A Method for Analyzing Fatty Acids in Cattle Hair, with Special Emphasis on Lauric Acid and Myristic Acid
Abstract
This study is aimed at improving a protocol for measuring fatty acids in cattle hair with respect to sensitivity, repeatability, and speed to increase its applicability as a biomarker. For the investigation, 14 hair samples from German Holstein cows are used. Alternative methods for grinding the hair (mortar vs mill), lipid extraction (modified Folch vs kit extraction), and solvent evaporation before injection on a gas chromatograph (evaporated vs unevaporated extracts) are tested. Hair ground with a mill compared to that with a mortar has smaller particles and a higher concentration of total lipids after extraction (p < 0.02). The kit used for lipid extraction is faster, and the amount of extracted total lipids and individual fatty acids, especially C12:0, is increased (p = 0.001). The analysis of unevaporated methyl ester extracts using gas chromatography (GC) analysis yields 5.8 and 1.3 higher amounts of C10:0 and C12:0, respectively, than those of evaporated extracts (p < 0.001). According to the results, the protocol for determining fatty acids in cattle hair can be improved by grinding the hair with a mill, extraction of lipids with a kit, and direct loading of methyl ester extracts in a gas chromatograph.
Practical Applications: The fatty acid profile of hair reflects the metabolic status of an animal for the previous 1–3 weeks, as these fatty acids are not influenced by diurnal and short-term fluctuations. An improved protocol is developed that increases the throughput of fatty acid analysis and improves its applicability for practical use. For breeding and animal welfare, the analysis of cattle hair is possible for more efficient evaluation of the hair fatty acid profile as a robust biomarker in a larger animal population.
1 Introduction
A new and innovative approach is the determination of the fatty acid profile of hair to assess the energy availability in lactating cows. In periods of positive energy balance, fatty acids are synthesized de novo and stored in different depots in the body, including in the hair. Therefore, fatty acids can be determined, for example, in hair. Compared to other tissues and body fluids, hair is easy to access in sufficient amounts, as it is not influenced by daily or day-time fluctuation. Thus, a hair sample reflects the metabolism of the last 1–3 weeks.1, 2 In a previous study, we found a relationship between hair fatty acids, reproduction, and milk protein production and suggested C12:0 as a useful biomarker to assess energy utilization.3 Furthermore, an additional study confirmed that C12:0 is related to energy intake and energy utilization in early lactating cows.4 Recently, cortisol as a stress indicator5 and salbutamol as an indicator of drug consumption6 were identified in cattle hair.
To determine endogenous metabolites in the hair, the hair first must be cleaned from exogenous contaminations. The following grinding step to obtain a powdery hair sample is crucial to reduce the particle size of the hair, which increases not only the extraction surface but also the homogeneity of the sample, thereby improving the repeatability of the results. For lipid extraction from hair, the use of liquid nitrogen during grinding is inevitable to protect samples from becoming overheated.
Compared to other tissues and body fluids, hair lipids contain free fatty acids, triacylglycerols, phospholipids, ceramide, cholesterol ester, phytosphingosine, squalene, and wax esters.7-9 To date, no extraction method is available that extracts all lipid classes from tissues, cells, or body fluids simultaneously.10 The most widely used method for total lipid extraction was developed by Folch, et al.11 This method was used, for example, for muscle,12 adipose tissue,13 and hair,3 as well as for legumes.14 For routine analysis, the Folch method requires many steps, which make this method time-consuming. A commercial kit for lipid extraction, which is easy and simple to handle, would reduce the number of steps and time and thereby improve the efficiency. Available kits extract lipid classes such as nonesterified fatty acids,15 cholesterol, and triacylglycerols.16 Verification of the concordance of extracted lipids and fatty acids would be necessary to replace an existing extraction method with a faster kit extraction.
The determination of a fatty acid profile by gas chromatography (GC) requires the methylation of the fatty acids. A typical method is the combination of sodium methylate and boron trifluoride in methanol,17 which leads to efficient derivatization results of free fatty acids, triacylglycerols, cholesterol esters, and phosphatidylcholines.18 However, boron trifluoride in methanol alone has difficulties trans-esterifying triacylglycerols and cholesterol esters,19, 20 which are components of hair lipids.
