Immunomodulatory activity of mannosylglycerate and two unnatural mannosyl-oligosaccharides obtained from microbial fermentation on RAW264.7 macrophages
Abstract
Mannosyl compounds have been widely used in nutrition, fodder, and vaccine adjuvant industries. In our previous study, the engineered strains for the biosynthesis of three mannosyl compounds including β-1,2-mannobiose (M2-β-1,2), β-1,2-mannotriose (M3-β-1,2), and mannosylglycerate (MG) have been developed. However, their biological activities have not been reported. Here, those three compounds were successfully purified after fermentation of the engineered strains, and their potential immunomodulatory activities on RAW264.7 macrophages were investigated with commercialized β-1,4-mannotriose (M3-β-1,4) as control. Our results showed that M3-β-1,2 and MG promoted the viability and phagocytic function of RAW264.7. Meanwhile, the cytokine TNF-α and interleukin-6 (IL-6) level of RAW264.7 macrophages were significantly enhanced upon the stimulation of M3-β-1,2 and MG compared with M3-β-1,4. Moreover, MG significantly stimulated macrophages to secrete IL-10 compared with other mannan oligosaccharides. Finally, this study proved that the immunomodulatory activity of M3-β-1,2 and MG on RAW 264.7 cells was mainly through mitogen-activated protein kinases and myeloid differentiation protein 88 (MyD88)-dependent signaling pathways. All these findings suggested that M3-β-1,2 and MG exhibited immunomodulatory activities in the innate and adaptive immune systems, thus facilitating the application potential in developing of mannosyl compounds as an immunomodulator available for food and pharmaceutical area.
1 INTRODUCTION
The immune system is a complex network of cells, cytokines, antibodies, and cell signaling cascades that continuously respond to external stimuli, with the ability of defense against various infectious diseases (Alamuru-Yellapragada et al., 2017; Mukherjee et al., 2013). Maintaining the stability of the immune system is very important for the health of the body. Increasing evidence confirmed that polysaccharides or oligosaccharides play an important role in maintaining immune system stability acting as immunostimulators to elicit immune cell responses or as immunosuppressors to suppress excessive immune responses (Deng et al., 2020; Liu et al., 2014; Zhang et al., 2020; Zheng et al., 2016). Mannan oligosaccharides (MOS) are functional carbohydrates particularly abundant in konjac and the outer cell-wall membrane of bacteria and yeast, they are composed of β-1,4, β-1,2, α-1,2, or/and α-1,6 linked d-mannosyl and d-glucosyl residues (Pangsri et al., 2015). Various studies have proven that MOS possesses versatile physiological functions, including anti-inflammatory, antioxidant, antiobesity, antitumor, prebiotic, treatment of Alzheimer's disease, and modulation of the immune system (Behera & Ray, 2016; Ferenczi et al., 2016; Hoving et al., 2018; Liu et al., 2021). Thus, MOS has potential extensive application in nutrition, fodder, and vaccine adjuvant industries (Janardhana et al., 2009; Mukherjee et al., 2013; Zeng et al., 2018). In particular, MOS has attracted extensive attention as an immunomodulator. For example, β-1,2-oligomannosides isolated from Candida albicans are capable of stimulating macrophages to produce pro-inflammatory cytokine tumor necrosis factor alpha (TNF-α), and the length of the mannosyl chain was found to be important for the immunostimulation effect (Jouault et al., 1995). Mukherjee et al. (2013) reported that a chemically synthesized acetylated trivalent β-1,2 linked mannodisaccharide-based compound showed considerable suppressive effects on Th2-type allergic inflammatory response. Therefore, follow-up research on the influence of the length of the saccharide chain and the type of glycosidic bonds of MOS on their immune regulation functions could provide a theoretical basis for further application of MOS in the field of food, healthcare, and biomedicine.
Mannosyl compounds contain a wider range of mannosyl derivatives including MOS and mannosyl derivatives, such as mannosylglycerate (MG). MG, a compatible solute with a widespread application, plays important role in protecting proteins in vitro and in vivo under salt, heat, and freeze-drying stresses (Borges et al., 2002; Cruz et al., 2006; Jorge et al., 2016; Schwentner et al., 2021). MG also possesses large potential applications in disease diagnosis and treatment. For example, Ryu et al. (2008) reported that MG was effective in suppressing the aggregation of soluble β-amyloid peptides into fibrils in Alzheimer's disease. Faria et al. (2013) reported that MG prevented aggregation of α-synuclein in a yeast model of Parkinson's disease. As a kind of mannosyl compound, the immune regulation function of MG has not been reported.
