Voxel-based and surface-based cortical morphometric MRI applications for identifying the epileptogenic zone: A narrative review
Abstract
Approximately 40% of patients with drug-resistant epilepsy referred for surgical evaluation have no epileptogenic lesion on MRI (MRI-negative). MRI-negative epilepsy is associated with poorer seizure freedom prognosis and has therefore motivated the development of structural post-processing methods to “convert” MRI-negative to MRI-positive cases. In this article, we review the principles, advances, and challenges of voxel- and surface-based cortical morphometric MRI techniques in detecting the epileptogenic zone. The ground truth for the presumed epileptogenic zone in imaging studies can be classified into lesion-based (MRI lesion mask or histopathology) or epileptogenicity-based ground truth (anatomical-electroclinical correlations or resections that lead to seizure freedom). Voxel-based techniques are reported to have a 13%–97% concordance rate, while surface-based techniques have 67%–92% compared to lesion-based ground truths. Epileptogenicity-based ground truth may be more relevant in the case of MRI-negative cases; however, the sensitivity and concordance rate (voxel-based technique 7.1%–66.7%, and surface-based technique 62%) are limited by the reliance on scalp EEG and qualitative analysis of seizure-onset pattern. The use of stereo-EEG and quantitative EEG analysis may fill this gap to evaluate the correlation between cortical morphometry results and electrophysiological epileptogenic biomarkers of the epileptogenic zone and help improve the yield of structural post-processing tools.
Plain Language Summary
Locating the epileptogenic zone (the brain area that is responsible for seizure generation) is important to diagnose and plan epilepsy treatments. An abnormal brain imaging (MRI) result can help clinical decision-making; however, around 40% of patients have normal MRI results (MRI-negative). We are reviewing the potential of two advanced MRI methods (voxel- and surface-based cortical morphometry) to localize the epileptogenic zone in the presence or absence of visible MRI abnormalities. We also describe the current challenge of applying the above methods in daily clinical practice and propose using advanced brain recording analysis to aid this translation process.
Key points
- The high rate of MRI-negative drug-resistant epilepsy (35%–44%) has pushed the development of cortical morphometric MRI methods (voxel- or surface-based) to convert "MRI-negative" cases to MRI-positive ones.
- We reviewed the advances of the cortical morphometric MRI studies compared to presumed epileptogenic lesions (radiological or histopathological-based) and epileptogenicity-based ground truths (epileptogenic zone based on anatomical-electroclinical correlations or resections leading to seizure freedom).
- Given the limitations of qualitative scalp EEG as a ground truth, the use of intracranial EEG e.g. stereo-EEG and its quantitative biomarkers may fill this gap to improve the yield of cortical morphometry techniques.
1 INTRODUCTION
Epilepsy is one of the most common neurological conditions, with a prevalence of 7.6 per 1000 population.1 Around a third of epilepsy cases are drug-resistant, for which, in selected patients, resective surgery is an effective treatment compared to the optimal pharmacological treatments.2 One of the primary factors that influence surgical success is epileptogenic lesion detection, which heavily depends on neuroimaging, particularly magnetic resonance imaging (MRI).3 Reports from newly diagnosed epilepsy cohorts described the rate of “MRI-negative epilepsy” from 65% to 81%; however, these were obtained across different age ranges, MRI field strengths, or mixed focal and generalized epilepsy cohorts.4-7 While only a small portion of MRI-negative cases are surgical candidates, MRI-negative epilepsy is associated with a less favorable prognosis for seizure control following epilepsy surgery compared to “MRI-positive” cases, as the epileptogenic zone (EZ) localization is more challenging (and potentially diffuse) in the absence of an epileptogenic lesion.8, 9 It also contributes to a significant portion of patients referred to tertiary epilepsy surgical centers, reaching 35%–44% of all cases.3, 10 Unlike concordant “MRI-positive” patients who can directly proceed to surgery, patients with MRI-negative epilepsy often require invasive electroencephalography (EEG) to proceed to surgery, which is resource-intensive and associated with non-negligible complication rates.11, 12 The absence of a focal abnormality on structural MRI is negatively associated with identifying a focal seizure-onset zone (SOZ) on stereo-electroencephalography (SEEG).13 Additionally, in a large SEEG series, MRI-negative status independently predicted unfavorable outcomes after SEEG-guided surgery.14
Lesion detection is influenced by several factors, including histopathologic abnormality, image quality, and reader expertise. Structural MRI is the first imaging technique performed in epilepsy presurgical evaluation before additional imaging techniques are considered. Besides the T1-weighted (T1-w) sequence, the addition of other sequences, for example, T2-weighted (T2-w), fluid-attenuated inversion recovery (FLAIR) or contrasts, for example, double inversion recovery (DIR), T2-relaxometry, and magnetization transfer ratio imaging (MTI) may improve the yield of visual detection.15 In 2019, the International League Against Epilepsy (ILAE) recommended the harmonized neuroimaging of epilepsy structural sequences-MRI (HARNESS-MRI) protocol composed of 3D millimetric T1-w, 3D FLAIR, and 2D sub-millimetric T2 images.16 Ultra-high field scanners (7T and beyond) provide even better signal-to-noise (SNR) and contrast-to-noise ratio (CNR) compared to the widely available 3T MRI, potentially allowing better visualization and delineation of smaller structures.17, 18 However, this comes with the cost of increased artifacts and potential incidental result detection, as the evidence that 7T MRI slightly improved pick-up rates of presumed epileptogenic lesions comes from small populations.18-20 At present, these ultra-high scanners are also not clinically readily available in many centers due to high costs, safety issues, and patient comfort.
