Interim analysis of the long-term efficacy and safety of azetukalner in an ongoing open-label extension study following a phase 2b clinical trial (X-TOLE) in adults with focal epilepsy
The X-TOLE Study Group presented in Appendix A.
Abstract
Objective
To report interim data from an ongoing, open-label extension (OLE) of a Phase 2b study (X-TOLE) of azetukalner in adults with focal onset seizures (FOS) receiving 1–3 antiseizure medications.
Methods
Eligible participants enrolled in the 7-year OLE at 20 mg azetukalner once daily with food. Long-term seizure outcomes included median percentage change (MPC) in monthly (28 days) FOS frequency from the double-blind phase (DBP) baseline and achievement of ≥50%, ≥75%, ≥90%, and 100% seizure reductions.
Results
285 participants completed the DBP, and 275 (96.5%) enrolled in the OLE. At the 24-month interim analysis (September 5, 2023), 182 participants had been treated for ≥12 months and 165 for ≥24 months; 152 (55.3%) continued in the study. The median (range) treatment duration in the OLE was 26.3 (0.1–46.6) months. MPC reduction was 83.2% at 24 months in the OLE vs. DBP baseline. For all participants who entered the OLE, 56.4% (155/275) and 44.4% (122/275) achieved a ≥50% seizure reduction, 28.4% (78/275) and 19.6% (54/275) achieved a ≥90% seizure reduction, and 22.2% (61/275) and 14.9% (41/275) achieved seizure freedom (100% seizure reduction) for any consecutive ≥6- and ≥12-month period, respectively. For those who reached ≥24 months in the OLE, seizure freedom was achieved by 34.5% (57/165) and 23.6% (39/165) for any consecutive ≥6- and ≥12-month period, respectively. The majority of treatment-emergent adverse events (TEAEs) were mild or moderate. The most common TEAEs were dizziness (21.8%), headache (15.3%), coronavirus infection (15.3%), somnolence (12.7%), fall (12.7%), and memory impairment (10.9%). Serious AEs were reported in 35 (12.7%) participants.
Significance
The efficacy demonstrated by azetukalner in reducing FOS seizure frequency in the DBP was sustained in this interim analysis. Azetukalner was generally well tolerated, with no new safety signals compared to the DBP. These data suggest sustained long-term efficacy and safety of azetukalner in a difficult-to-treat population.
Plain Language Summary
This long-term study assessed the safety and efficacy of azetukalner to treat focal seizures. Patients taking azetukalner daily with food for about 2 years had far fewer focal seizures with azetukalner than before taking the medication. For those who had been treated for 24 months, about a third were seizure-free for a consecutive 6-month period, and about a quarter were seizure-free for a consecutive 12-month period. Most side effects were mild or moderate, and these included dizziness, headache, and somnolence (sleepiness).
Key points
- Azetukalner 20 mg once-daily with food yielded long-term efficacy in this open-label extension (OLE) study with 60% retention at 24 months.
- Sustained monthly reduction in seizure frequency (median percentage change 80.2%–83.2%) from baseline was observed during OLE months 12–24.
- Seizure freedom for ≥6- and ≥12-month consecutive durations was achieved in 22.2% and 14.9% of OLE participants, respectively.
- Seizure freedom for ≥6- and ≥12-month consecutive durations was achieved in 34.5% and 23.6% of those treated for ≥24 months, respectively.
- Azetukalner continues to be generally well tolerated in the OLE, with adverse events consistent with prior results and other antiseizure medications.