Bovine hair can be collected without high manpower and is easy to store. Therefore, it offers a very good starting material for the determination of biomarkers. In two pilot studies,3, 4 we have shown that the content of de novo synthesized fatty acids, especially lauric and myristic acid, in hair was higher in cows, which had a better energy supply. This was associated with a better fertility, a higher milk protein yield,3 and higher energy utilization between the lactation weeks 1–6.4 From these two studies, we conclude that the fatty acid profile of bovine hair, in particular, the relative content of de novo synthesized medium chain fatty acids, is a suitable biomarker for the assessment of energy availability in cattle which is a physiological parameter that is difficult to measure. To validate this new and innovative approach in a bigger cow population, it is necessary to improve the protocol for fatty acid examination with respect to cutting down the time and the costs per sample. Therefore, it is crucial to improve the existing protocol to further examine the relationship between C12:0 and energy availability in bigger cohorts. The existing protocol for fatty acid analysis comprises the following steps: sample preparation, total lipid extraction, methylation of fatty acids, and GC. We tested different methods to optimize the whole procedure for cattle hair to save time and to increase the repeatability without compromising the sensitivity of fatty acid detection. Therefore, we examined the effects of the different methods i) for grinding of hair, ii) for total lipid extraction, and iii) for GC analysis with or without evaporation.
2 Experimental Section
2.1 Chemicals/Reagents
All solvents used were of HPLC grade or of the highest purity. N-heptane, chloroform, methanol, calcium carbonate, sodium sulfate, potassium carbonate, toluene, sodium hydrogen carbonate, and n-hexane were purchased from Carl Roth (Karlsruhe, Germany). C19:0 as the internal standard, boron trifluoride in methanol and the fatty acid extraction kit were purchased from Sigma-Aldrich (Taufkirchen, Germany). Sodium methylate was purchased from Merck (Darmstadt, Germany). Reference standards of fatty acid methyl esters for GC were purchased from Sigma-Aldrich (Taufkirchen, Germany), Biotrend (Köln, Germany), and Larodan (Solna, Sweden).
2.2 Hair Samples
Hair samples from 14 German Holstein cows (third lactation; 207 ± 120 days in lactation) were taken from the left ventral side of the foreleg in February 2017. All cows were from an experimental station (Leibniz Institute for Farm Animal Biology, Dummerstorf, Germany) and housed and cared for according to the German Animal Welfare Act.21 The hair samples were stored at −20 °C until further examination.
2.3 Fatty Acid Analyses of Hair
The existing protocol for fatty acid analysis comprises the following steps: sample preparation, total lipid extraction, methylation of fatty acids, and detection of the fatty acids using GC. Since this protocol is time-consuming, alternative methods of the protocol were tested to increase the throughput on the one hand and the amount of total lipids after extraction, as well as the amount of fatty acids after fatty acid methylation, on the other hand (Table 1). To assess the repeatability of the protocol, all examinations were performed in triplicate. Because of the low amount of hair from two cows, these samples were analyzed only in duplicate. Total lipids comprise all extracted lipids from hair. The sum of all fatty acids includes all fatty acids determined via GC from methyl ester extracts (see Table 2).
| Step | Examined methods | Evaluation parameter |
|---|---|---|
| 1) Grinding |
|
|
| 2) Total lipid extraction |
|
|
| 3) Methylation and GC analysis |
|
|
- a) is existing method and b) is alternative method.