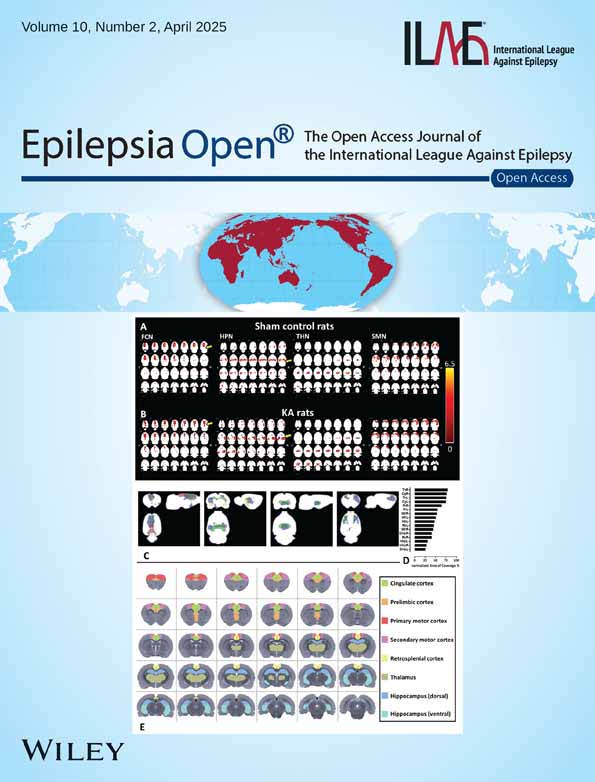
Cortical morphometry using voxel- or surface-based techniques may fill this gap by turning the “MRI-negative” status to “MRI-positive” by quantifying structural metrics, for example, volume, shape, and texture that are too subtle for human eyes. Since the introduction of both techniques, these methods have been utilized in epilepsy research mainly to aid in identifying an epileptogenic lesion or potential EZ. Appropriate “ground truth” selection is essential for adequately assessing and training quantitative MRI techniques. We propose two major groups of clinical ground truths in structural MRI post-processing studies: presumed epileptogenic lesions (radiological/histopathological-based) and epileptogenicity (EZ)-based (Table 1). Each ground truth has advantages and disadvantages. Although lesion borders can be clearly delineated from MRI (if visible) or histopathology, some lesions may be associated with extensive epileptogenic networks beyond the observed border, for example, focal cortical dysplasia (FCD) or hippocampal sclerosis (HS).21-23 Talairach and Bancaud24 defined the EZ based on the anatomical-electroclinical correlations using the observed seizure onset zone (SOZ), while Lüders et al.25 defined it as the minimal area that needs to be resected to achieve seizure freedom. The Bancaud definition allows the EZ to be hypothesized regardless of whether a resection can be safely performed (e.g., large, diffuse, or network-organized EZ). However, this view is limited by the spatial scope of the sampling electrodes, for example, in the case of SEEG, where only a limited number of electrodes can be implanted. Regarding postoperative results, in practice, not all patients can proceed to surgical resection, which is also a limitation of using histopathology for ground truth. This will also require long-term follow-up data to obtain seizure-free outcomes after a set amount of time (usually 12–24 months minimal). Moreover, it is hard to predict whether the actual resection margin overlaps exactly with the actual EZ itself. In clinical settings, the EZ hypothesis (and subsequently intracranial EEG planning) in MRI-negative cases is not made based on electrophysiology signals alone but also defined based on inputs of different modalities, such as neuropsychological testing, 18fluorodeoxyglucose positron emission tomography (18F-FDG-PET), subtraction ictal SPECT co-registered to MRI (SISCOM), magnetoencephalography (MEG), etc. This narrative review aims to describe the general advances of two cortical morphometrics MRI methods, that is, voxel-based and surface-based analysis, in detecting the presumed epileptogenic lesion and/or epileptogenicity-based ground truth (see Figure 1). We focused on cortical gray matter quantification techniques since epileptogenic activities obtained from WM contacts in SEEG recordings are less well-understood than GM, which warrants further investigation.26, 27 In closing, we also review current challenges and highlight future opportunities to apply quantitative electrophysiology methods from SEEG to improve the validation of these cortical morphometry techniques.
| Type | Ground truth | Advantages | Disadvantages |
|---|---|---|---|
| Lesion-based | MRI lesion mask |
|
|
| Histopathology |
|
|
|
| Epileptogenicity-based | EZ (anatomical electroclinical correlations) |
|
|
| EZ (resection that leads to seizure freedom) |
|
|
|
| Other | PET/SPECT |
|
|
- Abbreviations: EZ, epileptogenic zone; PET, positron emission tomography; SEEG, stereo-electroencephalography; SOZ, seizure onset zone; SPECT, single-photon emission computed tomography.
2 METHODS
We performed a literature search on PubMed under the following terms (epilepsy OR focal cortical dysplasia OR hippocampal sclerosis) AND (MRI OR imaging) AND (morphometr* OR voxel-based morphometr* OR surface-based morphometr* OR voxel* OR surface* OR volumetr* OR deep learning OR machine learning) which resulted in 3237 articles published from the year 2000 to 2024. We then selected original articles published in English based on their relevance to the general aim of this review and reviewed them grouped by the MRI techniques and ground truth used. (Articles that did not use a voxel-based or surface-based metric were not considered (e.g., studies using cortical/subcortical volumes, machine learning studies using the whole image as input, etc.).).
