Multiscale Materials Imaging and Spectroscopy for Battery Materials
Funding: This work was supported by National Research Foundation of Korea, RS-2023-00247245.
ABSTRACT
Multiscale imaging and spectroscopy play a pivotal role in understanding the structural, chemical, and dynamic behavior of battery materials, providing critical insights that drive advancements in performance, longevity, and safety. This review provides a comprehensive analysis of various imaging techniques, from macroscopic tools like x-ray tomography to nanoscale methods such as atomic force microscopy and transmission electron microscopy. By categorizing these techniques based on spatial resolution, the review highlights their applications in resolving key issues like electrode degradation, dendrite formation, and phase transitions during battery operation. Moreover, the integration of machine learning accelerates data processing, enabling multiscale correlations and predictive modeling. The review underscores the necessity of multiscale approaches to optimize battery performance, safety, and lifespan, showcasing how emerging methodologies contribute to next-generation energy storage technologies.
1 Introduction
Humanity has developed and applied batteries to various industries based on electrochemical reactions for a long time. Since Alessandro Volta invented the first battery in the early 1800s, battery technology has steadily advanced [1]. In the 19th century, chemical batteries such as the Daniell cell emerged, and in the 20th century, secondary batteries like nickel-cadmium batteries were developed and widely applied in industry [2]. Among these, secondary batteries, particularly lithium-ion battery systems, have become the most widely used and actively researched battery technology today, thanks to their high energy density, long lifespan, and lightweight properties [3, 4]. These batteries are essential energy storage devices used in various fields such as electric vehicles, portable electronic devices, and large-scale energy storage systems, with ongoing research and development aimed at further improving their performance.
Despite the increasing demand for batteries with higher capacity and stability, various battery systems still face several challenges. For instance, lithium-ion batteries can experience performance degradation and safety issues due to undesirable phase transitions during cycling, dendrite formation, and electrolyte decomposition [5-7]. Similarly, next-generation batteries such as sodium-ion batteries and solid-state batteries [8, 9] have their own unique challenges and technical limitations. Active research is being conducted to address these issues, with structural analysis and mechanism elucidation using various imaging techniques playing a crucial role. By utilizing advanced imaging technologies such as x-rays, neutrons, and electron microscopy to observe and analyze microstructural changes inside the batteries in real-time, researchers can understand the degradation mechanisms of each battery system and seek solutions for improvement [10-13].
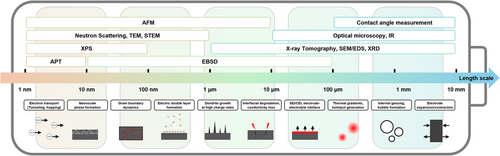
However, for battery systems, identifying the root causes of degradation remains complex due to the interplay of multiscale phenomena occurring at atomic, microstructural, and macroscopic levels. For example, issues such as dendrite growth at the micrometer scale and macroscopic problems like stress due to volume changes during cycling at the millimeter scale can arise in various battery systems [14-16]. Therefore, no single analytical tool can solve all these problems. To accurately identify and address the complex issues of each battery system, multi-scale analysis is essential. For example, electron microscopy can be used to observe dendrite formation at the micrometer scale, while X-ray or optical microscopy can analyze volume changes of electrodes where dendrite formed and associated stress at the millimeter scale [17, 18]. This multi-faceted approach plays a crucial role in accurately understanding the problems within the battery system, simulating actual operating conditions, and developing practical solutions. This review provides a comprehensive summary of various analytical methods for battery material analysis, categorizing them based on the scale of the sampled region. By classifying techniques according to the spatial scale they target—from nanometer to millimeter scales—this review encompasses not only imaging tools but also spectroscopy methods, offering insights into their latest research developments and applications for each scale.
2 Millimeter Scale
During battery charging and discharging, several important phenomena occur at the millimeter scale that can impact overall performance and longevity. Macroscopic structural changes such as electrode expansion and contraction due to lithiation and delithiation can lead to mechanical stress and eventual failure [19-22]. Additionally, gassing in liquid electrolyte systems can result in the formation of gas bubbles, disrupting ionic pathways [23]. Thermal effects also play a significant role, as uneven heating caused by internal resistance can create hot spots, potentially leading to thermal runaway if not properly managed [24]. Furthermore, non-uniform current distribution at this scale can result in localized overcharging or overdischarging, adversely affecting battery performance and lifespan. Understanding these macroscopic phenomena is crucial for improving battery design and ensuring safe and efficient operation.
Electrochemical impedance spectroscopy (EIS) is one of the most prominent and extensively utilized characterization techniques in battery research [25, 26]. Its non-destructive nature, seamless integration with cycling equipment, high sampling efficiency, and cost- and time-effectiveness make EIS an indispensable tool for analyzing battery systems. EIS operates by applying an alternating current (AC) signal over a range of frequencies, thereby inducing electrochemical phenomena and measuring the corresponding phase shift. This technique enables the characterization of bulk properties of cell components, the solid electrolyte interphase (SEI), charge transfer resistance, diffusion kinetics, ohmic resistance, and double-layer capacitance, among other electrochemical parameters. However, despite its widespread application, EIS is beyond the scope of this review. The primary limitation of EIS is its inability to selectively analyze specific regions within a battery, restricting its applicability to localized studies. Given this limitation, many battery studies employ EIS as a fundamental diagnostic tool, subsequently integrating more specialized characterization techniques, which are the primary focus of this review.
Another widely recognized and essential technique is nuclear magnetic resonance (NMR) spectroscopy [27-29]. NMR analyzes the chemical environment and coordination state of specific elements by utilizing the resonance phenomenon of nuclei interacting with a strong magnetic field and specific radio frequencies.
NMR is particularly useful for studying the interactions of lithium ions with solvents and anions in liquid electrolytes and can play a crucial role in identifying electrolyte decomposition components. Additionally, pulse field gradient NMR enables the measurement of lithium-ion diffusivity, while solid-state NMR can be employed to analyze SEI and CEI structures. Furthermore, in-operando NMR allows for real-time investigation of electrode phase transitions and structural changes during battery operation.
However, NMR has limitations in that it cannot selectively analyze specific regions and instead provides information as an average value across the bulk sample, making it challenging to study microstructural details. Moreover, due to the extensive scope of NMR and its differences from the other analytical techniques discussed in this review, it is not covered in detail in this paper.
2.1 Optical Microscopy
Optical microscopy is a fundamental imaging technique that uses visible light and a system of lenses to magnify small objects, making them visible to the naked eye or camera. This method is widely used in materials science to observe the surface morphology and microstructure of samples in real time. In battery research, optical microscopy allows for the visualization of dendrite growth, electrode surface changes, and other microstructural phenomena that occur during battery operation [30, 31]. The ability to provide real-time imaging makes this technique highly valuable for studying dynamic processes, such as the formation and evolution of battery materials under various conditions. This section explains how optical microscopy is applied in various battery material research through specific examples.
Dendrite growth in the electrodes is a major cause of battery performance degradation and reduced lifespan. This problem occurs in various materials, such as aluminum, lithium, and zinc, leading to voltage fluctuations, efficiency loss, and unstable cycling. Using in situ or operando optical microscopy, dendrite growth can be observed in real time, providing key insights into the mechanisms of dendrite growth and suppression [32-34]. These insights are crucial for developing strategies to prevent dendrite formation. Hao et al. visualized the growth of zinc dendrites during cycling using optical microscopy, offering direct evidence of how these structures degrade battery performance [35]. Similarly, Hao et al. and Sun et al. combined in situ optical microscopy with simulations in zinc-based batteries to reveal the mechanisms behind dendrite-free zinc deposition and identify strategies for inhibiting dendrite formation [36, 37]. Such observations are vital for developing dendrite-free electrode designs and understanding how dendrite growth progresses under various electrochemical conditions to enhance battery lifespan and stability.
Optical microscopy plays a critical role in monitoring surface changes and degradation in battery electrodes during and after cycling. For example, Bayaguud et al. used optical microscopy, alongside scanning electron microscopy (SEM), to monitor the surface morphology of lithium-sulfur battery electrodes after cycling, providing key insights into mechanical degradation and self-healing properties in flexible electrodes [38]. Additionally, Zhang et al. employed operando optical microscopy to visualize local state-of-charge variations in graphite electrodes, tracking how current concentration leads to surface degradation over time (Figure 1A) [39]. In studies on zinc anodes, Fear et al. used optical microscopy to observe surface changes such as non-uniform zinc deposition and corrosion during cycling (Figure 1B) [40]. These observations are essential for understanding how surface morphology evolves under electrochemical stress and offer valuable data for developing more durable and stable battery materials.
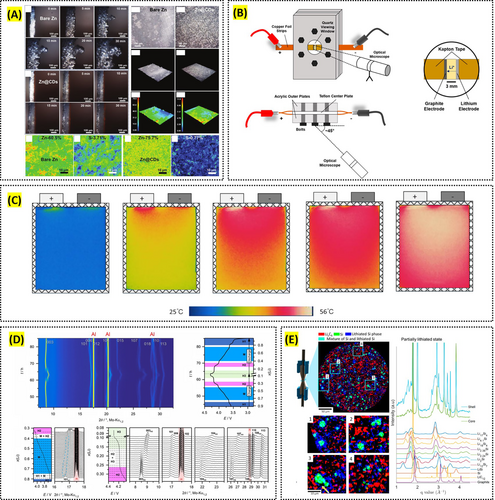
Reproduced with permission: Copyright 2022, John Wiley and Sons. Reproduced with permission: Copyright 2020, American Chemical Society. Reproduced with permission: Copyright 2019, Elsevier. Reproduced with permission: Copyright 2019, John Wiley and Sons. Reproduced with permission: Copyright 2019, American Chemical Society.