1 INTRODUCTION
KV7 voltage-gated potassium channels expressed in brain neurons play an important role in regulating neuronal excitability.1-4 Loss-of-function variants in the genes encoding certain neuronal KV7 channels have been associated with a range of neuronal hyperexcitability phenotypes, from an inherited benign form of epilepsy in newborns to a severe epileptic encephalopathy.1, 5 Conversely, molecules that promote the opening of brain KV7 channels may reduce seizure occurrence.3, 6
Azetukalner (XEN1101) is a novel, potent KV7 potassium channel opener in development for the treatment of focal onset seizures (FOS), primary generalized tonic–clonic seizures, and major depressive disorder.7-11 The pharmacokinetic profile of azetukalner, together with results of initial clinical safety and tolerability studies, supports once-daily (QD) dosing with food and no titration at treatment initiation.7, 9 In healthy volunteers, a single 20 mg oral dose of azetukalner was well tolerated and resulted in significant reductions in corticospinal and cortical excitability.12 The efficacy of azetukalner for the treatment of FOS was investigated in a Phase 2b placebo-controlled clinical trial (X-TOLE). X-TOLE evaluated 3 doses of azetukalner taken daily with food during an 8-week, randomized, double-blind phase (DBP). The baseline median monthly seizure frequency in the study population was 13.5 FOS. The study participants had tried and discontinued a median of 6 antiseizure medications (ASMs), and more than half (50.5%) were on 3 background ASMs at the time of study entry. The study met its primary efficacy endpoint, with azetukalner demonstrating a statistically significant, dose-dependent reduction from baseline in FOS, with median percentage reductions in monthly FOS frequency of 33.2% (p = 0.04), 46.4% (p < 0.001), and 52.8% (p < 0.001), in the 10 mg, 20 mg, and 25 mg groups, respectively, compared with 18.2% in the placebo group. Azetukalner was generally well tolerated, with treatment-emergent adverse events (TEAEs) that occurred with other commonly prescribed ASMs.7 The onset of action was rapid, with significant reductions in seizure frequency occurring within 1 week (10 mg p < 0.05; 20 mg p < 0.05; 25 mg p < 0.001 vs. placebo based on a post hoc pairwise comparison). Eligible participants could continue into a 7-year open-label extension (OLE) in which all participants began OLE treatment taking a 20 mg dose of azetukalner once daily with food. Here, we provide long-term efficacy and safety results from an interim analysis of the ongoing OLE (data cut September 5, 2023).
2 METHODS
2.1 Study design
The X-TOLE study was conducted at 95 sites across North America and Europe (NCT03796962; EudraCT, 2018–003221-29). The study design, methods, and primary efficacy and safety results of the DBP have been published.7 Briefly, the study enrolled a safety population of 325 adults. Participants needed to be 18–75 years old with a diagnosis of focal epilepsy per International League Against Epilepsy criteria13 for ≥2 years, experience ≥4 countable FOS per month during an 8-week minimum baseline period, and receive stable treatment with 1–3 ASMs. Daily seizure and treatment adherence was recorded in an e-diary, which was centrally monitored, in addition to monitoring at participating sites.7 In the 8-week DBP, participants were randomized to 1 of 3 doses of azetukalner (10, 20, or 25 mg) or placebo, taken once daily with food with no titration. Azetukalner is primarily metabolized by cytochrome P450 3A4 (CYP3A4), with minimal involvement from other cytochrome P450 enzymes, and plasma levels of azetukalner may decrease in the presence of CYP3A4 inducers. Randomization was stratified according to background use of CYP3A4 inducers (eg, carbamazepine, eslicarbazepine acetate) to ensure equal distribution across groups.7
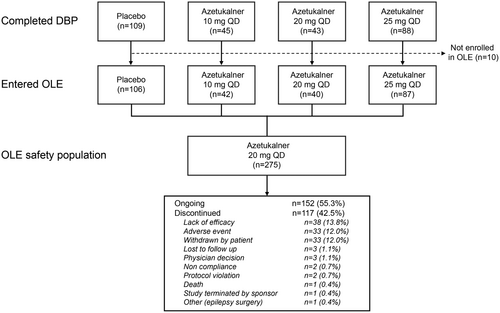
Participants who completed the DBP with a minimum of 80% study medication compliance and no important protocol deviations were eligible to enter the OLE phase, during which participants from all DBP arms began treatment with azetukalner 20 mg taken once daily with food (Figure 1). Azetukalner dose adjustments and changes in concomitant ASMs were allowed during the OLE. Assessments occurred at week 3 in the OLE (study day 77, week 11 from randomization) and at 3-month intervals thereafter for the first year. After the first year, on-site visits occurred at 6-month intervals, with teleconferences at 3 months between each on-site visit. The OLE phase is scheduled for completion after 378 weeks (7 years) of treatment, and no participant has yet completed the study.