The traditional preparation methods of MOS are enzymatic hydrolysis or chemical synthesis, which have the disadvantages of low hydrolysis efficiency, protection and deprotection, unsafety, nongreen, and time consumption (S. T. Li et al., 2019; S. Wang et al., 2018). In particular, MOS obtained by enzymatic hydrolysis is often a mixture of heterogeneous consistency and difficult to purify into a single component to study their functions. In our previous work, an alternative approach that converted mannose to three mannosyl compounds, including MOS of β-1,2-mannobiose (M2-β-1,2) and β-1,2-mannotriose (M3-β-1,2) with β-1,2 mannosidic linkages and MG using engineered microorganisms fermentation was provided (Tian et al., 2020). However, the immunomodulatory activity of these mannosyl compounds on macrophages is not clear yet. In the present study, the potential immunomodulatory activities of purified mannosyl compounds of MG, M2-β-1,2, M3-β-1,2, and commercial β-1,4-mannotriose (M3-β-1,4) were evaluated in vitro by assessing the TNF-α, IL-6, and IL-10 production and phagocytotic ability. In addition, the potential signaling pathways of these mannosyl compounds were investigated for underlying their immunomodulatory mechanism. This study aimed to provide the theoretical basis of microbial fermentation-derived mannosyl compounds for further application in the field of immunologic adjuvant, immune enhancer, or biomedicine.
2 MATERIALS AND METHODS
2.1 Materials and reagents
Dulbecco's modified eagle medium (DMEM), fetal bovine serum (FBS), streptomycin, and penicillin were obtained from Gibco (Invitrogen Corporation). Lipopolysaccharide (LPS), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Chemical Co. Mouse TNF-α, IL-6, and IL-10 enzyme-linked immune sorbent assay (ELISA) Kits were obtained from 4A Biotech Co. M3-β-1,4 was purchased from Megazyme. RNAprep pure cell/bacteria kit was obtained from TianGen Biotech Co. SYBR Green Real-time PCR Master Mix was purchased from Thermo Fisher. Antibodies against β-actin, phospho-p38 (p-p38), phosphor-Erk1/2 (p-ERK), and phospho-SAPK/JNK (p-JNK) were provided by Cell Signaling Technology. Antibodies against myeloid differentiation protein 88 (MyD88), IL-1 receptor-associated kinase (IRAK-1), and tumor necrosis factor receptor-associated factor 6 (TRAF-6) were obtained from Santa Cruz Biotechnology. All the reagents used in this study were of analytical grade.
2.2 Purification and characterization of mannosyl compounds
Crude MOS and MG were synthesized using recombinant Corynebacterium glutamicum strains via the fermentation process as described previously (Tian et al., 2020). The MOS was then purified sequentially using Ca2+ anion exchange chromatography and the preparative liquid chromatography. In brief, 40 ml of fermentation broth containing M2-β-1,2 and M3-β-1,2 was applied to Ca2+ anion exchange chromatography (450 × 40 mm), followed by eluting with ultrapure water at a flow rate of 1.5 ml/min. The elution (7.5 ml/tube) of each fraction was collected and detected using high-performance liquid chromatography (HPLC) apparatus equipped with a cation exchange column (Sugar-Pak™, 6.5 × 300 mm) with deionized water as a mobile phase and a refractive index detector. Then, the M2-β-1,2 and M3-β-1,2 samples were further separated by a preparative liquid chromatograph using a cation exchange column (Sugar-Pak™, 6.5 × 300 mm) with an injection volume of 60 μl, followed by eluting with ultrapure water at a flow rate of 0.4 ml/min. For MG, Ca2+ anion exchange chromatography and preparative thin-layer chromatography (TLC) were used to remove the impurities of the fermentation broth. For TLC, MG that was purified with Ca2+ anion exchange chromatography was run on silica gel plates (Silica gel 60 F254; Merck) with a solvent system composed of N-butanol, acetic acid, and water (2:2:1, v/v/v). Then, MG on the plates was scraped off and soaked in ddH2O. After centrifugation, MG was obtained in the supernatants and the concentration and purity of MG were detected using an HPLC apparatus equipped with a Bio-Rad HPX-87H column (300 × 7.8 mm) and a refractive index detector (Tian et al., 2020).
