Synthesis of KF/CaO as a catalyst for the production of bio-fuel from cracking of Cornus wisoniana oil
Abstract
This study aimed to prepare KF/CaO catalyst with high catalytic activity that can be applied to produce bio-fuel from Cornus wisoniana oil. The KF/CaO catalyst was characterized by Brunauer–Emmett–Teller surface area (BET), XRD, scanning electron microscopy (SEM), and temperature programmed desorption (TPD). The chemical compositions of C. wisoniana oil and bio-fuel oil were analyzed by GC–MS. The effect of the catalyst preparation conditions on catalytic activity was investigated. The optimal process conditions for the KF/CaO preparation were KF loading rate of 40%, calcination temperature of 600°C, and 4 h calcination time. Under the optimum process conditions, the bio-fuel yield reached 82.7 and 65% bio-fuel yield was obtained after being reused nine times. The bio-fuel oil obtained by catalytic cracking has similar chemical compositions to petroleum-based diesel, which exhibited good fuel properties.
KF/CaO was used as a catalyst for the production of bio-fuel from cracking of Cornus wisoniana oil.
Abbreviations
-
- BET
-
- Brunauer–Emmett–Teller surface area
-
- SEM
-
- scanning electron microscopy
-
- TPD
-
- temperature programmed desorption
1 Introduction
Growing interest in alternative energy sources chiefly emanates from issues relating to the shortage of traditional fossil energy and environment pollution. Among the options for alternative energy sources, biomass is the only renewable energy source that directly yields liquid fuels 1, 2. The researches of biomass focus on lignocellulosic material and triglycerides 3, 4. The well-known traditional biofuel that is currently used worldwide and is produced from biomass is biodiesel, which is associated with FAME produced by the transesterification of triglyceride-containing feedstocks 5, 6. But compared with petroleum-based diesel, biodiesel exhibits poor low temperature fluidity performances and calorific value, which could cause engine performance problems 7.
Catalytic cracking of triglycerides provides an alternative way of producing renewable bio-fuel products, which has significant advantages over transesterification, including lower processing costs, compatibility with engines and fuel standards, and feedstock flexibility. More importantly, the final product is a rich hydrocarbon fuel containing mixture of alkanes, alkenes, carbonyl compounds, and fatty acids, which are similar in composition to petroleum-based diesel.
Previous studies have reported that molecular-sieve catalysts, including ZSM-5, MCM-41, and Y zeolite 8-11, have been utilized to prepare rich hydrocarbon fuel from vegetable oils and animal fat by catalytic cracking process. However, high carboxylic acid has been detected in these rich hydrocarbon fuels, which has negative effect on the corrosion value, cold filter plugging point and freezing point of the bio-fuel. Therefore, screening suitable alkaline catalyst has become one of research directions.
In addition, almost all-commercial triglyceride are from edible oils such as soybean oil and rapeseed oil. But as bio-fuel use increases, demand for edible oil feedstocks will intensify and clash with the need to produce food. According to international bio-fuel standard, oils from woody oil plants have been defined as bio-fuel materials, such as Manioca, Cornus wisoniana, Camellia oleifera, and other non-food woody oil plants. Compared with oil crops, the woody oil plants relatively hold remarkable superiority in two aspects. Firstly, wood oil plants will not have competition with foodstuff. Secondly, woody plants enriched oils can produce novel fatty acid for feedstocks of bio-fuel 12, 13. Among many woody plant resources, C. wisoniana is a representative woody oil plant (oil content of its fruit is more than 30%) distributed in the south region of the Yellow River, China 14. As C. wisoniana can be planted on marginal land that is not suitable for other crops, it does not compete in the food chain. Hence, C. wisoniana oil is considered to be a potential raw material of bio-fuel.
In this study, the rich hydrocarbon bio-fuel has been prepared from C. wisoniana oil in the presence of KF/CaO as catalyst. Firstly, the structure and properties of KF/CaO catalyst were characterized by BET, XRD, SEM, and TPD. Subsequently, the preparation conditions of KF/CaO were optimized based on the yield of bio-fuel oil. To investigate the possibility of application of product, the compositions and fuel properties of bio-fuel oil also were analyzed.