2.2 Outcome analysis
In this interim analysis, long-term efficacy was assessed by calculating the median percentage change (MPC) in monthly FOS frequency from the DBP baseline and the percentage of participants with ≥50%, ≥75%, ≥90%, and 100% reduction from the DBP baseline in monthly FOS frequency for any consecutive ≥6-month and ≥12-month period in the OLE. The MPC analysis was conducted for the overall group, for a numerically constant cohort group, which consisted of participants who were treated for at least 24 months in the OLE and for those who were continuing in the study at data cutoff. Similarly, the calculation of the percentage of participants with ≥50%, ≥75%, ≥90%, and 100% seizure reduction was evaluated for all participants who entered the OLE and a second analysis for those who were treated for at least 24 months in the OLE. For continuing participants in the OLE, 100% reduction from the DBP baseline in monthly FOS frequency was also evaluated for ≥12 months from the last visit before the data cutoff. Long-term safety was assessed by frequency and severity of TEAEs and serious adverse events (SAEs). Additional safety outcomes included findings from physical, neurologic, and ophthalmoscopy examinations, clinically significant laboratory findings, vital signs, 12-lead electrocardiograph, and urological symptoms, as well as an increase in suicide risk via the Columbia-Suicide Severity Rating Scale.14
2.3 Statistical methods
Descriptive statistics were used to characterize outcome measures. The analysis of percentage change in seizure frequency from the DBP baseline was calculated based on monthly (28 days) FOS frequency.
2.4 Ethics
The study was conducted in accordance with the International Council for Harmonization Guidelines for Good Clinical Practice and all applicable local laws and regulations. The protocol and all amendments were submitted to the Ethics Committee/Institutional Review Board for review and approval before the enrollment of any participants. All participants provided written informed consent prior to the initiation of the study.
3 RESULTS
3.1 Participants
A total of 325 participants were randomized in the X-TOLE study (placebo, n = 114; 10 mg group, n = 46; 20 mg group, n = 51; 25 mg group, n = 114). Of the 285 participants who completed the DBP, 275 (96.5%) continued in the OLE (Figure 1). The characteristics of the participants who entered the OLE are similar to those of the overall X-TOLE study population (Table 1).7 As of September 5, 2023 (cutoff date for the current interim analysis), 152 (55.3%) remained in the study, 182 (66%) had been treated in the OLE for ≥12 months, and 165 (60%) had been treated for ≥24 months. The mean and median duration of treatment were 662 days (21.8 months) and 799 days (26.3 months), respectively (range, 0.1–46.6 months). Of all participants who entered the OLE (N = 275), 67 participants (24.4%) had dose reductions. The most common reasons given for discontinuation were lack of efficacy (n = 38, 13.8%), AEs (n = 33, 12.0%), and study withdrawal by the participant (n = 33, 12.0%).
| Characteristic | OLE population (n = 275) |
|---|---|
| Age at study entry, mean (SD), years | 41.1 (13.3) |
| Sex, n (%) | |
| Female | 138 (50.2) |
| Male | 137 (49.8) |
| Race, n (%) | |
| Black | 11 (4.0) |
| White | 250 (90.9) |
| Asian | 4 (1.5) |
| Other | 10 (3.6) |
| Ethnicity, n (%) | |
| Not Hispanic or Latino | 249 (90.5) |
| Hispanic or Latino | 22 (8.0) |
| Unknown | 4 (1.5) |
| Region, n (%) | |
| Europe | 166 (60.4) |
| North America | 109 (39.6) |
| BMI, mean (SD), kg/m2 | 27.0 (5.2) |
| Age at epilepsy onset, mean (SD), years | 18.1 (13.8) |
| Baseline seizure rate per month, median (IQR) | 13.5 (7.9, 30.3) |
| Number of ASMs tried and discontinued before study entry, mean (SD) | 6.5 (3.7) |
| Background ASM use, n (%) | |
| 1 | 23 (8.4) |
| 2 | 108 (39.3) |
| 3 | 144 (52.4) |
| CYP3A4 inducer useb, n (%) | 160 (58.2) |
- Abbreviations: ASM, antiseizure medication; BMI, body mass index; CYP3A4, cytochrome P450 3A4; DBP, double-blind period; IQR, interquartile range; OLE, open-label extension.
- a Baseline is DBP baseline.
- b CYP3A4 inducers included carbamazepine, eslicarbazepine acetate, oxcarbazepine, phenytoin, topiramate, or phenobarbital.