2.3 Cell culture and viability assay
RAW264.7 macrophages were cultured in DMEM medium plus 10% FBS, 100 µM penicillin, 100 µM streptomycin in a humidified atmosphere with 5% CO2 at 37°C. Then, the cytotoxic effects of mannosyl compounds on the viability of RAW264.7 cells were examined by MTT assay. In brief, RAW264.7 cells were seeded in a 96-well plate at a density of 1 × 105 cells per well overnight and then added into different concentrations of mannosyl compounds (62.5–250 µg ml−1 MG, 500–2000 µg ml−1 M2-β-1,2, 125–500 µg ml−1 M3-β-1,2, or 250–1000 µg ml−1 M3-β-1,4) or 100 ng ml−1 LPS for another 24 h cultivation. Subsequently, MTT solution was added into each well to a final concentration of 0.5 mg ml−1. After incubation for another 4 h, the supernatants were discarded, and 200 µl/well of DMSO was added. Finally, the absorbance was measured at 490 nm. The relative cell viability = OD490 of mannosyl compounds/OD490 of the control group.
2.4 Measurement of cytokine production
RAW264.7 cells were seeded in a six-well plate at a density of 6 × 105 cells per well and incubated at 37°C overnight. Then, the cells were treated with different concentrations of mannosyl compounds (125 and 250 µg ml−1 MG, 1000 and 2000 µg ml−1 M2-β-1,2, 250 and 500 µg ml−1 M3-β-1,2, or 250 and 500 µg ml−1 M3-β-1,4) or 100 ng ml−1 LPS for another 24 h. The supernatants were collected after centrifugation at 3000 rpm for 5 min at 4°C. The concentrations of IL-6, TNF-α, and IL-10 in the supernatants were measured using ELISA kits in accordance with the manufacturer's instructions.
2.5 Phagocytic activity assay
The phagocytic uptake ability of RAW264.7 cells was measured in accordance with the method described previously (Wu et al., 2017). The cells were preincubated in a 96-well plate at a density of 1 × 105 cells per well overnight. Then, they were treated with different concentrations of mannosyl compounds (62.5, 125, and 250 µg ml−1 MG, 1000 and 2000 µg ml−1 M2-β-1,2, or 125, 250, and 500 µg ml−1 M3-β-1,2) or 100 ng ml−1 LPS for another 24 h, followed by the addition of 100 µl of 0.1% neutral red and incubation for 1 h at 37°C. After three washings with cold PBS were performed, the cell lysis buffer glacial acetic acid/ethanol (1:1, v/v) was added and incubated for another 1 h at room temperature. Finally, the absorbance was measured at 490 nm.
2.6 Quantitative real-time polymerase chain reaction (qRT-PCR)
RAW264.7 cells were preincubated in a six-well plate for 12 h and treated with different concentrations of mannosyl compounds or 100 ng ml−1 LPS for another 24 h. Total RNA was extracted using RNAprep pure cell/bacteria kit. RT-PCR was performed on a LightCycler96 real-time system (Roche) with SYBR Green Master Mix. β-actin was used as the housekeeping gene for normalization and the expression of the target gene was calculated using the 2−∆∆Ct method (Livak & Schmittgen, 2001). The primers used for qRT-PCR are listed in Table 1.
| Forward | Reverse | |
|---|---|---|
| TNF-α | CCTCACACTCACAAACCACC | ATAGCAAATCGGCTGACGGT |
| IL-6 | TTGCCTTCTTGGGACTGATG | TTGCCATTGCACAACTCTTT |
| IL-10 | AATGCAGGACTTTAAGGGTT | GAGGGTCTTCAGCTTCTCAC |
| β-actin | GGCTCCTAGCACCATGAAGA | AGGGTGTAAAACGCAGCTCAG |
- Abbreviation: TNF-α, tumor necrosis factor alpha.