2 Materials and methods
2.1 Materials
Raw oil obtained from C. wisoniana fruit by cold pressing process. The fatty acid compositions of C. wisoniana oil were listed in Table 1. KF and CaO were purchased from Sigma–Aldrich Co., Ltd.
| Component | wt% |
|---|---|
| Hexadecanoic acid (C16:0) | 5.0 |
| Octadecanoic acid (C18:0) | 4.7 |
| Octadecenoic acid (C18:1) | 56.8 |
| Linoleic acid (C18:2) | 19.0 |
| Linolenic acid (C18:3) | 5.0 |
| Other fatty acid | 9.5 |
2.2 Catalyst preparation
The catalyst KF/CaO was prepared by impregnation method. Prior to immerse, the carrier of CaO 10.0 g was baked in muffle for 2 h at 500°C. Then CaO was immersed in 30–55 mL KF solution with certain amount (3, 3.5, 4, 4.5, 5, and 5.5 g) mechanical stirring for 5 h followed by 1–6 h calcination in muffle furnace at different temperature (300, 400, 500, 600, 700, and 800°C). The catalysts were preserved in dryer for use once again.
2.3 Characterization
The structure, composition, and morphologies of KF/CaO were examined by XRD (D/max2500PC diffract meter, RIGAKU, Japan) and scanning electron microscopy (SEM, XL-30ESEM instrument, PHILIPS, Holand). The specific surface area of KF/CaO was measured by the Brunauer–Emmett–Teller (BET) method with the instrument of NOVA 2000e (Quantachrome, USA). The basic properties of KF/CaO were measured by CO2-TPD (TP-5080, XIANQUAN) and Hammett indicator.
2.4 Catalytic cracking of C. wisoniana oil
Catalytic cracking of C. wisoniana oil was carried out in a homemade cracking reactor. C. wisoniana oil (200 g) after pretreated was taken into reactor and mixed with KF/CaO (1.2 wt%) catalysts that were prepared under different process conditions. A bimetallic thermometer at the bottom of the reactor determines the temperature change of the reactants. Catalytic cracking was carried out with C. wisoniana oil and KF/CaO catalyst at 500°C for 45 min. The yield of bio-fuel oil was calculated, which based on the carbon balance. The equation of bio-fuel yield is as follow.
Yield of bio-fuel = mass of bio-fuel oil/mass of raw C. wisoniana oil
2.5 Analysis of bio-fuel oil
Reaction products were periodically sampled and analyzed using GC–MS (BRUKER DALTONICS INCORPORATED) with HP-5MS type capillary column (30 m × 0.05 µm × 0.32 nm) and hydrogen flame ion detector. The initial oven temperature was 50°C with an equilibrium time of 2 min. Then the oven was heated at a speed of 5°C/min to 280°C, the temperature was remain for 20 min. Nitrogen as carrier gas, its velocity is 30 mL/min. A total of 0.1 µL sample was injected into the GC–MS. The samples were dissolved into hexane at a concentration of 0.1 g per 100 mL. The peak of light olefin was obtained compared with peaks of catalyzed by several KF modified catalyst loading different carriers.
3 Results and discussion
3.1 Characterization of KF/CaO catalyst
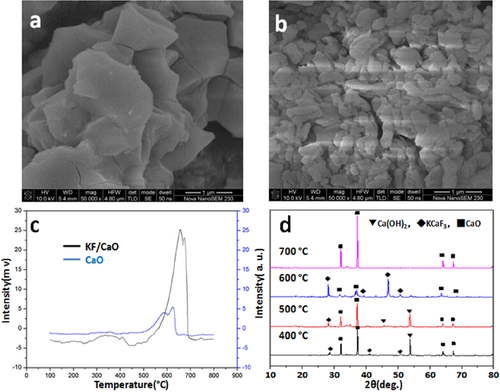
The SEM photograph (Fig. 1a) showed that the CaO carrier was tiny block structure. However, the CaO carrier (specific surface = 11.6 ± 0.5 m2/g) modified by KF (Fig. 1b) presented porous microstructure 15, which could significantly increase catalytic efficiency due to larger specific surface (107 ± 0.5 m2/g) and more basic sites (H = 15.0–18.4). As seen in Fig. 1c, the CO2-TPD was in agreement well with that of SEM pattern. The KF/CaO catalyst revealed strong basicity. According to the diffraction peak in XRD pattern (Fig. 1d), KF was successfully loaded on CaO carrier during the impregnation stage. The new crystal structure of KCaF3 formed at lower calcination temperature (approximately 400°C). With the calcination temperature increasing, the KCaF3 content increased until the calcination temperature reached 600°C. Subsequently, the KCaF3 was decomposed at higher calcination temperature and the intensity of CaO diffraction peak is accordingly increased.