3.2 Efficacy
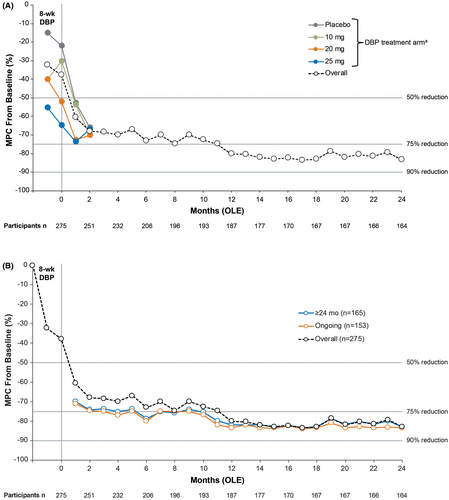
For ongoing OLE participants, monthly MPC reductions from the DBP baseline in FOS frequency improved over time from 61% (n = 266) at month 1, with MPC reductions (lower, upper quartile) from baseline of 68.5% (95.4, 37.0) at 3 months and 73.1% (100, 40.7) at 6 months (Figure 2A). At months 12, 18, and 24, the MPC reductions were 80.2% (100.0, 60.0), 83.0% (100, 50.9), and 83.2% (100, 53.2), respectively. For the constant cohort (treatment ≥24 months), the reductions in monthly MPC from baseline were 73.8% (100.0, 47.1) at 3 months and 78.8% (100.0, 49.2) at 6 months (Figure 2B). At months 12, 18, and 24, the constant cohort reductions were 82.1% (100.0, 62.8), 83.0% (100, 51.4) and 83.3% (100.0, 53.6), respectively. For ongoing participants, the reduction in monthly MPC from baseline was 75.1% (100.0, 47.4) at 3 months; 80.1% (100.0, 48.7) at 6 months; and 83.5% (100.0, 62.7), 83.5% (100, 53.8), and 83.5% (100.0, 53.6) at 12, 18, and 24 months, respectively.
In the OLE, 155 (56.4%) and 122 (44.4%) participants achieved a ≥50% seizure reduction for any consecutive ≥6-and ≥12-month period, respectively (Figure 3A). Seizure reductions of ≥90% were achieved by 78 (28.4%) participants for any consecutive ≥6-month period and 54 (19.6%) participants for any consecutive ≥12-month period. In participants who had been treated with azetukalner for 24 months or longer (n = 165), a ≥50% seizure reduction was achieved by 138 participants (83.6%) for any consecutive ≥6-month period and 115 (69.7%) participants for any consecutive ≥12-month period (Figure 3B). Seizure reductions of ≥90% for this population were achieved by 72 (43.6%) participants for any consecutive ≥6-month period and 52 (31.5%) participants for any consecutive ≥12-month period.
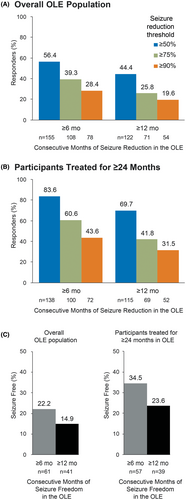
Seizure freedom (100% reduction in monthly FOS frequency) in the overall population was achieved by 61 (22.2%) and 41 (14.9%) participants for any consecutive ≥6-month period and ≥12-month period, respectively (Figure 3C). For participants who had been treated for ≥24 months in the OLE, seizure freedom was achieved by 57 (34.5%) participants for any consecutive ≥6-month period and 39 (23.6%) participants for any consecutive ≥12-month period. In ongoing participants (n = 152), 19.1% (29/152) experienced seizure freedom for ≥12 months since the last visit.
3.3 Safety
During the OLE, TEAEs were reported in 240 of 275 participants (87.3%) of the safety population (Table 2). Those reported most commonly (>10% of participants) were dizziness (21.8%), headache (15.3%), coronavirus infection (15.3%), somnolence (12.7%), fall (12.7%), and memory impairment (10.9%). Three participants reported urinary retention, 1 reported it as mild and 2 as moderate; no dose changes were made in any case. TEAEs that led to permanent treatment discontinuation (>2 participants) were dizziness (6 participants, 2.2%), somnolence (4 participants, 1.5%), and amnesia (3 participants, 1.1%). SAEs were reported in 35 participants (12.7%); SAEs reported in >1 participant were change in seizure presentation, reported in 6 participants (2.2%), and paresthesia, seizure, pneumonia aspiration, deep vein thrombosis, and fall, each reported in 2 participants (0.7%). There was 1 sudden unexpected death in epilepsy (SUDEP) reported, which was determined not to be related to the study drug. No signals of cardiovascular safety were identified as of the Sept 5, 2023, cutoff date. Overall, TEAEs were similar to those reported in the DBP, and no new safety signals were identified. At the end of the second year, participants recorded a mean (SD) weight change of −0.2 (8.8) kg.