Optical microscopy is also instrumental in examining the effectiveness of protective layers and coatings in stabilizing electrode surfaces and preventing dendrite growth and corrosion. For instance, Davis et al. observed through in situ optical microscopy that a nitrogen-doped graphene protective layer in zinc-ion batteries significantly mitigated hydrogen evolution and zinc hydrolysis [36, 41]. Similarly, Gao et al. used polyvinyl butyral (PVB) films acting as an artificial SEI to suppress side reactions and guide uniform zinc plating and stripping, with optical microscopy directly confirming improvements in surface morphology [37, 42]. Additionally, Yang et al. applied carbon dots as an interlayer in zinc batteries to regulate the Zn/electrolyte interface, where optical microscopy verified the formation of a dendrite-free zinc anode and enhanced surface stability [15]. However, optical microscopy cannot provide three-dimensional structural information and is limited by relatively low spatial resolution, highlighting the need for its integration with complementary high-resolution techniques discussed in after sections.
2.2 Infrared Imaging
Infrared imaging is a technique that detects and visualizes heat emitted from objects in the form of infrared radiation, which is invisible to the naked eye [43]. By capturing spatially resolved temperature maps, it provides valuable insights into the thermal behavior of materials. In battery research, infrared imaging at the millimeter scale has become a critical tool for assessing the thermal behavior and overall performance of battery systems. This non-destructive technique allows researchers to monitor temperature distribution and identify hotspots during charging and discharging cycles. Understanding these thermal dynamics is essential for improving thermal management strategies, ensuring safer and more efficient battery operation.
For example, Giammichele et al. used infrared thermography to evaluate the thermal management of cylindrical lithium-ion batteries, providing insights into how temperature fluctuates across the battery surface during charging and discharging cycles [44]. Wang et al. investigated temperature fluctuation and spatial distribution in a large, laminated lithium-ion power battery using infrared imaging, revealing how temperature varies with different discharge rates and depths (Figure 1C) [45]. Such information is crucial for improving thermal management strategies and ensuring the safety and efficiency of batteries in practical applications.
One of the unique strengths of infrared imaging at this scale is its ability to monitor batteries in real time, enabling the detection of defects and hotspots that could compromise battery performance. Duan et al. applied in situ infrared thermal imaging to identify defective lithium-ion batteries during high current charging, finding that the surface temperature of defective cells was significantly higher than that of healthy cells [46]. This capability is particularly valuable for quality control in battery manufacturing, as it allows for the early detection of potential failures before they impact the battery's operation in the field. Just et al. enhanced defect detection in battery electrode foils using infrared particle detection [47].
Furthermore, infrared imaging provides valuable data for understanding the thermal effects and chemical evolution within batteries during operation. Zhang et al. visualized self-heating processes in all-climate batteries using infrared thermography, helping to identify areas that require design improvements to enhance battery durability and safety [48]. Huang et al. monitored thermal effects in redox flow batteries, assessing the impact of charge–discharge cycles on temperature distribution and identifying regions prone to overheating [49]. In another study, Altin et al. investigated the thermal performance of silicon-modified Na0.67Fe0.5Mn0.5O2 cathodes using infrared thermal imaging, contributing to the optimization of battery design and thermal stability in next-generation energy storage systems [50].
While infrared imaging provides fast and accessible thermal analysis, it is inherently limited to surface-level observations and cannot reveal subsurface or internal temperature distributions. Therefore, its integration with complementary techniques—such as x-ray CT or neutron imaging—is often necessary to build a more complete picture of heat propagation and structural dynamics in battery systems.
2.3 X-Ray Diffraction
X-ray diffraction (XRD) is a technique used to determine the crystallographic structure, phase composition, and other structural properties of materials. When x-rays are directed at a crystalline material, they are diffracted in specific directions depending on the arrangement of atoms within the crystal. By measuring the angles and intensities of these diffracted beams, researchers can infer the material's atomic structure. At the millimeter scale, XRD is a crucial tool for characterizing the bulk structural and crystallographic properties of battery materials, particularly in understanding phase transformations and material stability. By analyzing diffraction patterns, researchers gain insights into how mechanical processing methods, such as high-energy ball milling, affect crystallite size, lattice strain, and overall materials performance [51-55].
For instance, Al-Tabbakh et al. applied XRD peak-broadening analysis to lithiated spinel materials, revealing that while reducing particle and crystallite sizes can enhance battery performance, increased lattice strain can lead to material degradation [56]. Such findings are essential for optimizing mechanical processing parameters to improve the durability and efficiency of battery materials. Fibriyanti et al. synthesized and analyzed the crystal structure of LiNiSixP1−xO4/C as a cathode material, demonstrating how XRD can aid in optimizing cathode materials for lithium-ion batteries [57].
In addition to structural analysis, XRD is highly effective for monitoring phase transformations in battery electrodes during operation. Operando XRD enables real-time observation of phase changes within materials like LiNiO2 and LiMn2O4 cathodes during charge–discharge cycles, providing critical data on how these transformations impact battery stability and performance. For example, Luo et al. studied the degradation mechanisms of a spinel LiMn2O4 cathode in different voltage windows using operando XRD, leading to the identification of specific degradation mechanisms that occur during cycling [58]. Such real-time insights are vital for designing cathode materials that can withstand the stresses of repeated cycling and maintain long-term stability in practical applications. Biasi et al. and Xiao et al. also utilized operando XRD to study phase transformation behavior and stability of cathode materials, contributing to the development of high-performance batteries (Figure 1D) [59, 60].
Moreover, XRD techniques are invaluable for the quantitative analysis of lithium distribution in battery electrodes and for understanding how concentration gradients develop during operation. By using energy-dispersive XRD, Yao et al. mapped the lithiation and delithiation processes within graphite electrodes, highlighting the challenges posed by steep lithium-ion gradients at high current rates [61]. This spatially resolved data is crucial for improving battery design, as it allows for the optimization of electrode composition and structure to ensure more uniform lithium distribution, thereby enhancing the safety, efficiency, and lifespan of lithium-ion batteries. Finegan et al. employed in situ high-energy XRD computed tomography to spatially resolve lithiation in silicon-graphite composite electrodes and discovered significant spatial heterogeneities, including the formation of core-shell structures in silicon particles where crystalline silicon cores remained surrounded by lithiated silicide shells. This finding highlights the under-utilization of electrode capacity and the critical role of stress-induced reaction retardation at the lithiation front, emphasizing the need for improved electrode design to optimize lithiation dynamics and material utilization (Figure 1E) [62].
While XRD provides robust information on crystal structure and phase evolution, it is limited in its sensitivity to amorphous components and surface phenomena. Additionally, spatial resolution is restricted compared to techniques like electron microscopy. To overcome these limitations and capture a more complete picture of battery behavior, XRD is often integrated with complementary methods and multifunctional analysis.
2.4 Contact Angle Measurement
Contact angle measurement is a technique used to assess the wettability of a material's surface by a liquid, typically a separator, electrode, or electrolyte in battery research. The contact angle is formed at the interface where the liquid, solid, and air meet, providing insights into the surface energy and interaction between the liquid and solid. A lower contact angle indicates better wettability, which is crucial for increasing the area of redox reactions through efficient electrolyte infiltration in battery separators and electrodes.
This technique is critical for evaluating how surface modifications can enhance the performance of lithium-ion batteries. For example, Liang et al. demonstrated that plasma-modified polypropylene membranes used as battery separators showed improved wettability, indicated by reduced contact angles after treatment [63]. These modifications led to better electrolyte infiltration and improved electrochemical performance. Understanding how contact angle correlates with surface energy and electrolyte compatibility is essential for optimizing separator materials and ensuring the reliable operation of batteries under various conditions [64].
In addition to separators, contact angle measurement is widely used to assess the surface properties of current collectors and electrodes, which play a crucial role in battery efficiency and longevity. Loghavi et al. showed that chemical modifications to aluminum current collectors enhanced their wettability, leading to better adhesion of the active material and improved cycling stability of the cathode (Figure 2A) [65]. Similarly, Jeon et al. studied the wettability of lithium-ion battery electrodes and found that non-uniform wetting can lead to issues such as uneven current distribution and lithium plating, compromising battery safety and performance [66]. By carefully controlling and measuring contact angles, researchers can develop more robust and reliable battery components that deliver consistent performance over extended cycles [67].

Reproduced with permission: Copyright 2019, Elsevier. Reproduced with permission: Copyright 2021, John Wiley and Sons. Reproduced with permission: Copyright 2023, John Wiley and Sons. Reproduced with permission: Copyright 2022, Springer Nature.
Moreover, contact angle measurement is instrumental in evaluating the interaction between electrolytes and solid components in batteries. Sun et al. investigated the wetting behavior of ionic-liquid-based electrolytes on different separators, revealing how variations in solvent and salt composition affect electrolyte distribution and battery performance (Figure 2B) [68]. Additionally, Beyer et al. studied the influence of surface characteristics on the penetration rate of electrolytes into model cells, providing insights into the mechanisms of electrolyte imbibition and factors that influence infiltration rates [69]. This understanding is vital for designing electrolytes and separators that optimize ion transport and enhance battery efficiency.
Although contact angle measurement offers straightforward and quantitative evaluation of wettability, it remains a macroscopic technique and does not capture sub-surface interactions or nanoscale interfacial structure. Therefore, it is often complemented by microscopic or spectroscopic techniques, such as AFM or XPS, to build a more comprehensive picture of interfacial phenomena in batteries.