3 VOXEL-BASED TECHNIQUES
Like pixels in two-dimensional images, the voxel is the smallest unit of a three-dimensional image. Voxel-based morphometry (VBM) allows the voxel-wise quantification of gray matter volumes (GMV) and concentration (GMC) in T1-w images.28 Common packages for VBM analysis include statistical parametric mapping (SPM, fil.ion.ucl.ac.uk/spm) including its computational anatomy toolbox (CAT, neuro-jena.github.io/cat), and FMRIB software library (FSL, fmrib.ox.ac.uk/fsl). The preprocessing steps of VBM include tissue segmentation into different classes (gray matter, white matter, and cerebrospinal fluid), spatial normalization, and spatial smoothing. Since the segmentation step is voxel-based, VBM results are prone to partial volume effects if a voxel contains multiple tissue types in areas with poor gray-white matter contrasts (e.g., gray-white matter junction, basal ganglia, thalamus, and brainstem). Tissue segmentation and classification can be improved by incorporating additional sequences, for example, T2-w or FLAIR images, or by improving the acquisition protocol, for example, magnetization-prepared two rapid acquisition gradient echoes (MP2RAGE) for T1-w scans.29, 30 Compared to the modulated GMV, the non-modulated GMC has been reported to be more sensitive to detecting structural changes, possibly due to the compensatory effect from the modulation step. Given that voxel-wise calculations may involve thousands of voxels at once, multiple comparison corrections, for example, family-wise error (FWE) rate or false discovery rate (FDR), are required to avoid false positives (type I error). Furthermore, in the individual versus group setting, the use of nonparametric statistical tests may be more appropriate to determine voxel-based analysis results.
In group settings, VBM has been implemented to observe structural differences in focal epilepsy, particularly unilateral TLE.31, 32 Reduced GMC has been consistently reported on the ipsilateral side in unilateral HS cases.33-35 GMC changes were also observed in extra-hippocampal structures either in the surrounding temporal lobe or extra-temporal regions, including the thalamus and parietal cortex.32, 36 Galovic et al.37 performed VBM on the postoperative TLE group and found that seizure-free patients had lower ipsilateral piriform cortex GMV post-surgery compared to the non-seizure-free group, showing the importance of piriform cortex resection in TLE surgery.
Huppertz et al.38 introduced the SPM-based morphometric analysis program (MAP), which is a commercial program primarily designed for FCD detection, although it can be used for other malformations of cortical development. MAP results in three types of morphometric maps measuring three structural metrics, that is, “junction map” (blurring of GM-WM junction), “extension map” (abnormal cortical gyration), and “thickness map” (cortical thickness). Similarly, improved acquisition with the MP2RAGE protocol has also been shown to increase the sensitivity of MAP-based algorithms in detecting FCD.39 An artificial neural network (ANN) has been incorporated into MAP18 for rapid FCD detection in MRI-negative cases.40
3.1 Voxel-based techniques versus presumed epileptogenic lesions
In MRI-positive cases, increased GMC was reported to have a 29% to 82% concordance rate with the ground truth, which was higher than decreased GMC (26%–30%), particularly in FCD cases.41-44 However, in MRI-negative FCDs, GMC increase was only concordant in 37.5% of the cohort.45 Focke et al. and Huppertz et al. demonstrated that voxel-based analysis (VBA) on normalized FLAIR signal intensity (nFSI) had 88% concordance with MRI-positive FCD cases and 97% with HS.46, 47 When compared to GMV, nFSI showed a higher concordance rate (13% vs. 60%) in MRI-positive FCD cases.41 Lindig et al.30 showed that multispectral VBAs also improved FCD (T1 + FLAIR and T2 + FLAIR) and HS (T1 + T2) detection. Gill et al.48 performed a multicenter validation of a deep-learning approach based on patch extraction that classified lesional and non-lesional voxels. This algorithm was validated across mixed MRI-positive and histologically validated MRI-negative FCD cohorts with a yield of 83% sensitivity and 89% specificity.
When compared to visual analysis to detect FCDs, MAP performed well for FCD type IIa but was comparable to visual analysis in detecting FCD type IIb.49 Wong-Kisiel et al.50 compared different types of MAP maps and found that the extension map exhibited higher sensitivity (74%) than the junction map (64%) in FCD detection. However, MAP-positive areas correlated not only with FCD but also with other pathologies (e.g., HS) and normal tissues.51 Martin et al.41 compared different VBA modalities and showed that the combination of nFSI and junction MAP had the best sensitivity and specificity of (18.2% both) in MRI-negative histologically verified FCD patients compared to the other combinations (among nFSI, junction map, GMC, and GMV). Similar to voxel-based analysis, the T2-based junction map also provided a better FCD delineation compared to T1-based maps.52 House et al.53 configured a CNN using MAP-based images (extension and junction maps), resulting in 74.1% detection of histologically confirmed FCDs. David et al.40 employed a MAP-based ANN trained on radiologically and/or histologically defined FCD, which had 81% sensitivity and 84% specificity after external validation. However, as this network employed an adult population as a healthy control, adjustment may be required for application in younger age groups. Performing MAP on 7T images yielded 25% more lesions than MAP on 3T images on the same patients.54 Similar to 3T, Chen et al.55 showed that the 7T junction map provided the best result compared to the extension and thickness maps (90.9% vs. 9.9% and 0% in 11 patients) (Table 2).