| Summary of TEAEs, n (%) | Azetukalner 20 mg (n = 275) |
|---|---|
| At least 1 TEAE | 240 (87.3) |
| At least 1 serious TEAE | 35 (12.7) |
| At least 1 TEAE leading to permanent discontinuation of study drug | 30 (10.9) |
| At least 1 serious TEAE leading to death | 1 (0.4) |
| Most common TEAEs (≥5% of overall OLE population), n (%) | |
| Dizziness | 60 (21.8) |
| Coronavirus infection | 42 (15.3) |
| Headache | 42 (15.3) |
| Fall | 35 (12.7) |
| Somnolence | 35 (12.7) |
| Memory impairment | 30 (10.9) |
| Weight increased | 26 (9.5) |
| Gait disturbance | 23 (8.4) |
| Fatigue | 22 (8.0) |
| Urinary tract infection | 22 (8.0) |
| Aphasiaa | 21 (7.6) |
| Change in seizure presentationb | 20 (7.3) |
| Nasopharyngitis | 17 (6.2) |
| Confusional state | 16 (5.8) |
| Disturbance in attention | 15 (5.5) |
| Balance disorder | 14 (5.1) |
| Paresthesia | 14 (5.1) |
| Tremor | 14 (5.1) |
- Abbreviations: OLE, open-label extension; TEAE, treatment-emergent adverse event.
- a Word finding difficulty or similar symptoms coded as aphasia.
- b Change in seizure presentation could include faster seizure onset, increased seizures, gelastic seizures, prolonged seizures, and seizure exacerbation.
4 DISCUSSION
Azetukalner was associated with continued safety and favorable long-term seizure outcomes in this interim analysis of the OLE of the X-TOLE trial in participants with highly treatment-resistant focal epilepsy. These findings extend and confirm the results from the 8-week DBP, during which treatment with azetukalner was associated with a significant and dose-dependent reduction in seizure frequency, with a large effect size compared with placebo.7 At the dose of 20 mg once daily (the same dose administered during the OLE), the MPC in FOS frequency decreased by 46.4% in the DBP.7 By the interim analysis cutoff in the OLE, the median change in monthly FOS frequency in those remaining on treatment at 12 months (68% of overall participants) decreased by 80%–83% from the DBP baseline over months 12–24. In the group of participants treated for ≥24 months (60%) and ongoing participants (56%), MPC in monthly FOS frequency decreased by 79%–84% and 81%–84%, respectively, from DBP baseline over months 12–24.
In the OLE, the ≥50% responder rates were 56.4% and 44.4% for consecutive periods of ≥6 and ≥12 months, respectively, among all participants who entered the OLE. In the subset of participants who had been treated for 24 months or longer, responder rates were greater, with 83.6% achieving ≥50% seizure reduction for any consecutive ≥6-month period and 69.7% achieving ≥50% seizure reduction for any consecutive ≥12-month period. Seizure freedom for at least 12 consecutive months was achieved by 14.9% of all participants in the OLE and by 23.6% of those who had been treated for at least 24 months. In ongoing participants, 19.1% were seizure free for ≥12 months since their last visit prior to the data cutoff. The seizure freedom rates with azetukalner are particularly promising when considering that the X-TOLE trial enrolled a difficult-to-treat patient population with a median baseline seizure frequency of 13.5 FOS per month, who had tried and stopped a median of 6 ASMs prior to study entry. While it is difficult to compare seizure freedom rates across studies due to different methodologies and study durations, other OLE studies have reported on seizure freedom rates. In an OLE of a Phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures, 100% seizure reduction for any consecutive ≥12-month period was experienced by 18.4% of the modified intent-to-treat population (median duration for the safety population was 53.9 months)15 compared with 14.9% (median duration, 26.3 months) in the present study. In a 4-year OLE study of Phase 3 randomized trials of adjunctive perampanel for patients with treatment-refractory focal seizures, seizure freedom rates of 12.8% (10/78 patients) were reported during the last 12-month interval at 4 years of treatment.16 In a 1-year study of adjunctive brivaracetam treatment in patients with focal epilepsy (n = 994), 12 months of sustained seizure freedom was achieved by 73 patients (7.3%).17 In our study, seizure freedom for at least 12 months since the last visit before the data cutoff in ongoing participants was achieved by 19.1% (29/152).