3.2 Catalytic cracking of Cornus wilsoniana oil
The effect of the preparation technical parameters of KF/CaO catalyst on the yield of biofuel was discussed. As shown in Fig. 2a, the biofuel yield increased with the increase of KF loading amount. The biofuel yield reached its maximum at 40% loading amount of KF, and then gradually decreased. The results implied that strong base sites are harder to create with low loading amounts. Meanwhile, the active sites are more dispersed on the CaO surface at a low loading of KF. However, if CaO is loaded with too much KF, the KF cannot disperse properly and the excess KF could cover the active sites on the composite surface, which caused the decrease of catalytic activity 16.
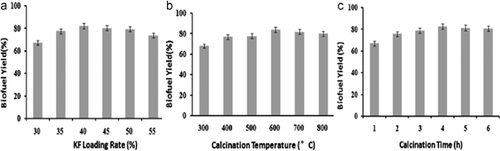
The relation between biofuel yield and calcinations temperature (Fig. 2b) is consistent with XRD pattern. At 600°C, the best crystal structure of KF/CaO was formed, and it obtained the best catalytic activity. The effect of calcination time on the yield of biofuel was shown in Fig. 2c. As calcinations time went on, more basic sites gradually formed, which improved the catalytic activity of KF/CaO. But if KF/CaO was calcinated for more than 4 h, the surface of CaO carrier formed compact structure due to modulizing. The compact surface texture blocked the formation of basic sites, which resulted in catalysts deactivation 17.
Based on the above results, the bio-fuel yield reached 82.7% when KF/CaO obtained under optimal process conditions acted as basic catalyst in cracking reaction. Subsequently, the reusability of KF/CaO and CaO are presented in Fig. 3.
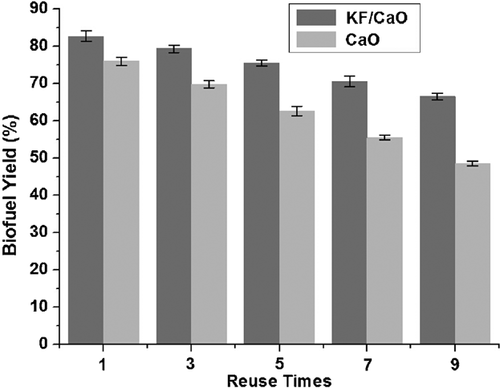
The results showed the loss of catalyst's mass in each recovery was minimal and after the ninth repetition the conversion ratio around 65% was maintained. On the contrary, not only catalytic activity but also reusability of CaO catalyst is lower than KF/CaO catalyst. This was mainly due to two reasons. On the one hand, the specific surface of KF/CaO is larger than CaO, which increased the contact between oil and catalyst, leading to improve catalytic effectiveness. On the other hand, based on the previous TPD characterization, KF and CaO combined to form KCaF3 crystal that provided more basic sites (activated center). In addition, free K+ and Ca2+ concentration in the biofuel was determined by atomic absorption spectrometry after removal of the catalyst CaO or KF/CaO. The result showed that the concentrations of dissolved K+ and Ca2+ from KF/CaO and CaO catalysts were 16.7(K+), 25.4 and 136 mg L−1, respectively. It indicates that KF/CaO catalyst is more stable than CaO in the reaction system as a result of the existence of KCaF3 crystal phase 18. Since less loss of active component, the lifetime of KF/CaO was longer than CaO, which consisted with the previous reusability test.
3.3 Compositions and fuel properties of bio-fuel oil from C. wisoniana oil
Through GC–MS analysis of cracking products (Fig. 4), the main chemical compositions were listed in Table 2, including alkane, olefin, ester, and acid. Most of them were short-chain hydrocarbons (C5-C22). It further proved the macromolecular of C. wisoniana oil was converted to small molecule hydrocarbons after cracking reaction, polymerization reaction, and isomerization.