| Type | Study | Demographics | Modalities | Features | Ground truth | Results |
|---|---|---|---|---|---|---|
| Voxel-based technique |
Huppertz et al. (2005) N = 25 |
Adults and pediatrics, ETLE & TLE, 14% MRI-negative | T1, FLAIR (1.5T) | Jun., Ext. | FCD (histology-confirmed) | Concordance 84% |
|
Bonilha et al. (2006) N = 11 |
Adults and pediatrics, ETLE & TLE | T1 (2T) | GMC | FCD (histopathology) | Concordance 81.8% | |
|
Colliott et al. (2006) N = 27 |
Adults, ETLE, 100% MRI-positive | T1 (1.5T) | RI | FCD | Concordance 78% | |
|
Focke et al. (2008) N = 25 |
Adults, TLE & ETLE, 0% MRI-negative | T1, FLAIR (3T) | nFSI | FCD (MRI-positive) | Concordance 88% | |
|
Wagner et al. (2011) N = 91 |
Adults and pediatrics, ETLE, 14% MRI-negative | T1 (1.5/3T) | Jun., Ext. | FCD II a (histology-confirmed) | Detection rate 82% | |
| FCD IIb (histology-confirmed) | Detection rate 92% | |||||
|
Huppertz et al. (2011) N = 103 |
Adults, TLE, 9.7% MRI-negative | T1, FLAIR (3T) | nFSI | HS (MRI-positive) | Concordance 97% | |
|
Pail et al. (2012) N = 18 |
Adults, TLE, 38.9% MRI-negative | T1 (1.5T) | GMC | HS/FCD (histologically-confirmed) | Concordance 72.2% | |
|
Martin et al. (2017) N = 144 |
Adults and pediatrics, TLE & ETLE, 85.3% MRI-negative | T1, FLAIR (3T) | GMV, GMC, nFSI, Jun. | FCD (MRI-negative based on histopathology) | Sensitivity 18.2%, specificity 18.2% | |
|
Wong-Kisiel et al. (2018) N = 39 |
Adults and pediatrics, ETLE, 30.8% MRI-negative | T1 (1.5/3T) | Jun., Ext. | FCD (MRI-negative based on histopathology) | Sensitivity 74% (ext)., 64% (jun.) | |
|
Gill et al. (2021)a N = 23 (validation) |
Adults and pediatrics, TLE&ETLE, 70% MRI-negative | T1, FLAIR (3T) | Signal intensity | FCD (MRI-positive & histology-verified MRI-negative) | Sensitivity 83%, specificity 89% | |
|
House et al. (2021)a N = 100 (validation) |
Adults and pediatrics, 100% MRI-negative | T1, FLAIR (3T) | Jun., Ext. | FCD (histopathology) | Accuracy 74.1% | |
|
David et al. (2021)a N = 58 (validation) |
Adults and pediatrics, 59% MRI-negative | T1, FLAIR (3T) | Ext., Jun., Thi. | FCD (MRI-positive & histology-confirmed MRI negative) | Sensitivity 81% specificity 84% | |
|
Isen et al. (2021) N = 42 (MRI-positive group) |
Adults, ETLE, 100% MRI-positive | T1, T2, FLAIR (3T) | GMC, nFSI | HS Majority (47.6%) |
Sensitivity 29% (Increased GMC) Sensitivity 26% (Decreased GMC) |
|
| Surface-based technique |
Hong et al. (2014)a N = 33 |
Adults, ETLE, 100% MRI-Negative | T1, FLAIR (3T/1.5T) | CTh, SD, Curv, RI, Gradient | Histology-confirmed FCD type II (3T) | Sensitivity 74% specificity 100% |
| Histology-confirmed FCD type II (on 1.5T) | Sensitivity 71% specificity 95% | |||||
|
Ahmed et al. (2015)a N = 31 |
Adults, ETLE, 77.4% MRI-negative | T1, FLAIR (3T) | CTh, GWC, SD, Curv., Jacobian distortion | FCD (MRI-positive) | Detection rate 85.7% | |
| FCD (histology-confirmed MRI-negative) | Detection rate 58% | |||||
|
Adler et al. (2017)a N = 27 |
Pediatrics, ETLE, 100% MRI-positive | T1, FLAIR (1.5T) | CTh, GWC, Curv, SD, FI, LCD, doughnut maps | FCD | Sensitivity 73% | |
|
Tan et al. (2018)a N = 28 |
Adults and pediatrics, TLE & ETLE, 64.3% MRI-negative | T1, FLAIR (3T) | CTh, SD, RI | FCD (MRI-positive & histologically confirmed negative) | Detection rate 82.0% | |
|
Jin et al. (2018)a N = 61 |
Adults and pediatrics, TLE & ETLE, 27.9% MRI-negative | T1, FLAIR (3T) | CTh, GWC, SD, Curv, LCD, doughnut maps | FCD (MRI-positive & histologically-confirmed MRI-negative) | Sensitivity 73.7% Specificity 90.0% | |
|
Mo et al. (2019)a N = 80 |
Adults, TLE, 31.3% MRI-negative | T1 (3T) | Curv, GM/WM blurring, hippo volume, temporal horn volume | HS (MRI-positive & negative) | Detection rate 97.9% (SVM) 95.8% (log.) | |
| HS (MRI-positive) | Detection rate 98.2% (SVM) 96.4% (log.) | |||||
| HS (histology-confirmed MRI-negative) | Detection rate 88.0% (SVM) 96.0% (log.) | |||||
|
Caldairou et al. (2021)a N = 56 (validation) |
Adults, TLE, 58.0% MRI-negative | T1, T2, FLAIR (3T) |
Columnar volume, T2 intensity, FLAIR/T1 intensity |
HS lateralization (histopathology & lesion mask) | Accuracy 93% (mixed MRI-positive & negative) | |
| Accuracy 76%–90% (MRI-negative, two validation cohorts) | ||||||
|
Spitzer et al. (2022)a N = 571 |
Adult and pediatrics, TLE & ETLE, 62.9% MRI-Negative | T1, FLAIR (1.5/3T) | CTh, GWC, Curv, SD, Int Curv, FI | FCD (MRI-positive & negative) | Sensitivity 67% | |
| FCD (MRI-negative, histology confirmation) | Sensitivity 54% | |||||
| FCT type II B & seizure-free | Sensitivity 85% |
- Abbreviations: CTh, cortical thickness; Curv., cortical curvature; ETLE, extra-temporal lobe epilepsy; Ext., extension map from MAP; GMC, GM concentration; GWC, GM-WM contrast; ICEEG, intracranial electroencephalography; Int. Curv., internal curvature; Jun., junction map from MAP; LCD, local cortical deformation; log, logistic regression model; nFSI, normalized FLAIR signal intensity; RI, relative intensity; SD, sulcal depth; SVM, support vector machine; TLE, temporal lobe epilepsy.
- a Machine-learning studies.