2.5 Differential Electrochemical Mass Spectroscopy
Differential electrochemical mass spectroscopy (DEMS) or online electrochemical mass spectroscopy (OEMS) is an exceptionally sensitive quantitative technique used to detect gas evolution or production during battery cycling. This technique collects gases generated during electrode cycling at specific potentials and time intervals. Since ionized gases are detected based on their mass-to-charge (m/z) ratio, DEMS exhibits exceptional sensitivity and selectivity during in-operando battery analysis [70]. By identifying the types of gases released, DEMS provides crucial insights into electrochemical reactions and mechanisms occurring during battery operation, including electrolyte decomposition, SEI formation, cathode degradation, dendrite growth, and thermal runaway (Figure 2C) [71].
Beyond its sensitivity, DEMS provides unique insights into the electrochemical reactivity of battery materials, particularly under aggressive conditions. Cao et al. investigated the decomposition mechanisms of lithium carbonate in lithium-air batteries by analyzing isotopically labeled carbon gases, such as CO2, CO, and O2 (Figure 2D) [72]. This finding indicates that a detailed understanding of decomposition pathways can aid in the development of highly efficient cathodes for lithium-based batteries. Li et al. examined the introduction of a hyaluronic acid gel electrolyte with high reduction stability, demonstrating its ability to suppress hydrogen gas generated by undesirable parasitic side reactions in electrolytes [73]. Thus, DEMS can evaluate whether electrolytes primarily facilitate lithium-ion transport rather than being consumed by corrosion or side reactions, which ultimately enhances coulombic efficiency.
Due to its highly precise gas detection capabilities, DEMS can identify the gases responsible for initiating thermal runaway, thereby helping to determine the optimal battery compositions to prevent catastrophic failures. To better understand electrochemical performance and gas production mechanisms, Wang et al. optimized the negative-to-positive (N/P) electrode ratio in battery cells by analyzing gas evolution through DEMS [74]. They monitored real-time gas behavior across different N/P ratio configurations, revealing that higher N/P ratios induce the evolution of H2, CO2, and CO gases.
Despite its high sensitivity, DEMS has inherent limitations. It provides spatially averaged gas detection, lacks imaging capability, and requires high-vacuum conditions that may alter volatile components. Furthermore, it cannot localize gas evolution within specific microstructural features. To overcome these drawbacks, DEMS is often integrated with complementary techniques such as ToF-SIMS for spatially resolved gas species analysis. Coupling DEMS with advanced imaging or modeling frameworks can enable correlative diagnostics of structure–reactivity relationships, thereby positioning DEMS as a critical pillar in multiscale battery material analysis.
3 Micrometer Scale
At the micrometer scale, several key phenomena occur that significantly impact battery performance and longevity. Electrochemical reactions at the electrode-electrolyte interface involve the formation and growth of the SEI layer on the anode surface, which is crucial for stabilizing the interface but can also consume electrolyte and active lithium. Additionally, degradation and the formation of new phases at the cathode interface can reduce both surface ionic and electronic conductivity, leading to diminished battery performance. Microstructural changes, such as the formation and propagation of cracks in electrode materials due to repeated cycling, lead to the loss of active material due to direct contact between the electrolyte and the active material [75-77]. Electrode particles can fracture from mechanical stress and volumetric changes, impacting both conductivity and capacity. Phase transitions, such as dendrite growth at high charging rates, pose risks of capacity decrease and short-circuiting the battery, while phase separation in cathode materials like LiCoO2 results in inhomogeneous lithium distribution and mechanical degradation [78-80].
3.1 X-Ray Tomography
X-ray tomography is a non-destructive imaging technique that utilizes x-rays to generate detailed three-dimensional (3D) images of a sample's internal structure. By rotating the sample and capturing multiple two-dimensional (2D) images from various angles, a comprehensive 3D reconstruction is produced [81]. This allows researchers to visualize and analyze internal features without the need to disassemble the object, typically achieving a resolution on the order of a few to several tens of micrometers. Moreover, by employing synchrotron x-ray sources, it is now possible to reach resolutions on the order of tens of nanometers. In the battery field, x-ray tomography enables 3D visualization of the internal structure of cells or electrodes without disassembly and can also capture real-time 3D images of internal changes during charge–discharge cycles.
For example, Lu et al. utilized x-ray tomography to reveal the three-dimensional microstructure of NMC111, demonstrating that microstructural heterogeneities markedly affect lithium-ion transport and overall LiB performance. Notably, they established a fully microstructure-resolved 3D model using a novel x-ray nano-computed tomography (CT) dual-scan superimposition technique, which captures features of the carbon–binder domain [82]. Similarly, Cabana et al. utilized soft x-ray tomography to localize even nanoscale battery reactions in 3D, providing crucial details for optimizing material properties and improving the overall efficiency of battery systems (Figure 3A) [83].
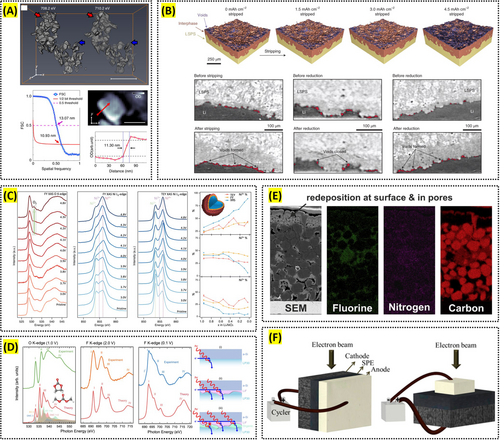
Reproduced with permission: Copyright 2018, Springer Nature. Reproduced with permission: Copyright 2021, Springer Nature. Reproduced with permission: Copyright 2024, Royal Society of Chemistry. Reproduced with permission: Copyright 2022, Springer Nature. Reproduced with permission: Copyright 2017, Elsevier. Reproduced with permission: Copyright 2018, American Chemical Society.
X-ray tomography offers a major advantage in that it can be performed operando under cell configurations closely resembling real operating conditions, enabling the capture of real-time 3D images of internal changes. Recently, this approach has been employed to observe internal transformations in various next-generation batteries. For instance, Lewis et al. used operando synchrotron x-ray computed microtomography to investigate the evolution of lithium/Li10SnP2S12 solid-state electrolyte interfaces during battery cycling, revealing that the loss and subsequent reconfiguration of interfacial contact are factors driving cell failure (Figure 3B) [84]. Sadd et al. employed operando synchrotron x-ray tomography to visualize, in real time, the lithium metal anode microstructural changes arising from the plating and dissolution process, thereby examining how dendrite growth and dissolution mechanisms vary with current density [85].
Although x-ray tomography offers the advantage of three-dimensional imaging of a battery's internal microstructure, achieving nanometer-scale resolution is challenging and typically requires expensive synchrotron-based instruments. Moreover, this technique provides limited chemical information. To address these limitations, an efficient multi-scale approach can be employed—for example, scanning the entire cell with micro-CT, followed by high-resolution synchrotron-based imaging of specific regions of interest. In addition, complementary analyses using Raman, XPS, TEM, or ToF-SIMS can provide chemical and nanoscale structural insights, thereby enabling a more comprehensive and synergistic understanding of the system.
3.2 X-Ray Absorption Spectroscopy
X-ray absorption spectroscopy (XAS) is a technique in which the intensity of x-rays absorbed by specific atoms is measured when a sample is irradiated with x-rays, thereby revealing the electronic structure, chemical state, and local atomic environment of the sample with typical spatial resolution ranging from tens to hundreds of micrometers. Depending on whether the signal is measured in transmission, fluorescence, or electron yield (TY) mode, XAS can provide information on either the bulk electronic structure and chemical state of a material or its surface properties. Moreover, by focusing on the region near the absorption edge—x-ray absorption near edge structure—or on the fine structures beyond the edge—extended x-ray absorption fine structure—one can acquire detailed insights into the oxidation state of metal ions and their interactions with neighboring atoms, such as coordination numbers and interatomic distances. XAS is widely employed in the analysis of battery materials, particularly to determine the oxidation states of transition-metal ions, their coordination environment, and interatomic distances within battery electrodes. Recently, in situ XAS has also been used to examine changes in the oxidation states of transition metals and the formation of interfacial chemical products during electrochemical cycling.
XAS is extensively applied to investigate the oxidation states of transition-metal ions in lithium layered oxide cathodes, as well as their coordination environments and interatomic distances within electrode materials. For example, Lijin An et al. used bulk-sensitive x-ray Raman spectroscopy (XRS) to show that in LiNiO2, charge compensation in the bulk is driven by Ni–O rehybridization rather than involving molecular O2. In contrast, fluorescence yield (FY) XAS indicated the emergence of molecular O2 in the outer ~200 nm of the cathode surface. By uncovering these phenomena, they highlighted the surface instability of LiNiO2 and underscored the critical importance of surface stabilization (Figure 3C) [86].
Operando XAS enables real-time monitoring of the oxidation state and coordination environment of transition-metal ions, as well as the formation of the SEI, during electrochemical cycling. For example, Liu et al. employed in situ and operando soft x-ray absorption spectroscopy to reveal distinct charge dynamics in NMC and LFP cathodes. They attributed the differences between the two systems, along with the relaxation effect observed in LiFePO4, to a phase transformation mechanism, the mesoscale morphology, and the charge conductivity of the electrodes [87]. In another example, Swallow, Jack et al. used operando soft x-ray absorption spectroscopy in total electron yield mode to track the SEI's chemical evolution on an amorphous Si anode, concluding that FEC additives rapidly healed SEI defects and thereby improved cycling performance (Figure 3D) [88].
