.07 for ripe fruits, 1.33 ± 0.03 for green fruits, 1.63 ± 0.02 for leaves, and 1.26 ± 0.01 for stems. The lowest zm-PS/GAE ratio was found in ripe fruits. It means, on average, polyphenolic compounds from ripe fruits had a smaller molecular weight than those from the other parts of the plant. The zm-PS/GAE ratio difference between green and ripe fruits was explained by the oxidative processes taking place during ripening. Those processes reduce the molecular weight of phenolic compounds. Also, there was a significant reduction in the TPC. For example, the spheric cultivar from field F2 reduces its TPC from 44 to 19 mg GAE/g (58 to 24 mg zm-PS/g) (Figures 1a,e). The reduction of TPC during ber ripening had been reported before (Huang et al., 2017).
On the contrary, the leaves contained a higher ratio, which means, the compounds with higher molecular weight are present in the leaves. Harvest location affects TPC, being F2 values greater than F1 except for stems, whereas F1 and F2 were not significantly different.
3.2 Ascorbic acid content
The Vitamin C content of fruits is shown in Figure 2. The ascorbic acid accounted for 323–466 mg/100 g in the green fruit and dropped to 251–349 mg/100 g in the ripe fruit. It was consistent with previous reports from 46 cultivars of New Mexico and 121 cultivars from China, reporting an ascorbic acid content in the range of 225 to 820 mg/100 g (Huang et al., 2017).
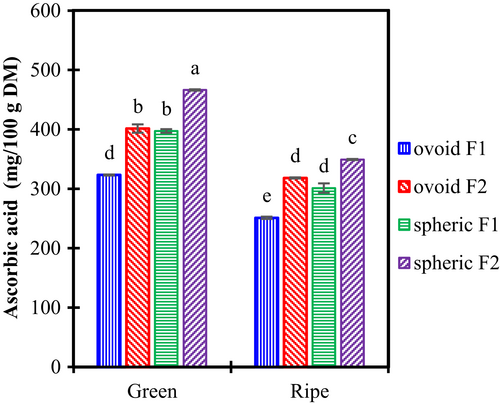
For each cultivar and harvest location, the ascorbic acid concentration decreased by 20%–25% during the ripening process. According to Zozio et al. Zozio, Servent, Cazal, et al. (2014) and Zozio, Servent, Hubert, et al. (2014), ascorbic acid concentration increased with sucrose concentration until a maximum value was reached. Then, both sucrose and ascorbic acid decreased. Hasan et al. (2022) reported similar behavior. It probably means that the fruits included in this study had been harvested at or after the maximum ascorbic acid concentration.
Table 2 describes several fruits considered rich in Vitamin C. Ber had an ascorbic acid content higher than many of those rich-vitamin C fruits, such as pineapple, kiwi, avocado, lemon, or guava. Ber concentration was approximately 1 to 7 fold-up the concentration of the fruits mentioned above.
| Fruit | Species | Ascorbic acid (mg/100 g) | Reference |
|---|---|---|---|
| Pineapple | Ananas comosus | 61.0 ± 0.1 | Valente et al. (2011) |
| Kiwi | Actinidia deliciosa | 91.0 ± 0.2 | Valente et al. (2011) |
| Avocato | Persea americana | 160 ± 1 | Shyla and Nagendrappa (2013) |
| Lemon | Citrus x limón | 177 ± 1 | Shyla and Nagendrappa (2013) |
| Guava | Psidium guajava | 198 ± 2 | Valente et al. (2011) |
| Amla | Emblica officinalis | 385 ± 1 | Valente et al. (2011) |
| Chinese jujube | Ziziphus jujuba | 387–555 ± 50 | Wojdyło et al. (2016) |
| Ber | Ziziphus mauritiana | 251–466 ± 8 | This work |
3.3 Total proanthocyanidin (PAC) concentration
Figure 3 shows the results for PAC concentration of Ziziphus mauritiana samples of green and ripe fruits, leaves, and stems from two harvest locations. PACs were expressed in terms of a reference compound (4'-O-methylgallocatechin, or 4-MGC) and using a standard prepared from the purified extract from the same fruit. The zm-PAC/4-MGC ratio for all samples was between 4–6 with no specific trend. The zm-PAC was used to represent a more realistic mass value because it contains a similar molecular weight distribution respecting the fruit.