Safety results from the OLE demonstrate that azetukalner is generally well tolerated during long-term treatment, with a tolerability profile consistent with that reported during the DBP. Besides coronavirus infection and falls, the most commonly reported TEAEs were related to the central nervous system (dizziness, headache, somnolence, and memory impairment). One case of SUDEP has been reported, which was not attributed to treatment. Serious AEs were infrequent at 12.7%, as were TEAEs related to urinary retention (1.1%), which have been reported with the use of KV7 channel openers.18 In total, 10.9% of participants discontinued the OLE due to a TEAE. No new safety signals were observed, and no signals of cardiovascular safety were identified.
OLE trials, such as this study, have inherent limitations that must be considered when interpreting the results. Key limitations are the open-label design and lack of a control group. In addition, there could also be selection biases since not all participants who enrolled in the DBP entered the OLE phase. This limitation is mitigated in the present situation by the fact that nearly all eligible subjects chose to receive double-blind treatment, and almost all (96%) of those completing the DBP continued into the OLE.7 As in all OLE studies, long-term assessment can be biased by participant dropout during the course of the OLE. Participants who perceive that they are doing well may preferentially remain on treatment. Moreover, at 24 months in the OLE, retention on azetukalner remained high at 60%, with only 13.8% and 12.0% of participants discontinuing the study due to lack of efficacy and due to AEs, respectively. In addition, our analysis of efficacy outcomes in a constant cohort group shows comparable improvements, indicating that the improvement is not solely due to attrition bias. The X-TOLE OLE is scheduled to continue for at least 7 years after the initiation of treatment, and further data on long-term safety and seizure outcomes will become available as the study progresses.
5 CONCLUSIONS
This interim analysis of the results of the X-TOLE OLE trial demonstrates that azetukalner continues to be associated with long-term efficacy in a highly treatment-resistant patient population with focal epilepsy, with no new safety signals reported compared to the DBP. The analysis supports its continued clinical development as a novel treatment option for FOS.
ACKNOWLEDGMENTS
The authors acknowledge the X-TOLE open-label trial investigators' contributions to data acquisition. A list of investigators is included in Appendix A. The authors thank medical writing and editorial support, conducted in accordance with Good Publication Practice 2022 Update (GPP 2022) and the International Committee of Medical Journal Editors guidelines, provided by Robin Smith, PhD, and Kirk W. Evanson, PhD, of the Curry Rockefeller Group, LLC, a Citrus Health Group, Inc., company (Chicago, Illinois), and funded by Xenon Pharmaceuticals Inc.
FUNDING INFORMATION
This study was funded by Xenon Pharmaceuticals Inc.
CONFLICT OF INTEREST STATEMENT
J. French receives salary support from the Epilepsy Foundation and from the Epilepsy Study Consortium for consulting work and/or attending scientific advisory boards for Acadia Pharmaceuticals, Access Industries, Acuta Capital Partners, AFASCI Inc., Agrithera, Inc., Alterity Therapeutics Limited, Angelini Pharma S.p.A., Autifony Therapeutics Limited, Axonis Therapeutics, Bain Capital, Beacon Biosignals, Inc., Biogen, Biohaven Pharmaceuticals, Bloom Science Inc., Bright Minds Biosciences, Inc., Camp4 Therapeutics Corporation, Capsida Biotherapeutics, Cerebral Therapeutics, Cerecin