XAS offers the advantage of easily probing the oxidation states and local electronic structures of transition metals, along with its availability for in situ analysis. However, because XAS provides electronic and structural information in an averaged form, it is difficult to capture fine inhomogeneities within the sample at high spatial resolution. To overcome this limitation, synchrotron-based methods can be employed to focus the x-ray beam to micrometer or nanometer scales, thereby enhancing spatial resolution. Additionally, integrating XAS with chemical imaging techniques such as x-ray microscopy or Raman imaging allows for the mapping of sample heterogeneities, enabling a more comprehensive interpretation of the observed phenomena.
3.3 SEM-EDS
SEM combined with energy dispersive x-ray spectroscopy (EDS) is a powerful analytical technique used to examine the surface topography and elemental composition of materials. SEM uses a focused electron beam to scan the sample's surface, producing high-resolution images with a typical resolution of around 1 to 10 nm, revealing fine details of the material's morphology. EDS complements this by analyzing the characteristic x-rays emitted from the sample when bombarded with electrons, enabling identification and quantification of elements with a spatial resolution of approximately 1 to 2 μm. SEM-EDS has been a vital tool in battery research for analyzing the structural and chemical properties of battery materials, and correlating these with performance and degradation behavior enables optimization of processing parameters. In particular, in situ SEM enables real-time imaging during operation, providing valuable insights into the morphological evolution of electrodes and interfaces [89-91].
SEM-EDS provides morphological and chemical insights into electrode materials under varying processing conditions, enabling the optimization of critical parameters. Tang et al. demonstrated that the morphological evolution of LiNi0.8Mn0.1Co0.1O2 (NMC811) during high-temperature sintering included dehydration, oxidation, and combination of the raw materials process, along with a significant reduction in particle size, thus offering a valuable reference for controlling the synthesis temperature [91]. Jaiser et al. employed cryogenic scanning electron microscopy with broad ion beam slope-cutting (Cryo-BIB-SEM) to investigate the drying process of graphite anodes under high drying rates; EDS and image analysis of segmented cross-sections showed homogeneous shrinkage of electrode films and highlighted capillary transport as a key factor influencing drying kinetics and binder distribution (Figure 3E) [92].
In situ SEM enables real-time observation of structural changes in battery materials under operating conditions, offering critical insights into dynamic morphological and chemical transformations. For example, Dienwiebel et al. combined aspects of in situ and ex-situ SEM/EDS measurements to gain detailed insights into the degradation mechanisms of silicon nanoparticle-based electrodes during charge–discharge cycling [93]. Golozar et al. used in situ SEM combined with EDS to detect and characterize the carbide nature of dendrites in Li-polymer batteries, which are known to cause short circuits and reduce cycle life [94]. By visualizing these processes as they occur, researchers can develop strategies such as applying mechanical pressure to ensure uniform lithium deposition and suppress dendrite growth or improving the SEI to minimize defects that act as initiation points to mitigate dendrite formation, thereby enhancing the safety and reliability of batteries.
SEM-EDS is a powerful technique for simultaneously analyzing the surface topography and elemental composition of materials. However, because it primarily provides information about the surface, characterizing deeper internal structures can be challenging. In addition, EDS generally has a spatial resolution on the order of a few micrometers, and detecting light elements such as Li is particularly difficult due to their low X-ray energies, resulting in reduced accuracy and sensitivity. These limitations can be addressed by employing focused ion beam-SEM (FIB-SEM) to directly section the sample for high-resolution 3D analysis of internal structures, or by using complementary techniques such as x-ray photoelectron spectroscopy (XPS), Auger electron spectroscopy (AES), and wavelength dispersive x-ray spectroscopy (WDS) to enhance light element detection and improve quantitative analyses.
3.4 Raman Imaging and Spectroscopy
Raman imaging and spectroscopy enable detailed characterization of surface molecular bonds by measuring the inelastic scattering arising from specific molecular vibrations, which are induced by changes in polarizability when near-infrared to visible lasers illuminate a sample. By using a relatively narrow wavelength range, these techniques can achieve lateral resolutions on the order of a few micrometers without requiring vacuum conditions or causing physical damage to the sample. In battery research, Raman spectroscopy is widely used to analyze interfacial compositions and track electrode changes, while recent advancements have significantly enhanced spatial resolution and enabled in situ observation in next-generation systems. Raman methods are widely employed to investigate the complex organic and inorganic composition of the SEI and to track defect sites or lithium intercalation in carbon-based electrodes exhibiting large polarizability changes. As an example, Gajan et al. demonstrated that by employing in situ Raman spectroscopy, one can track the compositional dynamics of the SEI on a tin electrode during Li-ion battery coin-cell cycling and thereby identify the origin of the tin electrode's irreversible capacity [95]. Cabañero et al. used in-operando Raman spectroscopy to observe that lithium carbide peaks emerge as the graphite D and G bands vanish upon full lithiation, thereby demonstrating that Raman spectroscopy can be employed to study the evolution of the lithium deposition reaction [96].
Current research is advancing lateral resolution to the nanometer scale through a technique called tip-enhanced Raman spectroscopy (TERS), and simultaneously enabling in situ analyses of surface transformations in next-generation batteries, particularly lithium–sulfur batteries. Nanda et al. employed TERS to observe the chemical composition and phase distribution of the surface SEI on amorphous silicon (a-Si) thin-film anodes, achieving a lateral resolution of less than 10 nm [97]. Lang et al. employed operando confocal Raman microscopy to investigate the reaction pathways and kinetics of the multistep Li–S redox processes, particularly tracking the rate and spatial distribution of polysulfide evolution during cell operation (Figure 4A) [98].
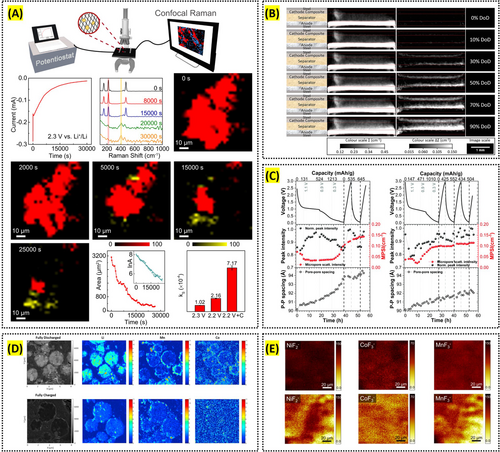
Reproduced with permission: Copyright 2022, Springer Nature. Reproduced with permission: Copyright 2023, John Wiley and Sons. Reproduced with permission: Copyright 2019, Royal Society of Chemistry. Reproduced with permission: Copyright 2015, Elsevier. Reproduced with permission: Copyright 2024, Elsevier.
Raman imaging and spectroscopy offer relatively high lateral resolution, making them well suited for characterizing various molecular structures and bonds. However, because visible light can be scattered back from depths of hundreds of nanometers within the sample, analyzing an SEI layer only a few nanometers thick remains challenging, and sensitivity to molecules with low polarizability or those exhibiting fluorescence is limited. In contrast, high-vacuum techniques such as XPS and ToF-SIMS enable vertical chemical composition analysis at nanometer-scale depth resolution. Consequently, employing these methods in a complementary manner is essential for achieving a comprehensive understanding of surface chemical composition and its distribution.
3.5 Neutron Imaging and Scattering
Neutron imaging is a technique used to visualize a sample's internal structure by detecting and recording the intensity distribution of neutrons transmitted through the sample, typically achieving a spatial resolution on the order of tens to hundreds of micrometers. Neutron scattering measures how incoming neutrons scatter off atomic nuclei or their immediate surroundings, providing structural and dynamic information based on scattering angles and energy changes, thereby sensitively capturing structural details at the nanoscale, ranging from a few nanometers up to hundreds of nanometers. Compared to x-rays, these neutron-based methods offer higher sensitivity to light elements such as hydrogen and lithium, making them particularly useful for tracking changes in lithium distribution within electrode materials and electrolytes. In addition, they are highly effective for observing structural changes and phase transitions in lithium-containing components such as lithium metal, electrodes, and the SEI.
Neutron imaging offers the advantage of determining the lithium distribution within electrode materials and electrolytes and, in particular, can be performed in situ to observe real-time changes in lithium distribution. Bradbury et al. used in situ neutron tomography to visualize lithium-ion transport through a solid electrolyte separator during the cycling of a solid-state Li–S battery (Figure 4B) [99]. Weydanz et al. employed neutron imaging to monitor the process of filling electrolyte into lithium-ion cells in real time, demonstrating that applying a vacuum can assist the process and accelerate it by about a factor of two [100].
Neutron scattering enables the observation of structural changes and phase transitions in lithium-containing components and is similarly well-suited for in situ analysis to capture real-time dynamics of structural and phase evolution. Risse et al. applied operando small-angle neutron scattering to investigate the precipitation and dissolution of sulfur, polysulfides, and lithium sulfide in microporous carbon structures of Li–S batteries in real time [101]. Jafta et al. used operando small-angle neutron scattering (SANS) to examine how the LiTFSI salt concentration in the electrolyte affects dynamic cell chemistry, including the mechanism of SEI formation and lithium intercalation, also in real time (Figure 4C) [102].