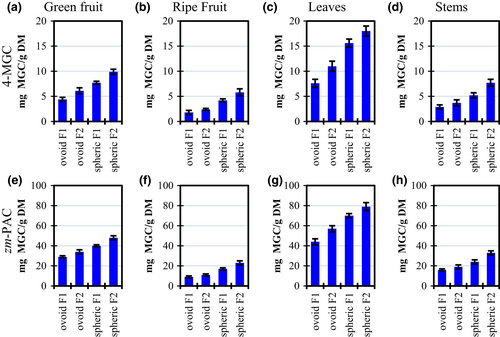
For example, PAC's highest value for leaves was 18 mg 4-MGC/g (Figure 3c), but the same sample corresponds to 79 mg zm-PAC/g because ber samples contain polymeric proanthocyanidins, not well represented by the 4-MGC monomer.
Table 3 compares the ber samples contained in this study with several other fruits. PACs in ber samples titer for 1.8–18 mg 4-MGC/g. Our highest PAC content was greater than the reported for many fruits such as avocado, apricot, figs, raisins, or blackberries. In contrast, ber PAC's content was lower than many fruits, especially from the Prunus genus, such as cherry, peach, and nectarine.
| Fruit | Specie | Country | mg/g DM | Method/standard | Reference |
|---|---|---|---|---|---|
| Avocado | Persea americana | USA | 1.1 | HPLC/blueberrry | Wang et al. (2010) |
| Apricot | Prunus armeniaca | Algeria | 2.24 | Photometric/leucoanthocyanidin | Ouchemoukh et al. (2012) |
| Apricot | Prunus armeniaca | Spain | 3.04 | HPLC/epicathechin | Redondo et al. (2017) |
| Figs | Ficus carica | Algeria | 2.18 | Photometric/leucoanthocyanidin | Ouchemoukh et al. (2012) |
| Raisins | Vitis vinifera | Algeria | 7.39 | Photometric/ leucoanthocyanidin | Ouchemoukh et al. (2012) |
| Cherry | Prunus avium | Spain | 10.54 | HPLC/epicathechin | Redondo et al. (2017) |
| Peach | Prunus persica | Spain | 13.79 | HPLC/epicathechin | Redondo et al. (2017) |
| Nectarine | Prunus persica | Spain | 59.89 | HPLC/epicathechin | (Redondo et al., 2017) |
| Blackberry | Rubus adenotrichos | Costa Rica | 2.50–9.26 | Photometric/4'-O-methyl-gallocatechin | Araya et al. (2017) |
| Pear jujube | Ziziphus jujuba | China | 1.5–5.5 | Photometric /Grass-seed | Wu et al. (2012) |
| Ber | Ziziphus mauritana | Costa Rica | 1.8–9.9 | Photometric/4'-O-methyl-gallocatechin | This study |
Although there are some differences between the results respecting the method and standard utilized, the values of Table 3 provide reference values.
3.4 Antioxidant activity
As mentioned before, the ascorbic acid content was remarkably high in Z. mauritiana, respecting many other vitamin C-rich fruits. Our samples contain high TPC as well. Figure 4a–d shows the antioxidant capacity by measuring DPPH antiradical activity. Our samples showed the antioxidant activity of 90–387 μmol TE/g. Those values were under some berries such as blackberry 551–2151 μmol TE/g (Araya et al., 2017), a well-known antioxidant-rich fruit. However, the antioxidant activity was over the reported for some common fruits. For example, antioxidant activity was 2–52 μmol TE/g in apples (Xu et al., 2016), and 0.4–1.0 μmol TE/g in oranges (M'hiri et al., 2017).