3.2 Voxel-based techniques versus Epileptogenicity-based ground truth
The rate of concordance between VBA on T1 (0%–31.8%), nFSI (7.1%–14.2%), and MAP (27.9%) with scalp-based electroclinical findings tends to be low (see Table 3).41, 56-58 When compared to the presumed EZ, the multispectral combination of T1 + FLAIR yielded a higher concordance rate than T1-w only (46.2% vs. 30.8%).59 Isen et al.43 defined the ground truth as a 4 mm sphere from SEEG contacts marked as involved in the SOZ and showed that nFSI had a higher sensitivity to detect the EZ at 28% compared to increased or even decreased GMC (22% and 17%). Using MAP on 7T scans, Wang et al.54 observed that 13 of 16 patients with visual or MAP-positive findings had full or partial concordance with SOZ from intracranial EEG.
| Type | Study | Demographics | Modalities | Features | Ground truth | Results |
|---|---|---|---|---|---|---|
| Voxel-based technique |
Salmenpera et al. (2007) N = 93 |
Adults, TLE and ETLE, 100% MRI-negative | T1 (1.5T) | GM density | Video-EEG | 9.0% concordance rate |
|
Focke et al. (2009) N = 70 |
Adults, TLE, 100% MRI-negative | T2, FLAIR (3T) | nFSI | Video-EEG (ictal/interictal) |
7.1% concordant localization 11.8% concordant lateralization |
|
|
Wagner et al. (2011) N = 91 |
Adults and pediatrics, ETLE, 14% MRI-negative | T1 (1.5/3T) | Jun., Ext. | Seizure freedom |
71.8% (MRI+ MAP+) 72.7% (MRI− MAP+) 71.4% (MRI+ MAP−) |
|
|
Riney et al. (2012) N = 22 |
Pediatrics, TLE & ETLE, 63.6% MRI-negative | T1, T2, FLAIR (1.5T) |
VBM-T1 VBM-FLAIR |
Ictal EEG, SPECT (N = 14/22) | 14.2% concordance rate (VBM-FLAIR) | |
|
Wang et al. (2015) N = 150 |
Adults and pediatrics, TLE & ETLE, 100% MRI-negative | T1 (1.5/3T) | Jun. | Seizure freedom (N = 50/150) |
90.0% (MAP+ region completely resected) 33.3% (MAP+ region partially/not resected) |
|
|
Martin et al. (2017) N = 144 |
Adults and pediatrics, TLE & ETLE, 85.3% MRI-negative | T1, FLAIR (3T) | GMV, GMC, nFSI, Jun. | Video-EEG, auxiliary PET, MEG |
31.8% concordance rate (GMC) 10.1% concordance rate (nFSI) 8.5% concordance rate (GMV) 27.9% concordance rate (MAP) |
|
|
Kotikalapudi et al. (2018) N = 13 |
Adults, ETLE, 100% MRI-negative | T1, T2, FLAIR (3T) | GMC, VBM-FLAIR | Video EEG, iEEG (n = 5), neuropsychology, auxiliary PET/CT (n- = 6) |
46.2% concordance rate (T1 + FLAIR) 30.8% concordance rate (T1) |
|
|
Wong-Kisiel et al. (2018) N = 39 |
Adults and pediatrics, ETLE, 30.8% MRI-negative | T1 (1.5/3T) | Jun., Ext. | Seizure freedom | 65.4% (Jun.), 66.7% (Ext.) lobar concordance | |
| Resection area | 66.7% (Jun.) 71.8% (Ext.) lobar concordance | |||||
|
Wang et al. (2020) N = 67 |
Adult and pediatrics, TLE and ETLE, 77.6% MRI-Negative | T1 (7T) | Jun. | SOZ (iEEG) (n = 16) | 81% concordance (from MAP-guided or visual review) | |
| Seizure freedom (n = 12) | 83% concordance (from MAP-guided or visual review) | |||||
|
Isen et al. (2021) N = 27 (MRI-Negative group) |
Adults, ETLE, 100% MRI-negative | T1, T2, FLAIR (3T) | GMC, nFSI | SOZ (SEEG) |
22% sens. 98% spec. (increased GMC) 17% sens. 97% spec. (decreased GMC) 28% sens. 98% spec. (increased FLAIR) |
|
| Surface-based technique |
Wagstyl et al. (2020)a N = 21 |
Pediatrics, ETLE, 33.3% MRI-negative | T1, FLAIR (3T) | CTh, GWC, Curv, SD, Int Curv, FI | SOZ (SEEG) | 62% Co-localization rate |
- Abbreviations: CTh, cortical thickness; Curv., cortical curvature; ETLE, extra-temporal lobe epilepsy; Ext., extension map from MAP; FI, FLAIR index; GWC, GM-WM contrast; iEEG, intracranial electroencephalography; Int. Curv., internal curvature; Jun., junction map from MAP; MEG, magnetoelectroencephalography; SD, sulcal depth; sens., sensitivity; spec, specificity; TLE, temporal lobe epilepsy.
- a Machine-learning studies.
Regarding postoperative seizure freedom, Bonilha et al.42 observed that surgical refractory is found in patients with excessive GMC increase from VBM-T1 beyond the visualized FCD border in 71% of 11 patients; however, those patients often had multifocal EEG findings. Resection of the area that was concordant with nFSI abnormalities was associated with 7.33 odds of having seizure freedom; however, this was an uncorrected result.41 Several studies have described the rate of seizure freedom after the resection of MAP-positive regions, with results ranging from 65% to 90%.49-51 Wang et al.51 reported that in MRI-negative patients, 90% of patients with complete removal of the junction MAP-positive region were seizure-free; however, 30% of patients without complete removal of the MAP-positive region were also seizure-free. Additionally, multiple MAP-positive regions were also observed in non-seizure-free patients, with the majority of the unresected MAP-positive region located ipsilateral to the resection.51 In contrast to the previous study, the rate of seizure freedom was higher in patients with partial rather than complete MAP-positive resection (78.6% vs. 57.1%).50 In 7T scans, 83% of 12 patients with resected positive findings from visual or MAP-guided review were seizure-free after a year.54
In summary, VBA incorporating nFSI showed increased sensitivity and accuracy compared to VBM on T1-w only, particularly in the MRI-positive cohort. The ability of the voxel-based method to detect lesions on MRI-negative or with epileptogenicity-based ground truth is less impressive, particularly for VBM on T1-w. There is evidence that complete resection of MAP-positive areas is correlated with seizure freedom; however, a larger study is required.