2.7 Western blot analysis
RAW264.7 cells were preincubated in a six-well plate for 12 h and treated with different concentrations of mannosyl compounds or 100 ng ml−1 LPS for another 24 h. After three washings with cold PBS were performed, the cells were lysed with RIPA lysis buffer (Beyotime) for 5 min and supernatants were obtained after centrifugation at 12,000 rpm for 5 min at 4°C. The denatured proteins in the above supernatants were separated using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto the nitrocellulose filter membrane (NC) membrane by electroblotting. The membrane was blocked with 5% BSA in TBST buffer (20 mM Tris-HCl, 500 mM NaCl, and 0.1% Tween-20) for 2 h at room temperature. Subsequently, the membranes were incubated with rabbit or mouse monoclonal antibody anti-p-ERK (1:2000), anti-p-p38 (1:1000), anti-p-JNK (1:1000), anti-β-actin (1:1000), anti-MyD88 (1:2000), anti-IRAK-1 (1:2000), anti-TRAF-6 (1:2000), and anti-GAPDH (1:1000) overnight at 4°C, respectively. After four washings with TBST for 5 min, the membranes were incubated with HRP-conjugated anti-rabbit or mouse secondary antibody for 1 h at room temperature. The expression levels of proteins were visualized using a chemiluminescence analysis system (SageCreation). The density of Western blot bands was corrected depending on the amount of β-actin or GAPDH and analyzed using Image J software.
3 RESULTS AND DISCUSSIONS
3.1 Purification and characterization of mannosyl compounds
To explore the influence of the length of saccharide chain and the types of glycosidic bond of mannosyl derivatives on their immunomodulatory activities for RAW264.7 cells, three kinds of mannosyl compounds, MG, M2-β-1,2, and M3-β-1,2 were prepared using recombinant C. glutamicum strains as described elsewhere (Tian et al., 2020). In the present study, 240.1 mg of MG was obtained in a 40 ml fermentation broth with the initial 20 g/L mannose and 5 g/L glycerate. In addition, 75 mg of M2-β-1,2 and 443.6 mg of M3-β-1,2 were obtained in a 40 ml fermentation broth with an initial mannose concentration of 40 g/L. After being purified with Ca2+ anion exchange chromatography, 93.3 mg of MG, 20.6 mg of M2-β-1,2, and 173.2 mg of M3-β-1,2 were obtained, and the purity increased to 38.9%, 27.4%, and 39.0%, respectively. Subsequently, after further purification was conducted with preparative TLC or a liquid chromatograph, the purity of MG, M2-β-1,2, and M3-β-1,2 reached 85%, 90%, and 90%, respectively. Finally, the yield of MG, M2-β-1,2, and M3-β-1,2 was 24.4, 13.1, and 41.6 mg, respectively (Figure 1 and Table 2).
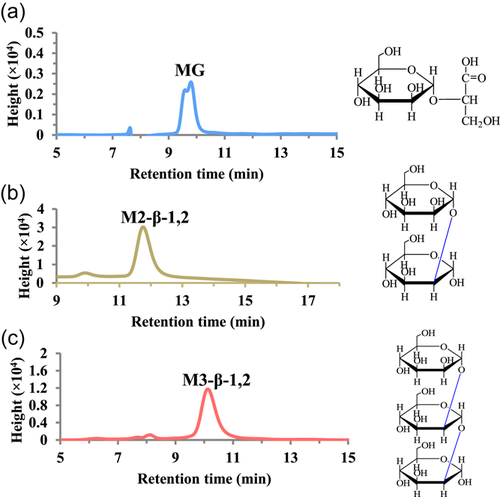
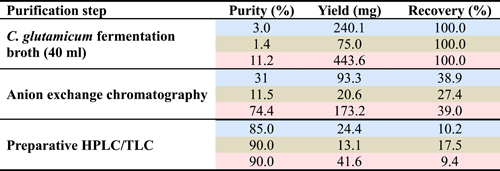 |
- Note: Data for MG, M2-β-1,2, and M3-β-1,2 are marked by blue, green, and red backgrounds, respectively.
- Abbreviations: HPLC, high-performance liquid chromatography; MG, mannosylglycerate; MOS, mannan oligosaccharides; TLC, thin-layer chromatography.