Neutron imaging and scattering are highly sensitive to light elements such as hydrogen and lithium, making them indispensable tools in the battery field, where lithium is essential. However, because a high-intensity neutron source (e.g., a research reactor) is required, experimental access is severely limited. In addition, analyzing heavier elements is more challenging than with x-ray-based methods, and the spatial resolution of neutron imaging is generally lower than that of x-ray CT. Consequently, combining these neutron-based approaches with x-ray-based scattering and high-resolution x-ray CT helps achieve a more comprehensive understanding of battery materials.
3.6 Time-of-Flight Secondary Ion Mass Spectroscopy
Time-of-flight secondary ion mass spectroscopy (ToF-SIMS) is a high-resolution surface analysis technique that provides elemental and molecular information at the micrometer (μm) to nanometer (nm) scale. It bombards the sample with a pulsed ion beam, ejecting secondary ions for mass-to-charge (m/z) analysis. This technique enables high-sensitivity chemical imaging and depth profiling, making it particularly valuable for studying surface chemistry and interfacial phenomena in battery materials. It can detect trace organic and inorganic species with high lateral resolution, but its limitations include its destructive nature and potentially altering volatile components under vacuum. Despite these challenges, ToF-SIMS is widely used to investigate the SEI, electrolyte decomposition, and ion diffusion, providing insights into performance degradation mechanisms.
ToF-SIMS enables detailed chemical mapping of electrode surfaces, allowing analysis of CEI composition and electrolyte decomposition. CEI plays a crucial role in battery performance, with its spatial heterogeneity influencing lithium-ion transport and long-term stability. Sui, Tan, et al. precisely mapped Li, Mn, and Co nanoscale distribution in Li-ion battery cathodes, revealing localized Li trapping during charge–discharge cycles. They enabled direct visualization of Li—a task challenging with conventional EDX techniques—thereby highlighting significant Li accumulation at grain boundaries and interfaces (Figure 4D) [103]. These findings provide critical insights into the degradation mechanisms and performance losses in Li-ion batteries and offer valuable guidance for designing improved battery materials.
Another key advantage of ToF-SIMS is its depth profiling capability, which allows for investigating lithium distribution and electrode degradation across depths. Using Tof-SIMS depth profiling on the anode, Lee et al. demonstrated that transition metal (TM) dissolution from the NCM811 cathode is more pronounced in anode-free lithium-metal batteries (AFLMBs) compared to conventional lithium-metal batteries (LMBs). The presence of TM species in the SEI accelerated electrolyte decomposition, exacerbated interfacial instability, and promoted self-discharge, ultimately contributing to the capacity degradation of AFLMBs over prolonged cycling (Figure 4E) [104]. These studies highlight ToF-SIMS as a powerful tool for understanding degradation mechanisms and improving electrode designs.
Despite its strengths, ToF-SIMS has limitations when applied to battery materials. The ultra-high vacuum (UHV) environment can alter the chemical states of volatile species, potentially affecting surface chemistry characterization. If combined with AFM, UHV conditions can also lower the z tolerance of the AFM tip in non-contact mode, making rough sample measurements challenging. Additionally, the high-energy ion beam used for depth profiling can cause ion beam mixing, reducing depth resolution. Quantitative analysis remains complex due to variations in ionization efficiency across different materials. To address these challenges, complementary techniques such as XPS and AES are often employed alongside ToF-SIMS for a more comprehensive analysis of battery material surfaces.
4 Nano and Atomic Scale
At the nanometer scale, several crucial phenomena occur that significantly influence battery performance and stability. The formation of an electric double layer at the electrode-electrolyte interface is essential for charge storage, particularly in capacitive components of the battery. Nanoscale structural changes, such as atomic rearrangement during lithiation and delithiation processes, affect material stability and performance, while grain boundary dynamics influence ionic and electronic conductivities. Diffusion processes, including nanoscale pathways for lithium-ion diffusion within electrode materials, dictate the rate capability and efficiency of the battery, and nanoscale electron transport mechanisms, like tunneling and hopping, determine the electrical conductivity of materials. Additionally, surface chemistry and reactions, such as catalytic surface reactions, significantly impact charge/discharge efficiency and side reactions, and the formation of nanoscale phases during cycling can affect the overall electrochemical performance and stability.
4.1 Atomic Force Microscopy
Atomic force microscopy (AFM) is a high-resolution imaging technique that offers exceptional resolution in the x, y, and z directions, typically down to 1, 1, and 0.1 nm. It scans a sharp tip across a material's surface, interacting with surface forces to generate topographical images and provide information about surface properties. In battery research, AFM is valuable for observing nanoscale changes in electrode materials, such as the formation of SEI layers, early-stage mechanical degradation, and structural changes during cycling. AFM can observe these processes in real time, aiding the understanding of battery material evolution during charge–discharge cycles. AFM-based methods like conductive AFM (C-AFM) and electrochemical strain microscopy (ESM) also enable the evaluation of electrical conductivity, ionic conductivity, and strain in materials, critical for battery performance. Recent AFM advancements offer improved resolution and the ability to monitor dynamic electrochemical processes, enhancing its role in analyzing next-generation energy storage materials.
The in situ and operando capabilities of AFM are crucial for understanding dynamic processes during battery operation. Breitung et al. used AFM to observe structural changes in nano-silicon-based electrodes, revealing insights into mechanical degradation and electrochemical reactions (Figure 5A) [105]. Becker et al. applied advanced scanning probe microscopy (SPM) techniques, including AFM, to gain localized information on the physical and chemical properties of lithium-ion batteries (LIBs), contributing to a better understanding of their degradation processes [106]. Also, analyzing the electrical, ionic conductivities, and mechanical properties in battery research is vital, particularly when studying the CEI/SEI layer, which significantly impacts the cell's stability and performance. Choi et al. utilized C-AFM to demonstrate that the conductive CEI, formed by the oxidative decomposition of an electrolyte additive, enables rapid and uniform lithiation and delithiation in LiFePO4 without morphological changes during cycling [107]. Similarly, Op de Beeck et al. used C-AFM to measure the electrical properties of electrode materials at the nanoscale, revealing the relationship between ion movement and conductivity [108]. Furthermore, Hong et al. employed nanoindentation to assess the SEI layer's thickness and elastic modulus on the lithium surface, showing how electrolyte additives influence these properties and cycle lives further (Figure 5B) [109].
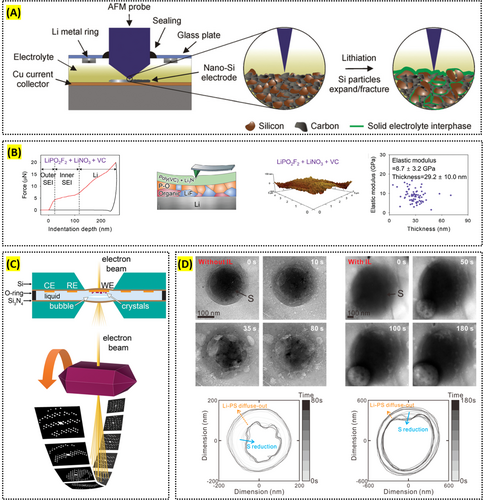
Reproduced with permission: Copyright 2024, John Wiley and Sons. Reproduced with permission: Copyright 2018, American Chemical Society. Reproduced with permission: Copyright 2020, American Chemical Society.
New techniques are continuously being developed to gather more information from battery surfaces where complex electrochemical reactions take place. In situ electrochemical atomic force microscopy (EC-AFM) is increasingly used to assess new battery components by enabling real-time observation of electrode surface changes during charge and discharge cycles. This technique integrates a potentiostat with a sealed electrochemical cell containing an electrolyte, allowing AFM imaging of surface morphology changes as cycling progresses. A major advantage is its ability to visualize surface modifications caused by factors like electrolyte additives and current density variations without cell disassembly. It also explains how electrolyte composition affects SEI degradation and battery performance. Additionally, AFM-based scanning electrochemical microscopy (SECM) has been used by Mascaro et al. to distinguish conductive and non-conductive regions on electrode surfaces, offering detailed electrical property measurements [110]. Shang et al. developed high-speed electrochemical AFM (EC-HS-AFM) to track dynamic processes in fast-charging battery materials, providing high-resolution imaging at faster speeds [111]. These advancements enable a more precise analysis of critical battery properties such as ionic transport and local mechanical deformation, which are essential for developing high-performance batteries [112, 113].
While AFM provides valuable insights for battery analysis, it faces several technical challenges that must be addressed for reliable measurements. A key issue is the samples' surface roughness, which, within the 20-μm z-range tolerance of standard AFM, can hinder accurate tip tracking, leading to imaging artifacts and making some regions unmeasurable. This problem is amplified in advanced AFM techniques like Kelvin probe force microscopy (KPFM) and ESM, where rough surfaces disrupt electrical tuning and affect electrochemical characterization. Additionally, battery samples' sensitivity to atmospheric exposure and moisture requires specialized AFM cells or glove box measurements, with precise pressure control needed when introducing liquids. In EC-AFM, gas bubbles and reaction byproducts can form, obstructing optical detection and compromising measurement accuracy. These challenges highlight the need for advanced experimental strategies, such as improved sample preparation and enhanced AFM modes, to overcome the limitations of AFM in battery research.
4.2 Transmission Electron Microscopy
Transmission electron microscopy (TEM) is a powerful imaging technique that uses electron beams transmitted through an ultra-thin specimen to produce high-resolution images. TEM operates on the principle of electron interactions with the sample, where accelerated electrons exhibit diffraction due to their wave nature, interacting with the lattice planes of the material and generating diffraction patterns that reveal the crystal structure. With a resolution typically ranging from 0.1 to 0.5 nm, TEM enables researchers to observe the atomic structure, defects, interfaces, and phase transitions within battery materials. This high spatial resolution is particularly valuable for studying structural changes and material degradation during battery cycling, providing critical insights into the mechanisms that affect battery performance, efficiency, and longevity.