Inc., Cerevel, Ceribell, Cognizance Biomarkers, Cowen and Company, LLC, Crossject, EcoR1 Capital, Eisai, Encoded Therapeutics, Engrail, EpiMinder, Epitel Inc., Equilibre BioPharmaceuticals, Genentech, Inc., Grin Therapeutics, Harmony/Epygenix, iQure Pharma Inc, IQVIA RDS Inc., Janssen Pharmaceuticals, Jazz Pharmaceuticals, Korro Bio Inc., Leal Therapeutics Inc., LivaNova, Longboard Pharmaceuticals, Marinus, Modulight.bio, Neumirna Therapeutics, Neurelis, Neurocrine, Neurona Therapeutics Inc., NeuroPace, Inc., NeuroPro Therapeutics, Neuroventis, Neurvati, Noema, Ono Pharmaceutical Co., Otsuka Pharmaceutical Development, Ovid Therapeutics Inc., Praxis, PureTech LTY Inc., Rapport Therapeutics, Inc., Receptor Holdings Inc., Rivervest Venture Partners, Sage Therapeutics, Inc., SK Life Sciences, Stoke, Stream Neuroscience, Supernus, Takeda, Taysha Gene Therapies, Third Rock Ventures LLC, UCB Inc., uniQure, Ventus Therapeutics, Vida Ventures Management, and Xenon Pharmaceuticals Inc. She has also received research support from the Epilepsy Study Consortium (funded by Eisai and UCB), Epilepsy Study Consortium/Epilepsy Foundation (funded by UCB), GW/FACES/One8Foundation, NINDS, and Praxis. She is on the editorial board of Lancet Neurology and Neurology Today. She is Chief Medical/Innovation Officer for the Epilepsy Foundation. She is the President and on the Board of Directors for the Epilepsy Study Consortium, Inc. She has received travel/meal reimbursement related to research, advisory meetings, or presentation of results at scientific meetings from the Epilepsy Study Consortium, the Epilepsy Foundation, Biohaven Pharmaceuticals, Cerebral Therapeutics, Cowen and Company, LLC, Harmony Biosciences, Longboard, Neumirna Therapeutics, Neurocrine, NeuroPace Inc., Neurvati, Praxis, Rapport, SK Life Science, Takeda, Ventus, and Xenon Pharmaceuticals Inc. Roger J. Porter is a consultant for Aeterna, Axonis, Cadent, Engrail, Longboard, Neurocrine, Otsuka, Passage Bio, and Xenon Pharmaceuticals Inc. Emilio Perucca receives speaker or consultancy fees from Eisai, GRIN Therapeutics, Sintetica, SK Life Science, Sun Pharma, Takeda, UCB Pharma, and Xenon Pharmaceuticals Inc. Martin J. Brodie has nothing to declare. Michael A. Rogawski has served as a consultant to Xenon Pharmaceuticals Inc. but received no fees in relation to this study. Cynthia Harden, Jenny Qian, Constanza Luzon Rosenblut, Christopher Kenney, and Gregory N. Beatch are employees of and own stock or stock options in Xenon Pharmaceuticals Inc.
ETHICS STATEMENT
The authors confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
APPENDIX A
The authors acknowledge the X-TOLE open-label trial investigators for their contributions to data acquisition:
Bassel Abou-Khalil, M.D., Vanderbilt University Medical Center, Nashville, Tennessee, USA; Sami Aboumatar, M.D., Austin Epilepsy Care Center (AECC), Austin, Texas, USA; Robert Armstrong, M.D., Asheville Neurology Specialists, Asheville, North Carolina, USA; Ricardo Ayala, M.D., Tallahassee Neurological Clinic, Tallahassee, Florida, USA; Juan Luis Becerra, M.D., Hospital Germans Trias i Pujol, Barcelona, Spain; Francesca Bisulli, M.D., Ph.D., IRCCS-Istituto delle Scienze Neurologiche di Bologna, Bologna, Emilia-Romagna, Italy; Victor Biton, M.D., Clinical Trials, Inc., Little Rock, Arkansas, USA; Christian Brandt, M.D., Bethel Epilepsy Centre, Bielefeld, North Rhine-Westphalia, Germany; Dulce Campos, M.D., Hospital Clínico Universitario Valladolid, Valladolid, Spain; Laura Canafoglia, M.D., Ph.D., Istituto Epilettologia Clinica e Sperimentale Besta, Milano, Italy; Micaela Chatman, M.D., Minnesota Epilepsy Group, P.A., St. Paul, Minnesota, USA; Samuel