3.2 In vitro immunomodulatory activity
3.2.1 Effects of mannosyl compounds on the viability of RAW264.7 cells
Macrophage cells are important immune cells of the innate immune system, it defend against invading pathogens through phagocytosis, killing pathogenic microorganisms, or releasing inflammatory cytokine (Liu et al., 2017; Mosser & Edwards, 2008). Murine macrophage cell line RAW 264.7 is usually used as an in vitro cell model to evaluate the immune modulation effect of candidate drugs (Ren et al., 2017). When the body is infected, macrophages could be quickly recruited and proliferated to play an anti-infective role. In the present study, compared with the control group of 0 μg ml−1 oligosaccharide added, the MTT assay showed that the three mannosyl compounds (M3-β-1,2, M2-β-1,2, and MG) had no obvious cytotoxic effects on RAW264.7 macrophages (Figure 2a). It is noteworthy that 500 μg ml−1 M3-β-1,2 and 62.5-125 μg ml−1 MG could significantly promote the viability of macrophages, giving maximum cell viability of 1.15-fold and 1.21-fold higher than that of the control group, respectively. By contrast, 500–1000 μg ml−1 M3-β-1,4 significantly inhibited the proliferation of macrophages (Figure 2a). In a previous study, Paulovicová demonstrated that treatment with synthetically prepared biotinylated mannooligosaccharides containing β-1,2-linked mannosyl units terminus could significantly increase the proliferation of RAW264.7, consistent with the findings of the present study (Paulovicova et al., 2019). Therefore, 125 and 250 μg ml−1 of MG, 1000 and 2000 μg ml−1 of M2-β-1,2, 250 and 500 μg ml−1 of M3-β-1,2, 250 and 500 μg ml−1 of M3-β-1,4 were selected for the following assays.
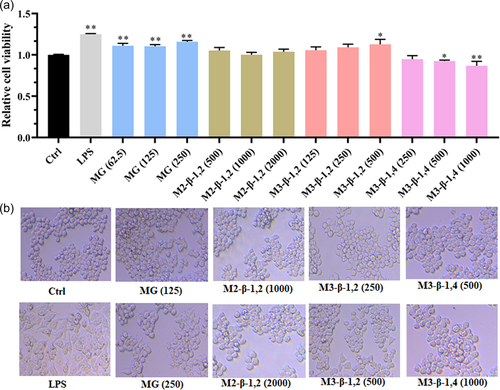
After macrophages are activated, cells increase in volume and content, and their immunomodulatory activity could be significantly improved. In this study, cell morphology was enlarged after being treated with two mannosyl compounds and LPS. As shown in Figure 2b, in the control group, the cells morphology was round, while when treated with 250 μg ml−1 MG or 500 μg ml−1 M3-β-1,2, most of the cells increased in size, showed pseudopods, and became irregular in shape with higher cell density than the control group, consistent with the results of MTT assay. For M2-β-1,2 and M3-β-1,4, no obvious changes in cell morphology were observed.
3.2.2 Effects of mannosyl compounds on phagocytic uptake of RAW264.7 cells
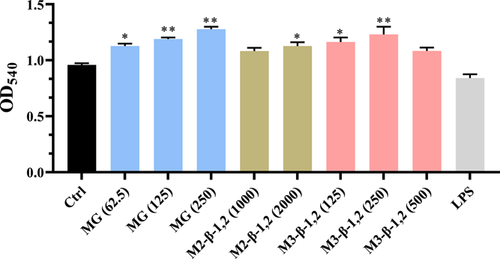
Phagocytosis is one of the important features of macrophages response to pathogens and foreign substances, and the enhancement of the phagocytic function of macrophages could be used as an index to evaluate the immunomodulatory activity of drugs (M. Wang et al., 2017). Neutral red is an effective cationic dye that could be applied to evaluate the phagocytic capacity of macrophages (Wu et al., 2017). In the present study, as shown in Figure 3, M3-β-1,2 was found to significantly increase the phagocytic activity of macrophages when its concentration varied from 125 to 250 μg ml−1. When treated with 250 μg ml−1 M3-β-1,2, the phagocytotic ability of macrophages exhibited a 1.3-fold increase compared with that of the control group. Similarly, compared with the control group, MG was found to significantly increase the phagocytic activity of macrophages under the concentration ranging from 62.5 to 250 μg ml−1, and the highest increase of 1.4-fold was obtained under 250 μg ml−1. By contrast, there was no obvious enhancement of phagocytic activity was found for M2-β-1,2 compared with the control group. Collectively, these results indicated that M3-β-1,2 and MG could efficiently induce the immune response of macrophage cells.