TEM provides atomic-level insights essential for understanding mechanisms of electrode degradation and for developing strategies to enhance the stability and performance of battery materials. For example, Yang et al. studied the atomic structure of lithium-ion conductors and the behavior of various cathode materials during electrochemical cycling, revealing preferred atomic sites and migration paths of elements like Ni, Co, and Mn [114]. Such insights are critical for developing future materials with improved safety and performance characteristics. Wu et al. detailed structural changes and core-shell-like phase distributions of degraded intermediate phases in overcharged cathode materials. These nanometer-scale phase distributions are difficult to define through other analytical methods, such as XRD. Understanding the correlation between the fine structures of active materials and electrochemical performance is of significance in designing more stable batteries [115].
In situ TEM enables real-time observations of electrochemical processes within battery materials. Du et al. monitored lithiation and delithiation processes in materials like CoS2 and silicon nanowires, capturing the formation and evolution of SEI and other key changes during battery operation [116]. These real-time observations are essential for understanding electrode degradation mechanisms and for developing strategies to mitigate these effects, thereby improving battery performance and longevity [117, 118]. Additionally, the use of liquid-electrochemical cells in TEM studies by Demortière et al. and Harris et al. allows for observation of electrochemical reactions in a more realistic environment, closely mimicking actual battery conditions (Figure 5C) [119, 120]. Yuk et al. utilized in situ graphene liquid cell electron microscopy (in situ GLC-EM) to demonstrate the direct visualization of lithium polysulfides in liquid and revealed that the addition of ionic liquid to the electrolyte significantly reduces lithium polysulfide diffusion and shuttle current, improving the cyclability and efficiency of lithium/sulfur batteries (Figure 5D) [121].
However, to conduct a TEM analysis, the battery materials to be analyzed must be resistant to the electron beam. For example, sulfide-based solid-state electrolytes and SEI layers exhibit a high degree of vulnerability to the electron beams, necessitating the utilization of a specific cryo-TEM holder or cryo-TEM for their analysis. Furthermore, for samples that are sensitive to atmospheric oxygen and moisture, the process of sample preparation and transfer into the TEM chamber inevitably induces damage to the samples. To address these challenges, there is an ongoing development of ultra-low temperature TEM and atmosphere-free systems, along with the advancement of high-performance detectors to facilitate the acquisition of detailed information even at the low electron dose rates.
4.3 Scanning Transmission Electron Microscopy
Scanning transmission electron microscopy (STEM) combines high-resolution imaging capabilities with advanced spectroscopic techniques, such as electron energy loss spectroscopy (EELS), to provide detailed elemental and chemical analysis at the atomic scale. With a resolution as fine as 0.1 nm, STEM allows researchers to observe and map the fine structure of materials, including interfaces and defects, in unprecedented detail. However, conventional STEM EDS is limited in detecting lithium ions, as lithium has only two electrons in its K-shell, preventing it from undergoing the ejection of inner shell electrons required for x-ray emission. To overcome this, combining EELS with high-angle annular dark-field STEM (HAADF-STEM) is particularly useful for mapping the distribution and chemical states of elements, including lithium, within battery electrodes. This combination is essential for studying lithium-ion behavior, phase transitions, and other critical processes influencing battery performance and longevity.
STEM offers unparalleled atomic-level precision in both imaging and elemental analysis, essential for developing and optimizing battery materials. By combining STEM with EELS, Muto et al. mapped the distribution and chemical states of critical elements such as lithium within battery electrodes at the nanoscale [122, 123]. Similarly, Nomura et al. used operando STEM-EELS to visualize the phase boundary movement in a lithium titanate anode by tracking the Li distribution (Figure 6A) [124]. This precise elemental mapping is crucial for understanding the behavior of lithium ions in different electrode materials, enabling the design of batteries with improved capacity and stability.
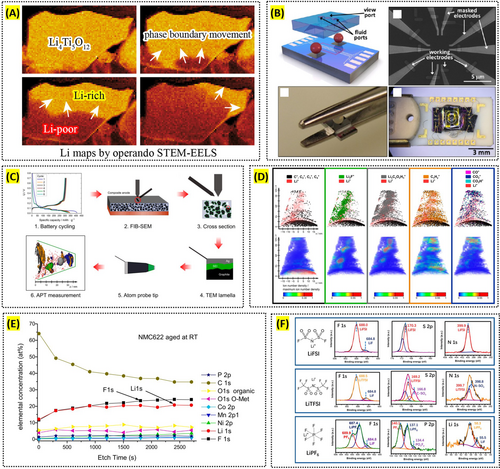
Reproduced with permission: Copyright 2025, American Chemical Society. Reproduced with permission: Copyright 2017, American Chemical Society. Reproduced with permission: Copyright 2023, American Chemical Society. Reproduced with permission: Copyright 2021, Elsevier. Reproduced with permission: Copyright 2022, American Chemical Society.
One of STEM's standout capabilities is its high spatial resolution, enabling the observation of single-atom dynamics within battery materials. Harrison et al. studied the behavior of lithium ions in solid electrolytes and electrodes, revealing atomic-level movements and interactions (Figure 6B). Such detailed observations are crucial for developing materials that facilitate faster ion transport and improve overall battery efficiency and durability [125, 126].
However, the acquisition of crystal structure information in STEM mode necessitates the use of high magnification to obtain the actual atom images, making it difficult to obtain crystal structure information over large areas. In recent years, an analytical method known as four-dimensional scanning transmission electron microscopy (4D-STEM) offers several significant advantages over traditional STEM techniques. Primarily, it possesses the capacity to capture a complete two-dimensional diffraction pattern at each scan position, resulting in a four-dimensional data cube. This capacity for versatile post-processing analyses enables researchers to extract a wide range of information from a single dataset. For instance, 4D-STEM can be used to generate virtual images, perform orientation mapping, and map strain distributions within materials by analyzing local lattice distortions from diffraction patterns [127, 128].
4.4 Atom Probe Tomography
Atom probe tomography (APT) is a high-resolution technique that provides three-dimensional compositional mapping at the atomic scale, with a spatial resolution on the order of 0.1 nm. It is based on field-evaporating atoms from a needle-shaped emitter and reconstructing their original positions using an inverse projection algorithm. APT is capable of detecting and quantifying the distribution of elements such as lithium with ppm-level detection sensitivity within battery materials, allowing for detailed analysis of microstructural degradation and performance loss. This technique is particularly valuable for studying buried interfaces and understanding how atomic-scale changes impact the overall efficiency and stability of batteries.
APT provides 3D compositional mapping essential for understanding microstructural degradation and performance of battery materials. Meng et al. and Maier et al. used APT to detect and quantify lithium distribution in electrode materials like lithium manganese oxide and LiNi0.8Co0.15Al0.005O2 cathodes, studying the precise locations of lithium and other elements within the material's structure [129, 130]. This level of detail is essential for identifying factors contributing to capacity fading and performance degradation in lithium-ion batteries, enabling the development of materials with improved stability and longevity [131, 132]. Choi et al. investigated the 3D elemental distribution in carbon-supported Pt, PtMn alloy, and Pt3Mn nanoparticles using atom probe tomography, revealing significant differences in Mn distribution after heat treatment, and compared the experimental data with evaporation simulations, providing valuable guidelines for applying atom probe tomography to heterogeneous carbon-supported nanoparticle systems [133].
APT particularly excels in analyzing internal interfaces at high resolution, providing critical insights into interactions at electrode/electrolyte boundaries. For instance, Singh et al. studied the LiFePO4-electrolyte interface, revealing the formation of interphase layers enriched with elements like fluorine and lithium [131]. This detailed interface analysis helps in understanding the formation and evolution of SEI, which is crucial for the stability and performance of lithium-ion batteries (Figure 6C,D) [134]. By examining the chemical composition and structure of these interphases, researchers can develop strategies to enhance battery efficiency and durability.
APT's unique advantage in analyzing all elements with equal detection sensitivity, including light elements like lithium, makes it invaluable for studying material degradation and electron migration in battery components. Barnett et al. observed lithium migration in carbon fiber electrodes, providing insights into how lithium distribution changes during cycling and affects electrode performance [132]. Additionally, Chen et al. quantified compositional fluctuations and phase transformations in high-nickel cathode materials, offering a deeper understanding of how these changes impact battery performance over time [135]. This information is crucial for designing electrodes that maintain structural integrity and electrochemical performance over prolonged use. However, a limitation of APT is its sensitivity to sample preparation, which can cause surface artifacts; overcoming this requires careful handling and optimized specimen preparation techniques.
4.5 X-Ray Photoelectron Spectroscopy
XPS is a surface-sensitive analytical technique that provides high-resolution analysis of the elemental composition, chemical bonding, and oxidation states of materials by measuring the kinetic energy of electrons emitted from a sample when irradiated with x-rays. XPS is particularly useful in battery research for studying the surface chemistry of electrodes and the formation of interfacial layers like the SEI. With a typical spatial resolution of 1–2 μm and an energy resolution of about 0.5–1 eV, XPS allows for precise identification of chemical states and subtle chemical changes at the surface, enabling detailed insights into the chemical reactions and degradation mechanisms occurring at the electrode surface. This information is critical for optimizing material properties to enhance battery performance, cycle life, and stability under various operating conditions.