| Standard extraction | Kit extraction | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | ϑ | Mean | SD | ϑ | ||||
| Saturated fatty acids | |||||||||
| C10:0 | 27.9 | ± | 9.0 | 0.92 | 31.2 | ± | 13.7 | 0.86 | n.s. |
| C12:0 | 20.2 | ± | 7.0 | 0.92 | 25.4 | ± | 11.4 | 0.95 | 0.001 |
| C14:0 | 117.1 | ± | 38.5 | 0.90 | 161.3 | ± | 67.3 | 0.94 | <0.001 |
| C15:0 | 2.8 | ± | 0.8 | 0.93 | 3.4 | ± | 1.1 | 0.91 | <0.001 |
| C16:0 | 73.8 | ± | 8.8 | 0.86 | 85.1 | ± | 13.3 | 0.89 | <0.001 |
| C17:0 | 2.6 | ± | 0.4 | 0.89 | 2.9 | ± | 0.5 | 0.89 | <0.001 |
| C18:0 | 41.8 | ± | 5.0 | 0.86 | 48.9 | ± | 8.0 | 0.87 | <0.001 |
| C20:0 | 11.4 | ± | 1.5 | 0.75 | 11.7 | ± | 2.4 | 0.91 | n.s. |
| C21:0 | 1.1 | ± | 0.2 | 0.56 | 1.0 | ± | 0.2 | 0.54 | n.s. |
| C22:0 | 8.6 | ± | 1.7 | 0.69 | 8.7 | ± | 1.9 | 0.86 | n.s. |
| C23:0 | 1.2 | ± | 0.3 | 0.54 | 0.8 | ± | 0.2 | 0.58 | <0.001 |
| C24:0 | 14.0 | ± | 3.8 | 0.85 | 16.6 | ± | 5.5 | 0.91 | <0.001 |
| C26:0 | 3.3 | ± | 1.0 | 0.70 | 3.5 | ± | 1.3 | 0.74 | n.s. |
| Monounsaturated fatty acids | |||||||||
| C16:1 | 2.5 | ± | 0.6 | 0.71 | 2.7 | ± | 0.7 | 0.77 | 0.025 |
| C18:1cis-9 | 17.7 | ± | 3.7 | 0.95 | 21.0 | ± | 4.6 | 0.87 | <0.001 |
| C18:1cis-11 | 9.3 | ± | 1.9 | 0.94 | 11.1 | ± | 2.2 | 0.88 | <0.001 |
| C20:1 | 2.5 | ± | 0.7 | 0.97 | 3.0 | ± | 1.0 | 0.96 | <0.001 |
| C22:1 | 0.8 | ± | 0.1 | 0.85 | 1.1 | ± | 0.2 | 0.58 | <0.001 |
| Polyunsaturated fatty acids | |||||||||
| C18:2n-6 | 12.1 | ± | 4.0 | 0.93 | 15.9 | ± | 5.3 | 0.88 | <0.001 |
| C18:3n-3 | 7.0 | ± | 1.2 | 0.91 | 8.4 | ± | 1.7 | 0.89 | <0.001 |
| C20:3n-3 | 1.3 | ± | 0.3 | 0.37 | 1.6 | ± | 0.4 | 0.75 | <0.001 |
| Branched chain fatty acids | |||||||||
| iso C14:0 | 2.4 | ± | 0.9 | 0.84 | 3.2 | ± | 1.3 | 0.95 | <0.001 |
| iso C15:0 | 0.6 | ± | 0.2 | 0.92 | 0.8 | ± | 0.3 | 0.66 | <0.001 |
| anteiso C15:0 | 1.8 | ± | 0.7 | 0.48 | 1.1 | ± | 0.4 | 0.59 | 0.004 |
| SFA | 332.0 | ± | 71.9 | 407.8 | ± | 122.7 | <0.001 | ||
| MUFA | 34.0 | ± | 6.4 | 40.0 | ± | 8.2 | <0.001 | ||
| PUFA | 21.6 | ± | 3.6 | 27.3 | ± | 5.1 | <0.001 | ||
- n.s., not significant. SFA = sum of C10:0 + C12:0 + C13:0 + C14:0 + C15:0 + C16:0 + C17:0 + C18:0 + C20:0 + C21:0 + C22:0 + C23:0 + C24:0 + C26:0 + iso C14:0 + iso C15:0 + anteiso C15:0 + iso C16:0. PUFA = sum of C18:2tr + C18:2n-6 + C18:3n-3 + C20:3n-3 + C20:4n-6 + C22:6n-3. MUFA = sum of C14:1 + C16:1 + C17:1 + C18:1trans-9 + C18:1trans-11 + C18:1cis-9 + C18:1cis-11 + C20:1 + C22:1.