3.2.3 Effects of mannosyl compounds on cytokines generation and messenger RNA (mRNA) expression of RAW264.7 cells
Cytokines, a kind of small protein molecule, are secreted by monocyte-macrophages and lymphocytes when stimulated by a heterologous substance, and they play an important role in enhancing and regulating the body's immune response (Liu et al., 2017). Among them, TNF-α has been recognized as an important host regulatory molecule with tumor necrosis activity, and it is functional in initiating immune regulation and promoting immune cell activity (C. Wang et al., 2020). In the present study, the TNF-α level of macrophages was low in the untreated control group (32.5 pg ml−1), whereas its concentration was significantly enhanced by the stimulation of mannosyl compounds (Figure 4a). As a positive control, the highest TNF-α concentration of 4360.8 pg ml−1 was obtained in the LPS-treated group. In particular, the highest TNF-α levels in macrophage cells were 1017.5, 118, and 1111.5 pg ml−1 under the MG, M2-β-1,2, and M3-β-1,2 dosages of 250, 2000, and 500 μg ml−1, respectively. By contrast, no observable increase in the secretion of TNF-α by macrophages was found under the treatment of M3-β-1,4 even at a high loading dosage of 500 μg ml−1. The gene transcription levels of TNF-α were examined by RT-PCR to further confirm the effects of MG and M3-β-1,2 on the mRNA expression of cytokines in macrophages. Similar to the case of the protein expression of TNF-α, the mRNA levels of TNF-α were significantly and concentration-dependently increased by MG (125 and 250 μg ml−1) and M3-β-1,2 (250 and 500 μg ml−1) compared with those in the normal control group (Figure 4b).
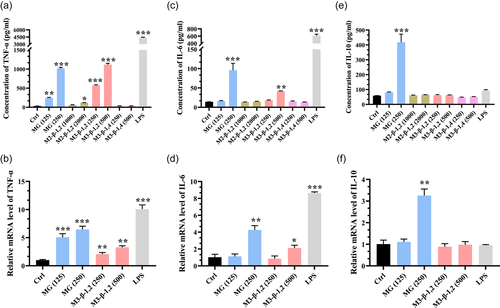
IL-6 is an important regulator of host defense responses for differentiation, maturation, and activation of macrophages (A. Li et al., 2012). According to Figure 4c, the production of IL-6 secreted by macrophages was 14.12 pg ml−1 in the untreated control group. Meanwhile, the highest IL-6 concentrations of 95.82 and 41.48 pg ml−1 were detected under the treatment of 250 μg ml−1 MG and 500 μg ml−1 M3-β-1,2 respectively, which was significantly increased compared with the untreated control group. By contrast, M2-β-1,2 and M3-β-1,4 could not stimulate the secretion of IL-6 by macrophages. In addition, 250 μg ml−1 MG or 500 μg ml−1 M3-β-1,2 markedly increased the mRNA levels of IL-6 (Figure 4d). These results suggested that MG and M3-β-1,2 had a strong immunostimulating activity.
With respect to anti-inflammatory substances, IL-10 has been recognized as a master anti-inflammatory cytokine exhibiting immunosuppressive effects necessary for inflammatory resolution (Sun et al., 2019). As shown in Figure 4e, the production of IL-10 by macrophages was significantly enhanced to 417.52 pg ml−1 by the stimulation of 250 μg ml−1 MG, which was 7.06-fold higher than that of the untreated control, indicating that MG exhibited excellent immunomodulatory activity. Moreover, the mRNA levels of IL-10 were significantly increased by 250 μg ml−1 MG (Figure 4f). Autengruber et al. (2014) reported that MG significantly reduced neutrophilic lung inflammation induced by particle exposure, consistent with the result of the present study. Collectively, M3-β-1,2 and MG showed stronger immunostimulating or immunomodulatory activity than M2-β-1,2 and M3-β-1,4, indicating the length of the mannosyl chain and the type of glycosidic bounds influence the function of MOS. Consistent with our finding, several similar conclusions have been reported, for example, glycoconjugate formulas with terminal β-mannosyl-units showed more potent of cytokines induction than other formulas and acetylated trivalent β-1,2-linked mannodisaccharide based compound showed suppressive effects on the allergic inflammatory response (Mukherjee et al., 2013; Paulovicova et al., 2019). Besides, degrees of polymerization of oligomannosides were strongly associated with the level of TNF-a in RAW264.7 cells (Jouault et al., 1995).