XPS is a powerful tool for analyzing the surface chemistry of battery materials. Dedryvère et al. used XPS to investigate interfacial mechanisms in nanosilicon electrodes, revealing crucial insights into the reactions of surface oxides, lithium-silicon alloying processes, and the composition and thickness of the SEI layer [136]. Such information is essential for designing electrodes that can maintain stability and performance over multiple charge–discharge cycles.
One of XPS's unique strengths is its capability for depth profiling, allowing researchers to study the composition and structure of interfaces layer by layer. Winter et al. employed depth profiling XPS to analyze the SEI layer on commercial graphite anodes, providing a detailed model of the film structure through sputter depth profiling [137]. This method helps in understanding how different components and impurities are distributed within the SEI, which is crucial for improving the long-term stability and performance of lithium-ion batteries [138]. Additionally, using both soft and hard x-ray photoelectron spectroscopy, researchers like Malmgren et al. can vary the probing depth to study more surface-sensitive and bulk-sensitive interactions, offering a comprehensive view of the electrode/electrolyte interfaces [139]. Bondarchuk et al. addressed common challenges in XPS analysis of LiNixMnyCozO2 (NMC) cathodes, including spectral confusion and ambiguous peak fitting, and introduce a novel method to accurately quantify the Ni2+/Ni3+ ratio, emphasizing the role of LiF over nickel and manganese fluorides in the formation of the cathode-electrolyte interphase (Figure 6E) [140].
XPS is highly effective for identifying and quantifying chemical phases, making it ideal for studying electrochemical reactions and degradation mechanisms in battery materials. It has been used to monitor the redox behavior of transition metals in electrodes, such as reversible conversion reactions during lithium insertion and extraction. Understanding electrode material changes during cycling and developing strategies to mitigate degradation is essential. For instance, Martinez et al. used XPS to compare SEI layers in sodium-ion and lithium-ion batteries, providing insights into the performance differences between the systems [141]. XPS helps guide the development of more durable and efficient battery materials by revealing the chemical composition and changes in the SEI. However, a key limitation of XPS is the potential for misleading artifacts caused by residual lithium salts, which can affect the accuracy of SEI component identification. Bao et al. highlighted this issue, emphasizing the need for complete salt removal and control experiments to mitigate such artifacts (Figure 6F) [142]. Addressing these challenges through improved sample preparation and controlled analysis can enhance the accuracy and reliability of XPS in battery research.
5 Multiscale Integration
In the study of battery materials, understanding phenomena across different scales—from the atomic to the macroscopic—is crucial for optimizing performance and ensuring longevity. However, integrating data from various scales poses significant technical and practical challenges. This section explores the complexities of multiscale integration, the role of machine learning in overcoming these challenges, and the current trends and future directions in multiscale materials imaging and spectroscopy platforms.
5.1 Challenges
Integrating and interpreting information from different imaging tools across various scales is challenging. This process is crucial for correlating structural, chemical, and dynamic changes in battery materials but is often hindered by technical and practical obstacles.
Technically, one of the most prominent challenges is aligning and registering the same region across different imaging techniques. Each tool often has its unique coordinate system, field of view, and resolution. For instance, correlating micrometer-scale observations from x-ray tomography with nanometer-scale insights from SEM or TEM requires precise navigation and alignment. Any misalignment can lead to errors in data interpretation, making it difficult to establish a meaningful correlation between structural and compositional features.
Each imaging technique requires unique preparation methods, making it necessary to extensively adapt battery materials for different analytical approaches. For example, SEM and TEM may require thin sections or polished cross-sections, while x-ray tomography typically demands intact samples. These variations can alter the material's structure or introduce artifacts, complicating the comparison of data obtained from different techniques. Maintaining the integrity of the same region across multiple preparations is particularly challenging. Also, imaging conditions such as vacuum requirements for SEM and TEM or sample wetting for neutron scattering and some optical techniques can alter the material's properties. Capturing the same state of the material under these differing conditions is a significant challenge. For in situ studies, maintaining comparable states across tools while the battery operates adds further complexity.
The multimodal data obtained from different imaging techniques often vary in format, scale, and dimensionality. Each imaging tool has inherent limitations that can affect the reliability of multiscale integration. For example, x-ray-based techniques may struggle with low atomic number elements, while TEM can be limited in analyzing larger sample areas. Bridging this gap to ensure that observations at one scale are consistent and complementary with those at another scale requires careful calibration and interpolation techniques. Additionally, differences in contrast mechanisms between imaging modalities can complicate the interpretation of overlapping features. Combining such diverse datasets demands careful consideration of the strengths and weaknesses of each tool.
5.2 Multi-Modal Correlative Characterization
Elucidating the relationship between structural and chemical features of battery materials and their corresponding properties is a challenging task. To address this complexity, it is essential to perform correlative analyses by employing multiple characterization techniques—preferably on the same location of a sample or, at minimum, under the same material configuration. This approach ensures that morphological information and various physical properties can be directly compared and interpreted in a comprehensive manner. In particular, in situ multi-modal analysis is crucial for capturing the dynamic changes that occur during repeated battery cycling, such as structural evolution, performance fluctuations, and degradation. In this review, we highlight three representative examples of multi-modal correlative characterization currently being explored: x-ray tomography–based methods, FIB–SEM–based methods, and SPM–based methods.
X-ray tomography enables 3D structural visualization of materials over a wide range of length scales, spanning from the nanometer to the millimeter regime. Its non-destructive nature under in situ conditions makes it a powerful tool for monitoring the real-time structural evolution of battery materials, which can then be correlated with other in situ techniques that probe additional structural or physicochemical changes. For instance, Ziesche et al. investigated a commercial Li/MnO2 primary battery using x-ray tomography, visualizing electrode cracking and other forms of structural degradation in real time. They further employed neutron tomography to quantify changes in lithium diffusion processes and electrolyte wetting behaviors associated with electrode degradation (Figure 7A) [143]. In another study, Fordham et al. examined the aging processes of individual EV cells by combining x-ray tomography with infrared thermography, ultrasonic mapping, and synchrotron x-ray diffraction, thereby providing complementary insights into the mechanisms of cell degradation [144].
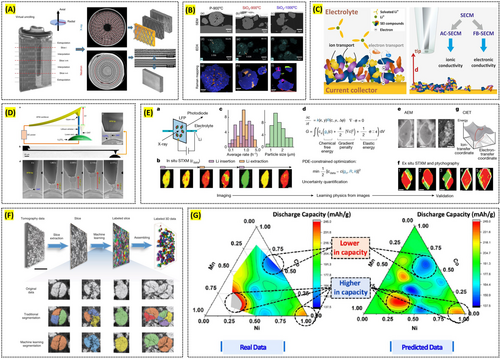
Reproduced with permission: Copyright 2020, Springer Nature. Reproduced with permission: Copyright 2025, Elsevier. Reproduced with permission: Copyright 2022, John Wiley and Sons. Reproduced with permission: Copyright 2020, Springer Nature. Reproduced with permission: Copyright 2023, Springer Nature. Reproduced with permission: Copyright 2020, Springer Nature. Reproduced with permission: Copyright 2021, American Chemical Society.
Focused ion beam scanning electron microscopy (FIB–SEM) offers high-resolution imaging of surface topography, microstructure, and roughness at the nano- to micrometer scale. It can be directly coupled with various chemical imaging techniques such as confocal Raman imaging, ToF-SIMS on the same area of a sample, enabling a detailed understanding of both microstructural and chemical/molecular characteristics. An additional advantage lies in the ability to perform sequential FIB milling and SEM, chemical imaging to construct a 3D representation of the structure and chemistry of battery materials. For example, Nisar et al. utilized correlative Raman–SEM microscopy to characterize secondary phases in LiNi0.5Mn1.5O4 (LNMO), which had previously been difficult to detect due to their low phase fraction and overlapping reflection issues (Figure 7B) [145]. This approach not only identified various secondary phases formed under different synthesis conditions but also revealed their impact on electrochemical performance. In another study, Liu et al. combined ToF-SIMS with FIB to uncover the complex chemical architecture of the cathode–electrolyte interphase (CEI), ultimately concluding that reduced surface reactivity is a key requirement for stable high-rate battery operation [146].
SPM techniques provide nanoscale imaging of surface morphology while simultaneously measuring diverse properties such as ionic conductivity, electronic conductivity, and work function of battery materials. By collecting data from different SPM modes at the identical sample location, researchers can directly correlate multiple properties at the nanoscale. Furthermore, coupling SPM with spectroscopic methods such as Raman, XPS, or ToF-SIMS allows the establishment of relationships between nanoscale morphological or physical properties and the chemical composition of the material. For instance, Santos et al. were the first to combine the feedback and multi-frequency alternating-current modes of SECM to quantitatively assess local electronic and ionic properties of the SEI under various formation conditions and electrolytes (Figure 7C) [147]. In another study, de Beeck et al. applied C-AFM and secondary ion mass spectrometry (SIMS) sequentially on the same LiMn2O4 (LMO) sample, thereby demonstrating a direct correlation between the presence of nanosized conductive pathways and local lithium concentration [108]. Additionally, Zhang et al. used environmental TEM to observe, under in situ conditions, the growth of lithium dendrites from an AFM tip, which enabled them to measure the yield strength of a single lithium whisker (Figure 7D) [148].