2.3.1 Grinding Methods
Before grinding, the hair was cleaned of exogenous lipids with n-heptane, evaporated under a stream of nitrogen and dried at 35 °C for 5 h.
Grinding Using a Mortar: A total of 900 mg of cleaned hair was cut into 0.5 cm pieces and ground by hand with a mortar and pestle under liquid nitrogen for 3 min.
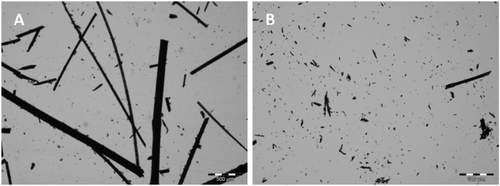
Grinding Using a Mill: A total of 900 mg of cleaned hair was added to a 25-mL stainless steel milling cup with two 10-mm stainless steel balls, cooled down with liquid nitrogen and inserted in a mixer mill (MM400, Retsch, Haan, Germany) at a vibration frequency of 30 Hz for 2 min. Since the milling process produces heat, the duration of milling was first optimized to ensure no chemical changes in the fatty acid profiles. To visualize the particle size of the ground hair, microscopic analysis was performed using an Olympus BX43 microscope (Olympus, Hamburg, Germany) equipped with an UC30 color camera (Olympus) and CELL^D image analysis software (OSIS, Munster, Germany). The existing extraction method was used to compare the two grinding methods. The best grinding method was selected for examination of the lipid extraction method.
2.3.2 Lipid Extraction Methods
Existing Extraction Method: The existing extraction method is a modified Folch method that has been described previously.3, 11 In brief, total lipids of 150 mg mill-ground hair were extracted using 8 mL chloroform/methanol (2:1, v/v). Lipids are retained in the chloroform layer. For quantifying fatty acids, the methyl ester of C19:0 was added as an internal standard to each sample before lipid extraction.
Kit Extraction Method: The Fatty Acid Extraction Kit from Sigma-Aldrich (Sigma-Aldrich, Taufkirchen, Germany) was used as an alternative method for extracting fatty acids from the hair. According to the kit protocol, 150 mg mill-ground hair was treated with 3 mL extraction solvent consisting of chloroform/methanol (2:1, v/v) and containing the ethyl ester of C19:0 as an internal standard for the quantification of fatty acids. After vortexing and a reaction time of 4 min, 0.5 mL of an aqueous buffer of the kit (composition is not disclosed by the company) was added and the sample was vortexed again. Subsequently, the extraction solution was poured into a syringe system containing a filter (provided in the kit). The eluted solvent contains the chloroform phase with total lipids. The solvents of the extracted lipids were then evaporated with a stream of nitrogen. Then, the total lipids were weighed. Since the reaction time for the extraction solvent was not specified in the kit protocol, it was optimized first to obtain the highest amount of total lipids. The kit method was evaluated after methylation of the extracted fatty acids and fatty acid detection using GC without solvent evaporation prior to injection. The best total lipid extraction method was used for examination of the evaporating methods.
2.3.3 Methylation of Fatty Acids and GC Analysis
As described previously,3 the fatty acids of all lipid extracts were methylated in a two-step reaction. In the first step, the saponification, 150 µL toluene and 1 mL sodium methylate were added to the lipid extract and the sample was shaken for 10 min in a water bath of 60 °C. In the second step, the methylation, 0.5 mL of a boron trifluoride in methanol solution (14%, w/v) was added and the sample was shaken for additional 10 min at 60 °C. The methylation was stopped by adding 1 mL saturated sodium hydrogen carbonate solution. Afterward, the fatty acid methyl esters were extracted with 2 mL n-hexane and subsequently dried with sodium sulfate and potassium carbonate (10:1, g/g) for 1 h. The tubes were capped immediately after the solvent had been added. To check if the methylation was complete, total lipid samples and associated methyl ester extracts were loaded on thin-layer chromatography using benzene-diethyl ether-acetic acid (85:15:1.5, v/v/v)17 as developing system and 1,4-dichlorofluorescein for visualization.
The prepared methyl ester extract was divided into two portions for subsequent GC to examine the influence of evaporation on the fatty acid profile. One portion was directly injected on the column. From the other portion, the solvent was evaporated before the sample was injected.