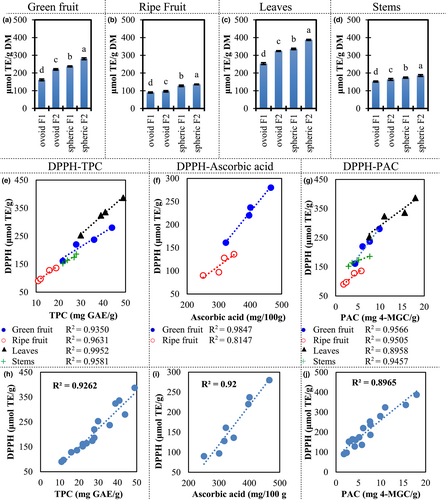
To understand the contribution of the different types of antioxidants evaluated in this study (TPC, PAC, and ascorbic acid) to the antioxidant activity, we evaluated the linear regressions of those compounds respecting the TE values for green and ripe fruits, leaves, and stems (Figure 4e–g). Curiously, in our samples, the trends of TPC, ascorbic acid, and PAC were very similar. Also, the R2 values for all the linear regressions showed a good fit (0.8147–0.9952). More important, most coefficients of determination are over 0.9. Also, the same behavior is observed when all samples are combined in a single regression (Figure 4h–j).
Previous studies including Ziziphus mauritiana and other species of the genus did not show a fit as high as in our experiments (Samirana et al., 2017; Yahia et al., 2020). Also, the phenolic compounds or a subgroup of the phenolic compounds usually show the best fit.
In the conditions included in this study, the biosynthesis and degradation of TPC, PAC, and ascorbic acid seemed to be related or at least occurring in parallel. However, it does not mean we can extrapolate our results to any growth, maturity, ripening, or harvesting conditions.
3.5 Antibacterial activity
Finally, we studied the fruit-derived standards zm-PS and zm-PAC containing the purified fraction of TPC and PAC, respectively, in terms of their capacity of inhibiting bacteria. We included two Gram-positive and two Gram-negative bacteria. Figure 5 shows the results of the antibiogram expressed as % Relative Inhibition, respecting chloramphenicol (positive control).
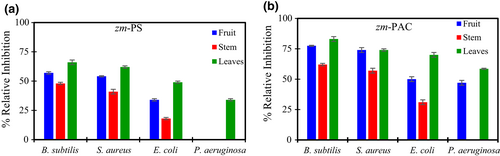
Both standards (zm-PS and zm-PAC) inhibit the two Gram-positive bacteria included in this study (B. subtilis and S. aureus). zm-PAC inhibition is stronger than zm-PS, and zm-PAC from fruits and leaves reaches values around 75% of the relative inhibition in the Gram-positives mentioned above.
Both zm-PS and zm-PAC from either fruit, stems, and leaves inhibit the growth of Gram-negative E. coli and P. aeruginosa. The other Gram-negative evaluated in this work is inhibited by both zm-PS and zm-PAC from leaves and the zm-PAC from the fruit; however, this one is not inhibited by the standards from stems or the zm-PS from the fruit. Therefore, the standards extracted from Z. mauritiana have an interesting spectrum of action.
4 CONCLUSIONS
There was a dependence on the main antioxidant nutritional quality parameters of ber fruits to the maturity conditions. The ber fruits, which are endemic to Asia, had been previously adapted to live in Costa Rica, and this is the first assessment of nutritional parameters. This fruit is rich in vitamin C and TPC. The concentration of ascorbic acid and total polyphenols was even higher than the one reported in bers grown in other countries such as Thailand, India, Pakistan, and others. From the two cultivars growing in Costa Rica, the spheric cultivar is richer in TPC, and PAC than the ovoid cultivar. Additionally, the farm location makes some differences between the metabolite productivity. There was a high correlationship between all TPC, PAC, and ascorbic acid with the antioxidant activity. The TPC and PACs had an interestingly wide antimicrobial spectrum, being active against B. subtilis, S. aureus, and E. coli, and some of them were against P. aeruginosa as well.
ACKNOWLEDGMENTS
We thank Mr. Luis Pineda from Cultivo de Guinda Cangelito, for kindly providing the biological materials for this study, and Mr. Adrián Cerdas-Pereira for proofreading the manuscript.
FUNDING INFORMATION
This research has been supported by Universidad Nacional, Heredia, Costa Rica, through the project SIA 89–22 (LAFIT: Phytochemistry laboratory).
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