4 SURFACE-BASED TECHNIQUES
Surface-based morphometry (SBM) enables vertex-based cortical segmentation and structural comparison of the cortical surface.60, 61 SBM tessellates the cortical surface into polygons and performs statistics at each meeting point of the polygons (vertices). This principle allows the quantification of cortical and subcortical morphology without being affected by the partial volume effects, unlike VBM. Tissue segmentation using SBM may also increase the chance of detecting signals from subtle abnormalities, particularly in convolved areas, for example, bottom-of-sulcus. This is achieved by performing boundary delineation of the GM-WM junction and pial surface, which appreciates cortical geometry and sulcal folding. FreeSurfer (surfer.nmr.mgh.harvard.edu) is the most used package for SBM, but other packages, such as CAT12 or CIVET (bic.mni.mcgill.ca/ServicesSoftware/CIVET), also allow surface-based quantification. Besides FreeSurfer, FastSurfer is a deep-learning package to perform surface-based segmentation.62 The principal steps of SBM include image registration and normalization, smoothing, surface extraction, surface inflation, and surface mapping. The registration step in SBM is performed by transforming the convoluted cortical surface into a sphere to establish a surface-based coordinate system. However, since FreeSurfer's prior is based on healthy brains, there is a need for manual quality assurance after post-processing steps, as the surface reconstruction is sensitive to morphological alteration, for example, the presence of large cortical lesions or prior resection, which is often encountered in drug-resistant epilepsy cases, though not in MRI-negative cases.
Cortical morphologies are affected by the underlying cytoarchitecture; for example, FCD is associated with abnormal neuronal morphology and density, resulting in cortical thickening. The most commonly applied surface-based metric in SBM is cortical thickness, which is determined as the average of the shortest distance from each vertex to the other surface (GM/WM boundary or pial surface). This aims to bisect the horizontal layers of the cortical column. The largest epilepsy imaging study (ENIGMA-epilepsy) utilized cortical thickness to explore the spectrum of TLE. Left-side mesial TLE-HS demonstrated more extensive cortical thickness reductions in the temporal and extratemporal lobes compared to right mesial TLE-HS.63 A longitudinal study showed that widespread progressive cortical thinning presents in focal epilepsy patients, particularly in the ipsilateral lobes of the epileptic focus.64
Other morphological parameters of SBM include cortical curvature, gyrification index, and sulcal depth. In bottom-of-sulcus dysplasia (BOSD), the affected sulci were often deeper compared to healthy controls.65 Other features include texture-based features, for example, GM-WM contrast and FLAIR intensity. Exploiting multiple features can be more advantageous in detecting lesions rather than depending on a single parameter; for example, volume reduction alone is not sensitive enough to detect HS.66 The multi-center epilepsy lesion detection (MELD) is an initiative to employ a machine-learning classifier that incorporates surface-based morphological and texture features to quantify the local variation to detect occult FCDs.67
4.1 Surface-based techniques versus presumed epileptogenic lesions
Given the unique hippocampal morphology, surface-based segmentation can be very useful for quantifying hippocampal volume and other surface-based metrics for subtle HS detection. Using GM/WM blurring, hippocampal curvature, and hippocampal and temporal horn volumes, Mo et al.68 developed a support vector machine (SVM) and logistic regression model that detected 98.2% and 96.4% of MRI-positive HS, respectively. On the other hand, the logistic regression model performed better than the SVM in detecting MRI-negative HS (96.0% vs. 88.0%). Caldairou et al.69 developed a surface-based classifier that predicted 93% of HS lateralization in mixed MRI-positive and histopathology-confirmed MRI-negative patients employing columnar volume, T2 intensity, and FLAIR/T1 intensity. Compared with columnar volume, the combination of T2 and FLAIR/T1 signal intensities performed better to lateralize in the MRI-negative-only cohort, with an accuracy of 76%–90%.
Similarly, surface-based parameters are very useful to detect FCD, given that histologically, FCD is characterized by cortical disorganization and associated with cellular morphological abnormalities (large dysmorphic neurons, balloon cells on FCD IIb) and pathological changes (gliosis due to astrocyte proliferation and hypertrophy). A single surface-based feature is often sensitive but not specific to the lesioned area, given the presence of variations in the healthy brain; for example, the motor cortex is thicker than the somatosensory cortex.67, 70 Combining multiple features, such as cortical thickening and GM-WM blurring, for example, yielded the most optimum sensitivity (92%) and specificity (96%) when compared to the lesion mask in MRI-positive FCD cases.71 Multiple parameters may also aid differentiation between FCD type II subtypes radiologically; for example, FCD IIb demonstrated increased cortical thickness, sulcal depth, and FLAIR intensity compared to FCD IIa in a multimodal MRI study.72 Similar to FCD, the combination of multiple surface-based parameters may also aid lesion identification in HS. In a pediatric HS cohort, the ipsilateral temporal lobe showed increased GM-WM contrast and FLAIR intensity compared to the contralateral side.73 Incorporating multi-modal imaging is also helpful, as not all features are abnormal in all lesions, and extra-lesional noise artifacts are less likely to present in multiple modalities.