Unevaporated Extracts: After the methyl ester extract had been dried with sodium sulfate and potassium carbonate, as described above, 1 mL of the extract was transferred into a vial for direct injection into a CP SIL 88 capillary column (100 m × 0.25 mm, Agilent Technologies) for separation and detection of fatty acids in a capillary gas chromatograph Clarus 680, which is equipped with a flame-ionization detector (Perkin Elmer Instruments, Shelton, USA). The following temperature program was applied: 150 °C for 5 min, followed by a stepwise increase of temperature by 2 °C min−1 until 200 °C were reached. 200 °C were held for 10 min, followed by further increase of the temperature by 1 °C min−1 to 225 °C, which was held for 15 min. The injection volume of the methyl ester extracts was 3 µL, and the split ratio was 15:1.
For the identification of the single fatty acids, we used the reference standard mixture “Sigma FAME C4-C24” and the methyl esters of C19:0, C22:4n-6 (Sigma-Aldrich, Taufkirchen, Germany), C18:1cis-11, C22:5n-3, C18:2cis-9, trans-11 (Matreya, PA, USA), C18:4n-3, iso C14:0, iso C15:0, anteiso C15:0 and iso C16:0 (Larodan, Solna, Sweden) which were loaded on the column and run under the same GC column time program as the samples. The retention time for every single fatty acid of the reference standard mixture was used to identify the corresponding fatty acid in the hair sample.
For the quantification of fatty acids, we used C19:0 as an internal standard which was added to each sample and the reference standard mixture of all fatty acids as an external standard. A five-point calibration with the reference standard mixture was performed before the experiment started. The five calibration concentrations for every single fatty acid ranged between 16 and 415 µg mL−1 solvent. To ensure a correct identification and an accurate quantification of each fatty acid during the GC run of several hair samples, the reference standard mixture was injected into the column after every fifth sample for a separate run. Furthermore, the control of blanks was performed before every sequence of analyses. The limit of quantification of a fatty acid was a signal-to-noise ratio of 10:1. The lower threshold for accurate quantification was 0.34 µg fatty acid per 100 mg hair. To assess the repeatability of the protocol, analyses of hair samples were performed in triplicate.
Evaporated Extract: The remaining 1 mL methyl ester extract was filtered, and the solvent was evaporated in a vacuum centrifuge (Scanvac, Lynge, Denmark) at 2,000 rpm and 30 °C for 30 min. Then, the solvent was completely evaporated. Subsequently, the fatty acid methyl esters were dissolved in 100 µL n-heptane and injected into a column for the GC analysis using the same conditions as described above. We used 1 µL for injection and a split of 25:1.
2.4 Statistical Analyses
The statistical software SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used for data analyses. Figures were prepared with R version 3.3.2.22 All statistical tests were considered significant at a threshold of p ≤ 0.05. To identify differences between the “old” and “new” method, differences between the two methods were compared with Wilcoxon signed rank tests for dependent datasets. The repeatability ϑ of the total lipids, sum of all fatty acids, and individual fatty acids for each method were estimated using the variance σ² of the components sample and error [ϑ = σ² sample / (σ² sample + σ² error)].
3 Results
3.1 Comparison of Grinding Methods
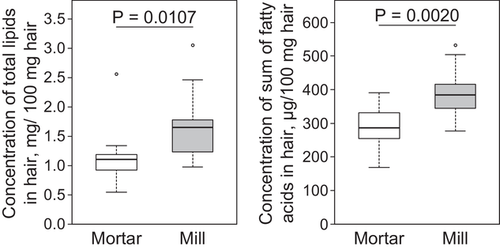
After grinding the hair with a mortar, the hair still showed its structure. When a mill was used for grinding, the hair was cut into small powder-like particles (Figure 1). Due to the increased surface of the mill-ground hair, significantly higher concentrations of total lipids were extracted, and a greater sum of all fatty acids was obtained compared to the mortar-ground hair (p < 0.011; Figure 2). In addition, the repeatability of the total lipid amounts obtained could be increased to 0.89 if mill-ground hair was used compared to mortar-ground hair. The repeatability for the sum of all fatty acids was also high (ϑ mortar = 0.93; ϑ mill = 0.88).