Destefano, M.D., Department of Neurology, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA; Carlo Di Bonaventura, M.D., Ph.D., Sapienza University of Rome, Rome, Italy; Toufic Fakhoury, M.D., Bluegrass Epilepsy Research, Lexington, Kentucky, USA; Evan Fertig, M.D., Providence Brain & Spine Institute, Portland, Oregon, USA; Nathan Fountain, M.D., University of Virginia, Charlottesville, Virginia, USA; Antonio Gambardella, M.D., Dipartimento Scienze Mediche e Chirurgiche, Catanzaro, Italy; Michael Gelfand, M.D., Ph.D., University of Pennsylvania-Perelman Center for Advanced Medicine, Philadelphia, Pennsylvania, USA; Antonio Gil-Nagel (Prev: Aledo M.D, Ph.D.), Hospital Ruber Internacional, Madrid, Spain; Khalid Hamandi, MB BS, MRCP, Ph.D., Cardiff, Cardiff, Wales, UK; Heidi Henninger, M.D., Maine Medical Center, Scarborough, Maine, USA; Shahram Izadyar (Prev: Beach), M.D., FAES, SUNY Upstate Medical University, Syracuse, New York, USA; Sergii Kharchuk, M.D., Medical Center of Limited Liability Company “Harmoniya Krasy” Kyiv, Ukraine; Pavel Klein, M.B., B.Chir., Mid-Atlantic Epilepsy and Sleep Center, Bethesda, Maryland, USA; Kenneth Laxer, M.D., CPMC Research Institute, San Francisco, California, USA; Rebekka Lehmann, M.D., Epilepsy Center Berlin-Brandenburg, Berlin, Bavaria, Germany; Holger Lerche, M.D., Ph.D., University of Tubingen, Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research, Tubingen, Baden-Württemberg, Germany; Andrew Lerman, M.D., Visionary Investigators Network, Miami, Florida, USA; George Li, M.D., Medsol Clinical Research Center, Port Charlotte, Florida, USA; Kore Liow, M.D., FACP, FAAN, Hawaii Pacific Neuroscience (HPN), Honolulu, Hawaii, USA; Irene Garcia Morales, M.D., Ph.D., Hospital Clinico, Madrid, Spain; Dean Naritoku, M.D., University of South Alabama, Mobile, Alabama, USA; Alberto Pinzon-Ardila, M.D., Ph.D., The Neurology Research Group, LLC., Miami, Florida, USA; Chiara Pizzanelli, M.D., Ph.D., Azienda Ospedaliero Universitaria Pisana, Pisa, Italy; Rodrigo Rocamora, M.D., Ph.D., Hospital del Mar, Barcelona, Spain; Juan Rodriguez-Uranga, M.D., Centro de Neurologia Avanzada, Sevilla, Andalusia, Spain; Joanne Rogin, M.D., Minneapolis Clinic of Neurology, Golden Valley, Minnesota, USA; Felix Rosenow, M.D., University Clinic Frankfurt-Epilepsy Center, Frankfurt, Hesse, Germany; Ahmed Sadek, M.B.B.Ch, M.S., FAAN, FACNS, FAES, Neurological Services of Orlando, P.A., Orlando, Florida, USA; Rosa Ana Saiz-Diaz, M.D., Hospital Universitario 12 de Octubre, Madrid, Spain; Juan Carlos Sanchez, M.D., Hospital Vithas La Salud, Granada, Spain; Andreas Schulze-Bonhage, M.D., Ph.D., University Clinic Freiburg, Freiburg, Breisacher, Germany; Pedro Serrano-Castro, M.D., Ph.D., Hospital Regional De Malaga, Malaga, Province of Malaga, Spain; Jose Serratosa, M.D., IIS-FJD (Fundacion Jimenez Diaz), Madrid, Spain; Michael Sperling, M.D., Thomas Jefferson University, Philadelphia, Pennsylvania, USA; Claude Steriade, M.D., NYU Comprehensive Epilepsy Center, New York, New York, USA; William Tatum, D.O., Mayo Clinic–Jacksonville, Jacksonville, Florida, USA; Christian Tilz, M.D., Krankenhaus Barmherzige Brüder Regensburg, Regensburg, Bavaria, Germany; Rafael Toledano, M.D., Hospital Ramón y Cajal, Madrid, Spain; Manuel Toledo, M.D., Ph.D., Vall d'Hebron Hospital, Barcelona, Spain; Vicente Villanueva, M.D., Hospital Universitario y Politécnico La Fe, Valencia, Spain; David Vossler, M.D., UW Valley Medical Center, Renton, Washington, USA.
Open Research
DATA AVAILABILITY STATEMENT
Due to the sensitive nature of the data and the need to protect participant confidentiality, the data will not be shared.