There have been several efforts to produce automated classifiers based on SBM to detect subtle lesions, particularly FCD. Hong et al.70 used an FCD-specific automated classifier based on morphology and intensity features, which detected 71% (1.5T) and 74% (3T) FCD type II based on histopathology and lesion mask. However, this method also detected at least one extra-lesional cluster in 50% of cases, particularly characterized by abnormal sulcal depth. The MELD classifier demonstrated that GM-WM contrast and FLAIR intensity were most discriminatory for FCD detection in lesional MRI compared to other parameters, with an overall sensitivity of 70%–73%.67, 74, 75 In a multi-center validation, Spitzer et al.76 demonstrated that the MELD classifier showed sensitivity and specificity of 67% and 54%, respectively, on mixed MRI-positive and -negative FCD cases. This algorithm provided the best detection rate for FCD type IIB (72.7%) while also detecting 69% of MRI-negative histologically proven FCD.76
4.2 Surface-based techniques versus Epileptogenicity-based ground truth
Thesen et al. showed concordant cortical thickness changes with intracranial EEG and MRI in two seizure-free patients.71 Using a surface-based classifier, Hong et al. showed that 6 of 10 patients with detected clusters who were allocated for surgery were seizure-free; however, only three patients had no extra-lesional clusters, and EEG anomalies were detected in the rest of the detected extra-lesional clusters.70 However, 4 of 5 patients without detected clusters from the classifier were also seizure-free. Using the MELD classifier, Spitzer et al.76 showed that the sensitivity of the algorithm increased from 67% to 85% if performed on carefully selected MRI-positive type IIb FCDs who were seizure-free.
Wagstyl et al. conducted a retrospective study in an SEEG cohort showing that the MELD algorithm-based classifier trained on MRI-positive patients could detect clusters with 62% co-localization with focal SOZ in 21 patients.77 Seizure freedom was achieved by 69.2% of patients with concordant SEEG-classifier results; however, 37.5% of patients without concordance were also seizure-free.77 It must be noted that they applied a more extensive circumferential margin from contacts exhibiting ictal activities from the VBM study by Isen et al. (up to 10 mm vs. 4 mm).43 MELD has been trialed to complement SEEG electrode implantation planning, which helped in altering the scheme in one of 19 patients.78 The algorithm also detected the cluster involved in the EZ in 15.8% of patients, although these clusters had been identified as implantation targets before the additional information from the algorithm was added. In summary, surface-based methods could capture variations of cortical morphology, which correlated with epileptogenicity-based ground truth, although fewer studies were trying to address this correlation, particularly in different clinical cohorts (e.g., adults, TLE patients). However, whether this method is applicable in clinical settings requires further investigation, as the initial evidence was obtained from a small population set.
5 CURRENT CHALLENGES IN IMPROVING CORTICAL MORPHOMETRICS MRI TECHNIQUES
The effort to translate cortical morphometrics methods to detect the presumed EZ (which correlates with a surgical resection plan) into the clinical setting faces several challenges and limitations. First, epilepsy is a clinically diverse condition. FCD (particularly type Ic) is the most common underlying pathology in a mixed MRI-negative cohort, followed by gliosis, hamartia-gliosis, and HS.79 The proportion of subtle FCD that are missed by visual examinations was reported to be around 50%–80%, particularly for small BOSD.65 At least 30% of all TLE cases are MRI-negative, with HS and encephaloceles as the two most frequent underlying pathologies in this MRI-negative group.8 The sensitivity rate may vary depending on the underlying pathology and location, e.g., left and right HS.80, 81 Some techniques and algorithms were mainly trained to detect only a specific pathology, for example, FCD, and may not be suitable for detecting other pathologies.38, 67
Second, the reported yield of VBM and SBM in epilepsy literature is highly variable due to technical aspects. It is understood that introducing additional sequences (FLAIR or T2-w) besides T1-w (on 3T images) improved results as it enhanced tissue classification and possibly captured better structural differences.30, 43, 59, 67 However, false-positive rates are common, for example, as reported by Martin et al. and Wang et al. for VBM (68%) and MAP (27%) studies, respectively, emphasizing the need to apply adequate multiple comparison correction and human re-interpretation.41, 51 The diverse availability of post-processing software and algorithms, even in the case of version updates, may also lead to slight variations in the results.82, 83 There is a lack of studies directly comparing the yield of voxel- and surface-based techniques to the same cohort in epilepsy, particularly MRI-negative. However, given that both techniques apply different principles in measuring gray matter morphology, some authors argued that the relationship is complementary based on data from other diseases.84
Third, the definition of performance metrics for these methods (sensitivity, specificity, accuracy) is not standardized. There is a lack of consensus in defining “complete” and “partial” concordance; for example, a study used a 5% overlap threshold between lesion masks with VBM results.85 Introducing a border zone surrounding the lesion, for example, will increase the sensitivity rate compared to strictly confining on the manual lesion mask (67% vs. 59%).76 Non-invasive EEG studies also reported the presumed EZ as “lobar” and “hemispheric,” which covered a broad cortical area that may extend beyond the actual EZ.56 A study defined the level of concordance based on the extent of morphometry findings compared to the ictal onset zone into “concordant” (matched exactly), “concordant plus” (smaller ictal onset area), or “concordant minus” (larger ictal onset area).54 Several SEEG studies provided a more precise presumed EZ approximation, for example, by drawing a sphere within a certain radius from the contacts involved in the SOZ.43, 77 However, SEEG is limited in terms of coverage, and there is a chance that the implantation scheme will not capture the whole EZ. Using postoperative seizure freedom as ground truth also requires careful interpretation as there are two major postoperative classifications of seizure freedom (Engel and ILAE class), which require more prospective data regarding their inter-rater reliability.86
Many cortical morphometry studies often included patients from only one center, particularly in early-phase development studies. The use of limited patient populations/centers for automated algorithm development may introduce the issue of overfitting, where the algorithm works best using the data it was trained on but performs less when introduced to a new set of data. There have been efforts to conduct multi-center collaboration for ANN validation, such as for MELD or MAP18 classifiers, to address this limitation.40, 76 However, these were conducted retrospectively, which introduces a possible source of bias, particularly for SEEG planning, as MRI findings may influence the SEEG implantation scheme. The only trial that utilized a surface-based algorithm was conducted on a small sample size, which may not reflect the actual effect on the whole population.78 Future directions may incorporate performing this algorithm on ultra-high field images as the higher SNR leads to higher image quality but also poses additional challenges for algorithm development.54, 55 Other directions may involve using multi-modal parcellations involving functional or diffusion MRIs or even synthetic MRI images. We also highlight that deep learning models are associated with interpretability issues as the algorithms work in a “black box” manner, making it difficult for clinicians to retrospect the reasoning behind the results. An effort to complement the deep learning technique with interpretable results, such as the list of extracted voxel- or surface-based features, may overcome this limitation.