5.3 Role of Machine Learning
Machine learning (ML) encompasses a broad set of algorithms that learn patterns from data to make predictions or automate tasks. By integrating ML with various imaging methods, researchers can process and analyze detailed morphological data to gain critical insights into the structural evolution and degradation mechanisms of battery materials. In the context of battery imaging, supervised learning methods train models using labeled image datasets to classify features such as particle boundaries or failure regions, while unsupervised methods cluster similar patterns to reveal hidden structures without prior labeling. Deep learning, particularly convolutional neural networks (CNNs), has become instrumental in processing imaging data due to its ability to automatically extract hierarchical features—such as edges, textures, and spatial correlations—from raw images. For operando imaging, where data are often noisy and time-resolved, ML models can denoise frames, detect dynamic events like dendrite initiation, and track morphological evolution across time-series data.
For example, ML algorithms have been applied to map the 3D architecture of lithium-ion electrode particles, correlating grain morphology with electrochemical behavior [149]. This approach enhances the understanding of particle performance and failure modes, ultimately guiding the design of more efficient batteries [150].
At the mesoscale, machine learning aids in analyzing microstructural data to track phase transitions and morphological changes that occur during battery cycling. Deep learning techniques are used to segment 3D representations of battery electrodes, providing detailed insights into structural changes during operation [151]. The segmentation of x-ray tomographic images using ML models enables faster and more accurate analysis of materials distribution and inhomogeneities, which are critical for understanding degradation processes and optimizing battery design [152].
In operando studies, which monitor batteries in real time during cycling, ML significantly enhances the interpretation of dynamic imaging data. For instance, machine learning models can track lithium metal morphology during galvanostatic cycling, identifying hotspots and failure nuclei in real time [153]. This application allows for more effective monitoring of structural changes such as dendrite formation and particle detachment, offering insights into battery failure mechanisms and guiding the development of more robust electrode designs. By conducting machine learning on in situ STXM image data of carbon-coated LiFePO4 cathode nanoparticles, Zhao et al. revealed spatially heterogeneous reaction kinetics and identified the thickness of the surface carbon coating as a key factor underlying this heterogeneity (Figure 7E) [154]. This finding illustrates how machine learning can be leveraged to deconvolute multiple contributing factors from in situ experimental observations, thereby enabling the extraction and comprehension of complex physicochemical phenomena.
Machine learning also facilitates multiscale analysis by correlating data across different length scales, from nanoscale electrode particles to macroscale battery components. ML algorithms are used to analyze particle detachment in lithium-ion battery cathodes at multiple scales, providing valuable information on how microstructural changes impact battery performance (Figure 7F) [149]. This multiscale approach enables a comprehensive understanding of battery materials, from atomic-level interactions to the behavior of entire electrode assemblies. For instance, Hong et al. introduced the Materials and Molecular Modeling, Imaging, Informatics, and Integration (M3I3) materials platform, which aims to accelerate the discovery and design of new materials by integrating multiscale imaging, molecular modeling, and informatics with machine learning, showcasing global efforts and demonstrating how this approach can enhance the understanding of structure–property relationships for advanced materials development, particularly in energy and information applications (Figure 7G) [155].
One of the key strengths of machine learning in battery imaging lies in its ability to integrate data across different spatial and temporal scales. By learning correlations between nanoscale features and macroscale phenomena, ML models can provide a holistic interpretation of battery behavior. This enables the construction of predictive frameworks that connect local morphological evolution to global material performance. For instance, ML-assisted segmentation of x-ray tomographic data at the electrode scale, combined with particle-level mechanical analysis, has been used to predict large-scale cracking and delamination tendencies [156]. Such multiscale fusion approaches allow researchers to transcend the limitations of individual imaging methods and gain insight into emergent material properties that arise from cross-scale interactions.
Finally, ML has proven essential in predicting battery material behavior under various conditions by integrating experimental imaging data with simulations. For example, CNNs are combined with finite element methods to model the thermal properties of composite phase change materials, helping optimize battery pack design [157]. By simulating material behaviors and integrating data from multiscale imaging techniques, ML enables faster discovery and optimization of materials for next-generation battery systems.
5.4 Trend of Multiscale Materials Imaging and Spectroscopy Platform
Advances in imaging technologies are transforming our understanding of battery materials across multiple scales, providing insights critical for optimizing their performance and longevity. Among these innovations, x-ray microscopy stands out as a non-destructive, multiscale characterization tool capable of bridging the resolution gap from the micrometer to nanometer scale. According to Marco Rossi, XRM's ability to visualize internal structures with high resolution offers unparalleled opportunities to monitor battery degradation processes, such as dendrite growth and electrode delamination, in real time [158]. This technique's non-invasive nature makes it invaluable for in situ and operando studies, which are essential for correlating structural changes with electrochemical performance.
The integration of x-ray tomography with other modalities is another growing trend. Paul R. Shearing highlights how combining x-ray tomography with scanning techniques enables researchers to study the evolution of electrode microstructures during cycling [159]. Such studies have revealed critical insights into the effects of mechanical stress and particle fractures on battery capacity loss. These developments underscore the importance of multiscale imaging in capturing the interplay between macroscale electrode architecture and microscale particle behavior.
Despite these advances, challenges remain in achieving seamless integration across scales. Haegyeom Kim emphasizes the need for robust computational tools and algorithms to process and correlate data obtained from different imaging techniques [160]. ML is emerging as a transformative solution in this domain, offering tools for automated segmentation and pattern recognition in complex imaging datasets. Arnaud Demortière demonstrates how artificial neural networks can accurately analyze multiphase tomographic images, paving the way for more efficient and scalable battery characterization [161].
Additionally, in-line metrology is gaining attraction to accelerate battery development. Yijin Liu explores the transition from traditional in situ experimentation to real-time imaging during manufacturing processes [162]. This shift is enabling researchers to directly observe how production parameters influence battery performance, ensuring consistency and quality in commercial applications. Innovations in synchrotron x-ray techniques and correlative imaging platforms are also contributing to this trend, allowing for simultaneous investigation of chemical, mechanical, and thermal phenomena in operational batteries.
Looking ahead, the integration of multimodal imaging with computational modeling holds significant promise. By combining high-resolution imaging data with predictive simulations, researchers can develop comprehensive models of battery behavior under various operating conditions. This approach not only aids in material discovery but also accelerates the design of next-generation batteries with improved energy density, safety, and durability.
6 Summary and Outlook
6.1 Summary
This review highlights the critical role of multiscale imaging and spectroscopy techniques in advancing our understanding of battery materials. By addressing phenomena from the millimeter to atomic scale, these tools offer comprehensive insights into the structural, chemical, and dynamic behaviors of battery components. Techniques such as x-ray tomography, scanning electron microscopy, x-ray diffraction, and atomic force microscopy have proven indispensable for non-invasive analysis, real-time monitoring, and failure diagnostics in battery systems. Integrating these imaging techniques using ML further enhances the ability to process complex datasets, making it easier to understand degradation mechanisms and optimize battery design. The ability to study batteries at various scales has deepened our understanding of key processes like lithiation, dendrite growth, and phase transitions, which are critical for improving battery performance, longevity, and safety. Overall, the multiscale approach remains a cornerstone in the development of next-generation energy storage systems.
6.2 Outlook
Batteries involve interconnected phenomena across length scales ranging from millimeters to nanometers, and these multi-scale processes collectively govern both battery operation and degradation. Consequently, the ability to observe and correlate events happening at different scales is vital, underscoring the need for further advancements in microscopy techniques and image processing. Looking ahead, the following research directions are proposed for progress in the field of multiscale imaging and spectroscopy for battery materials.
We categorize the future development direction of the microscopy technique into three main areas: in-operando microscopy, higher resolution paired with faster acquisition times, and correlative imaging. In-operando microscopy, in particular, enables researchers to observe dynamic changes within batteries under near-realistic operating conditions, providing deeper insights into how ion transport, material deformation, and interfacial reactions evolve during charge–discharge cycles. Equally important is the pursuit of higher spatial resolution—on the order of nanometers—alongside faster acquisition rates to capture rapid transformations, such as dendrite growth or transient phase changes. Meanwhile, correlative imaging that integrates multiple techniques offers a more comprehensive view of key structure–property and property–property relationships, ultimately contributing to a better understanding of how microstructural features influence overall performance.
In the realm of image processing, the importance of machine learning has become increasingly evident. As operando and correlative imaging techniques evolve, the volume and complexity of the resulting data grow exponentially, making it both difficult and time-consuming to manually process all datasets. By leveraging machine learning, researchers can rapidly segment large and complex datasets and can also elucidate intricate correlations between material properties and structural characteristics. Furthermore, recent studies integrating experimentally obtained images into modeling and simulation indicate that machine learning–based image processing will create substantial synergy with these computational approaches, potentially accelerating both data interpretation and the design of more advanced materials.
Continued advancements in microscopy techniques and image processing will be instrumental in driving battery technology forward. By enabling clearer multiscale insights and deeper data analysis, these developments offer the potential to significantly enhance battery performance, safety, and longevity. Through refined imaging approaches and the integration of machine learning, researchers can more effectively diagnose and mitigate degradation mechanisms, accelerating the design of next-generation electrochemical energy storage systems.
Author Contributions
Youngwoo Choi: draft preparation and revision. Gumin Kang: draft preparation, review and editing. Seonghyun Kim: draft preparation, review and editing. Yoonhan Cho: draft preparation, review and editing. Jaewhan Oh: draft preparation, review and editing. Dongho Kim: review and editing. Jacob Choe: review and editing. Jong Min Yuk: review and editing. Pyuck-Pa Choi: review and editing. Yongsoo Yang: draft preparation, review and editing. Sung-Yoon Chung: draft preparation, review and editing. Chi Won Ahn: review and editing. Jongwoo Lim: review and editing. Seungbum Hong: supervision, review and editing.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2023-00247245).
Conflicts of Interest
The authors declare no conflicts of interest.