3.3 Effect of mannosyl compounds on mitogen-activated protein kinases (MAPKs) signaling pathways in macrophages
Macrophages could be activated by several intracellular signal transduction pathways, and among them, MAPKs play an important role (G. Wang et al., 2016). MAPKs belong to the serine/threonine-specific protein kinases family and regulate the release of cytokine when macrophage cells are stimulated by various extracellular stimulants, including polysaccharides and oligosaccharides (Dong et al., 2020; Q. Z. Li et al., 2019; Wu et al., 2017). MAPKs are involved in three cascades, including extracellular activated signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 (G. Wang et al., 2016). Recent studies have reported that oligosaccharides or polysaccharides could induce cytokine secretion in macrophages accompanied by phosphorylation of ERK, JNK, and p38 (He et al., 2021; Wu et al., 2017; Zhou et al., 2015). In this study, the levels of phosphorylated ERK, JNK, and p38 in RAW 264.7 cells after being treated with mannosyl compounds were evaluated using Western blot. As shown in Figure 5a,b, after stimulation by MG (at 125–250 μg ml−1), a remarkable increase in the phosphorylation level of JNK, p38, and ERK was observed in a dose-dependent manner compared with the untreated control group. Meanwhile, M3-β-1,2 increased the phosphorylation of p38 and ERK, whereas M3-β-1,4 had no obvious effect on the phosphorylation of the three MAPKs.
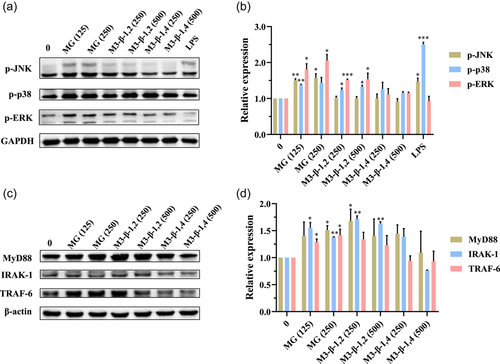
MyD88 is one of the key adaptor molecules that could integrate and relay signals to transcription factors upon activation of pattern recognition receptors (Seo et al., 2016). IRAK-1 belongs to the IL-1 receptor-associated kinases family, and it could associate with MyD88 to activate the nuclear factor kappa-B signaling pathway. When IRAK-1 was activated, TRAF-6 was recruited and it further activated a series of downstream kinases (Asehnoune et al., 2004). In this study, Western blot was used to investigate the stimulation effect of M3-β-1,2 and MG to the MyD88-dependent (MyD88/IRAK-1/TRAF-6) signaling pathway in RAW264.7 cells. According to Figure 5c,d, the expression levels of MyD88, IRAK-1, and TRAF-6 protein had a significant increase in a dose-dependent manner after being stimulated by M3-β-1,2 and MG. The above results suggested that MG and M3-β-1,2 induced the cytokines production of macrophages via MAPKs and MyD88-dependent pathways.
4 CONCLUSIONS
In this study, two kinds of MOS including M2-β-1,2 and M3-β-1,2 with β-1,2 mannosidic linkages and MG were biosynthesized and purified from recombinant C. glutamicum strains. The immunomodulatory activities of these three mannosyl compounds were evaluated compared with M3-β-1,4. The results showed M3-β-1,2 and MG promoted the viability and phagocytic function of RAW264.7. In addition, M3-β-1,2 and MG showed stronger immunomodulatory activities in terms of stimulating the production of TNF-α and IL-6. The IL-10 in macrophages was also significantly enhanced by MG. Finally, we have shown that the immunomodulatory activity of M3-β-1,2 and MG on RAW 264.7 cells was mainly functioned through MAPK and MyD88-dependent signaling pathways.
AUTHOR CONTRIBUTIONS
Juanjuan Liu: Data curation; validation; writing − original draft; writing − review & editing. Jiangang Yang: Writing − review & editing. Yan Zeng: Writing − review & editing. Chaoyu Tian: Methodology; writing − review & editing. Yan Men: Writing − review & editing. Yuanxia Sun: Project administration; supervision.
ACKNOWLEDGMENTS
This study was supported by the National Key R&D Program of China (No. 2021YFC2101400), Biotechnology Innovation Capacity Improvement Project (No. TSBICIP-KJGG-003), Youth Promotion Association of the Chinese Academy of Sciences (No. 2021176).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
None declared.