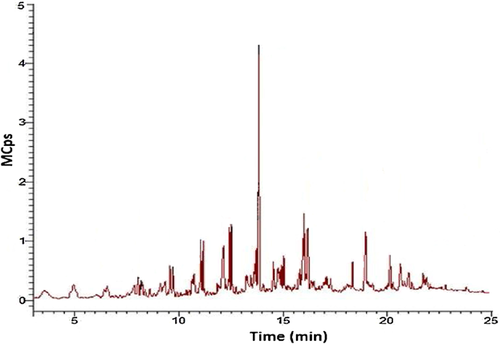
| Name | Molecular formula | Hold time (min) | Relative content (%) |
|---|---|---|---|
| 1-Octene | C8H16 | 3.334 | 1.791 |
| 2,5-Dimethylhexane | C8H18 | 3.440 | 2.141 |
| Butylcyclopentane | C9H18 | 4.741 | 1.758 |
| Nonane | C9H20 | 4.879 | 3.577 |
| Decylene | C10H20 | 6.369 | 1.2 |
| Decane | C10H22 | 6.516 | 1.376 |
| Heptanoic acid | C6H13COOH | 7.641 | 1.652 |
| 5-Undecene | C11H22 | 8.008 | 1.722 |
| Undecane | C11H24 | 8.146 | 1.437 |
| Cycloundecane | C11H22 | 8.216 | 1.222 |
| 2-Dodecene | C12H24 | 9.566 | 2.753 |
| Dodecane | C12H26 | 9.697 | 2.593 |
| 3,5,5-Trimethyl caproic acid | C8H17COOH | 10.6 | 1.85 |
| 1-Tridecene | C13H26 | 11.035 | 2.944 |
| Tridecane | C13H28 | 11.152 | 2.994 |
| Decenoic acid | C9H17COOH | 11.984 | 3.777 |
| 2-Ethyl-1-dodecene | C14H28 | 12.414 | 3.888 |
| n-Tetradecane | C14H30 | 12.521 | 4.111 |
| (E)-7-Tetradecenyl acetate | (E)-C15H30 | 13.632 | 2.613 |
| (Z)-7-Tetradecenyl acetate | (Z)-C15H30 | 13.708 | 2.798 |
| Pentadecane | C15H32 | 13.811 | 16.195 |
| 1-Hexadecene | C16H32 | 14.529 | 2.001 |
| n-Hexadecath | C16H34 | 14.937 | 1.791 |
| 8-Heptadecene | C17H34 | 15.937 | 3.846 |
| Heptadecane | C17H36 | 16.177 | 6.07 |
| cis-10-Heptadecenoic acid | C16H31COOH | 18.877 | 3.623 |
| Heneicosane | C21H42 | 20.122 | 1.179 |
| 1-Docosene | C22H44 | 22.719 | 1.813 |
The main properties of the bio-fuel oil obtained from C. wisoniana oil are shown in Table 3. For comparison purposes, Table 3 also gives the specified values for the petroleum-based diesel and biodiesel from transesterification reaction. The result showed that the bio-fuels derived from C. wisoniana oil possess acceptable values of the given fuel properties when compared with those for the petroleum-based fuel and biodiesel. By using basic catalyst, the cracking products showed a relatively low acid value. In addition, the bio-fuel oil exhibited better low temperature fluidity and oxidation stability because it contained abundant short-chain hydrocarbons.
| Property | Bio-fuel oil | Diesel | Biodiesel |
|---|---|---|---|
| Caloric value (KJ g−1) | 41.52 | 46.15 | 39.53 |
| Acid value (mg KOH/g) | 3.29 | ≤5 | ≤0.8 |
| Kinematic viscosity (mm2 s−1) | 3.51 (20°C) | 3–8 (20°C) | 1.9–6.0 (40°C) |
| Cold filter plugging point (°C) | −18 | 0 | −5 to −10 |
| Freezing point (°C) | −25 | −19 | −5 |
| Cetane number | 47 | ≥45 | ≥49 |
| Oxidation stability (110°C)/h | 8.69 | — | ≥6 |
4 Conclusions
The results in this work showed that the catalytic cracking of C. wisoniana oil with KF/CaO generates the bio-fuel oil. The chemical composition of the bio-fuel oil is similar to petroleum-based diesel. GC–MS analysis of the products has shown that the principal chemicals are short-chain hydrocarbons in the bio-fuel oil. Therefore, the bio-fuel oil exhibited good low temperature fluidity such as the cold filter plugging point and freezing point. Moreover, the acid value of product was reduced dramatically by using KF/CaO as a basic catalyst. In conclusion, the bio-fuel oil will have a wide application prospect due to its good fuel properties.
The authors acknowledge the financial support received from “948” Project of Chinese State Forestry Administration (2010-4-10), National Natural Science Foundation of China (31101388), National Science Foundation for Post-doctoral Scientists of China (2014M550419), Natural Science Foundation of Jiangsu Province (BK2011468), Zhenjiang Industrial support program (GY2011006), Jiangsu Province Key Lab Foundation (BM2008206001), Jiangsu University Research Foundation for Young Scholars (08JDG039), and Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
The authors have declared no conflict of interest.