3.2 Comparison of Lipid Extraction Methods
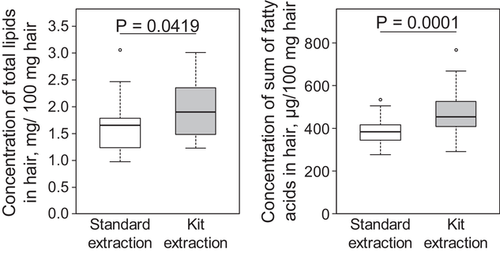
The sum of all fatty acids, as well as the concentration of most single fatty acids extracted with the kit, was higher compared to the existing extraction method (p < 0.001, Figure 3). The concentrations of saturated, monounsaturated, and polyunsaturated fatty acids extracted with the kit were 1.2, 1.2, and 1.3 times higher compared to the existing lipid extraction method, respectively. The concentrations of C12:0, C14:0, C16:0, and C18:0, as well as C18:2n-6 and C18:3n-3, which are important for the metabolism of ruminants, were 1.2 to 1.4 times higher with the kit (p < 0.002; Table 2). In addition, the repeatability was also improved with the kit extraction method independently of the method used for grinding the hair (e.g., measurements of C12:0 ϑ standard = 0.92; ϑ kit = 0.95; Table 2).
3.3 Comparison of Evaporating Methods
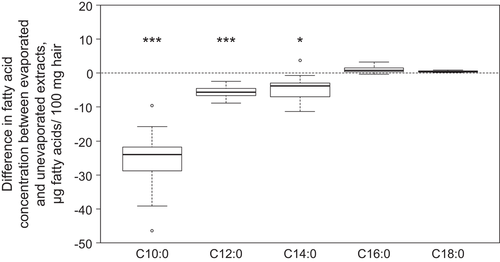
Although the unevaporated and evaporated methyl ester extracts were produced from the same total lipid sample, the measured total fatty acid concentration was 1.1 times higher in the unevaporated sample (p < 0.001). With evaporated and unevaporated samples, similar concentrations were determined for saturated fatty acids longer than 16 carbon atoms. For medium chain fatty acids, the concentrations of C10:0 and C12:0 were 5.8 and 1.3 times higher, respectively, in the unevaporated compared to the evaporated samples (p < 0.001), respectively (Figure 4).
4 Discussion
Earlier, we reported the first evidence that the fatty acid profile in hair was suitable to assess the energy availability for reproduction and milk protein production during early lactation in dairy cows.3 Furthermore, we could show that the fatty acids in hair were related not only to energy intake but also to energy utilization.4 Especially, the de novo synthesized fatty acid lauric acid was identified as a potential biomarker for energy availability. Since the existing protocol to determine fatty acids in hair is labor-intensive and time-consuming, the method for fatty acid analysis must be optimized to allow a wider application for throughput of more samples. Therefore, the objective of this study was the optimization of the fatty acid analysis protocol for cattle hair to reduce the time needed for lipid extraction and improve the amount of fatty acids extracted and the repeatability. We compared the existing protocol and alternative procedures for grinding, lipid extraction, and evaporation of the solvents before GC analysis.
First, the milling process was optimized to obtain a homogeneous sample without compromising lipid quality. A vibration mill was used to grind the cattle hair to prepare it for further analysis. This method had been used before for the detection of cortisol and ethyl glucuronide5, 23 in hair. After milling, the lipid extraction was more efficient and resulted in higher lipid yields compared to hair ground with a mortar. This is consistent with observations on human hair, where extensive grinding led to higher measurable amounts of ethyl glucuronide in the hair.23, 24 Although Burnett et al.25 did not cool down their samples for the detection of cortisol in cattle hair, we recommend cooling the hair samples during milling for subsequent fatty acid analysis because protection of the fatty acids from decomposition, especially polyunsaturated fatty acid, is crucial.26
The fatty acid extraction kit overcomes a long reaction time and reduces the number of steps during lipid extraction from 11 to 6. While the extraction of lipids from hair lasted 1 day using the existing protocol,3 the lipid extraction kit extracted hair lipids within 10 min. Furthermore, the number of samples that can be handled simultaneously using first the vibration mill and subsequently the kit was increased threefold compared to the existing method. In addition, the kit is more conveniently handled than other known lipid extraction methods27 because it also reduces hazardous conditions for human health.