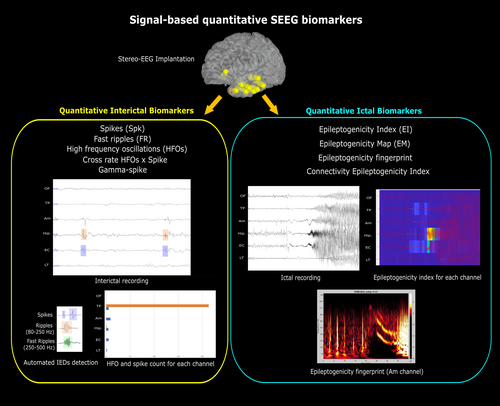
6 QUANTITATIVE EEG MARKERS AS ADDITIONAL GROUND-TRUTH
Using the EEG-based SOZ as the ground truth in MRI-negative cases has the advantage of being available for all patients irrespective of surgical decision. However, it faces a limitation due to the qualitative nature of SOZ interpretation, which may be rater-dependent. While intracranial EEG is not considered necessary if there is a “clear” surgical target, when indicated, quantitative SEEG biomarkers may be implemented to complement SOZ (and subsequently the EZ) determination in an objective manner. Currently available quantitative markers span from signal to network analysis from ictal and interictal recordings. Ictal markers, for example, epileptogenicity index,26 epileptogenicity map,87 epileptogenicity triad,88 epileptogenicity fingerprint,89 epileptogenicity rank,90 and connectivity epileptogenicity index,91 aim to characterize contacts associated with SOZ, thus revealing involved structures during the ictal period. The epileptogenicity index is a semi-quantitative technique that enables the classification of epileptogenic networks into the EZ, propagation zone, and non-involved cortex and has been used to quantify epileptogenicity properties in TLE, tuberous sclerosis complex, periventricular nodular heterotopia, polymicrogyria, and focal cortical dysplasia.26, 92-94 This method was also used to complement visual SOZ analysis as ground truth for 18FDG-PET results in malformations of cortical development.95 Other quantitative EEG measures, for example, epileptogenic zone fingerprint and epileptogenicity map, have also been utilized as ground truth for SISCOM and PET studies.96, 97 Besides ictal markers, interictal quantitative EEG markers, for example, corrected high-frequency oscillations (HFOs) or cross-rate between HFOs and interictal epileptiform discharges (IEDs), have demonstrated potential, although further evidence is required.98, 99 A meta-analysis showed that removing areas generating HFOs has been reported to be correlated with seizure freedom, particularly for fast ripples (250–500 Hz).100 Besides those signal markers, connectivity/network analysis for the interictal to ictal phase has also been introduced, including eigenvector centrality and neural fragility metrics.101, 102 Other network-based markers for the interictal period include the “source-sink” method,103 which is based on the hypothesis that the EZ is inhibited by other brain regions, as well as normative and network modeling for interictal recordings.104, 105
Although at present there are no perfectly accurate biomarkers for the presumed EZ, the addition of quantitative EEG measures may improve the objectivity and reproducibility of the epileptogenicity-based ground truth—and, therefore, the accuracy of cortical morphometry results (see Figure 2). This approach may be combined with other techniques utilizing SEEG electrodes, including cortical stimulation, to improve the definition of the epileptogenicity zone and, thus, the ground truth for cortical morphometry. The launching of open-source SEEG data such as iEEG.org,106 OpenNeuro,107 or Pennsieve (https://pennsieve.io/) also bears the potential to conduct a multi-center collaboration, which is necessary given that data from a single institute is often limited in providing statistical powers and thus the generalizability of the result.
7 CONCLUSION
The significant prevalence of MRI-negative drug-resistant epilepsy has led to advances in voxel- and surface-based methods to improve the detection of the presumed EZ. Some voxel- and surface-based algorithms have been developed and validated in multi-center settings and incorporated into clinical trials, although more work is required before these can be implemented in clinical settings. The incorporation of quantitative EEG markers may increase the objectivity and reproducibility of ground truth determination, which in turn will complement the optimization of these cortical morphometry techniques.
AUTHOR CONTRIBUTIONS
JB searched the literature and drafted the manuscript, tables, and figures. AN, BS, ML, PK, and TJO commented on and edited the text, including suggestions for additional sections and references. All authors read, edited, and approved the final manuscript prior to submission.
ACKNOWLEDGMENTS
JB is supported by the Monash University. AN is supported by an NHMRC Investigator Grant (APP2009152). TJO is supported by an NHMRC Program Grant (APP1091593) and an Investigator Grant (APP1176426). PK is supported by an NHMRC Investigator Grant (GNT2025849). Open access publishing facilitated by Monash University, as part of the Wiley - Monash University agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST STATEMENT
TJO's institution has received speaker/consultancy fees and research funding from Eisai, LivaNova, Supernus, Zynerba, Biogen, and UCB Pharma outside the submitted work. PK's institution has received speaker/consultancy fees from Eisai, LivaNova, and UCB Pharma outside the submitted work. AN's institution received speaker/consultancy fees from Eisai, LivaNova, and UCB Pharma outside the submitted work. The remaining authors have no conflict of interest to disclose.
ETHICS STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.