In addition to saving time, the amounts of extracted total lipids, sum of all fatty acids, and major individual fatty acid concentrations were higher when the kit was used. Especially, the concentrations of C12:0, C14:0, C16:0, and C18:0, as well as C18:2n-6 and C18:3n-3, were higher when the kit was used compared to the existing method. This improvement was crucial for establishing C12:0 as a robust biomarker for the energy availability of dairy cows on a farm, as previously suggested.4
The combination of acidic and alkaline catalysts for transmethylation of fatty acids overcomes the long reaction time of the acidic catalysts and esterifies the fatty acids nearly completely. Previously, complete transmethylation of a standard lipid solution of triacylglycerols, di- and monoacylglycerides, cholesterol, cholesterol ester, and free fatty acids using sodium methylate and boron trifluoride in methanol has been shown.19 Nevertheless, methylating all lipids of a sample simultaneously is difficult, especially triacylglycerols, free fatty acids, N-acyl lipids, and very short chain fatty acids.18, 28
Although we did not expect a loss of fatty acid methyl ester during evaporation of solvents by vacuum centrifugation, our results show that the evaporation of methyl ester extracts using a vacuum centrifuge leads to a loss of 84%, 20%, and 2% for C10:0, C12:0, and C14:0 fatty acids, respectively. Generally, short chain fatty acids and their esters are highly volatile and readily soluble in water.29 Therefore, low reaction temperatures, anhydrous conditions, and short reaction times, as well as minimal number of work steps, are suggested for handling fatty acids,26, 30 particularly to minimize losses of C8:0 and C10:0.31 Regarding C12:0 and other fatty acid methyl esters shorter than 16 carbons, indications exist that a strong stream of nitrogen leads to the loss of methyl esters.32 Earlier, Lepage and Roy33 reported recoveries of 75% and 92% for C12:0 and C14:0 methyl esters, respectively, when extracts were dried under a stream of nitrogen.
The injection of the fatty acid methyl esters in the n-hexane layer directly onto a gas chromatograph column is commonly used for the detection of fatty acids with a chain length of 4–10 carbons in milk and ruminal fluids.18 This approach should also be done for the detection of C10:0, C12:0, and C14:0 in other samples. The specific settings of the gas chromatograph allowed the detection of low concentrations of fatty acids of a chain length between 10 and 26 carbons (limit of quantification >0.34 µg per 100 mg hair) in unevaporated extracts.
5 Conclusions
In summary, optimization of fatty acid analysis with special emphasis on lauric acid and myristic acid (C12:0, C14:0) can be achieved by (1) grinding the hair with a mill, (2) extracting the total lipids with an extraction kit, and (3) directly injecting the methyl ester extracts for GC analysis. Using the optimized protocol, we were able to increase the throughput of samples for the fatty acid analysis of bovine hair three times compared to the existing method. The milling procedure led to a higher homogenization allowing subsequently robust measurements of fatty acids. Despite the short time for fatty acid extraction using a kit, higher concentrations of fatty acids could be extracted. Omitting the evaporation process did not only save the analysis time but further increased the detectable amount of C12:0 and other fatty acids, which is crucial for the application of C12:0 as a robust biomarker. The presented method now allows a higher throughput of samples, which is necessary to further evaluate the hair fatty acid profile as a biomarker for energy availability and later application in cattle breeding.
Acknowledgements
The authors thank Maria Dahm and Birgit Jentz, Institute of Muscle Biology and Growth, Leibniz Institute for Farm Animal Biology (FBN), for their excellent technical assistance with fatty acid extraction and GC. The project is supported by the German Government's Special Purpose Fund held at Landwirtschaftliche Rentenbank (Project Number: 807799).
Conflict of Interest
The authors declare no conflict of interest.




