Chemical composition, thermal stability and antioxidant properties of tea seed oils obtained by different extraction methods: Supercritical fluid extraction yields the best oil quality
Abstract
A comparison study has been performed for tea seed oils (TSO) extracted from different methods and sources, which were oil from cold-pressing of tea seeds [TSO(CP)], oil from supercritical fluid extraction of seeds [TSO(SCF)], and oil from supercritical fluid extraction of tea seed cake [TSCO(SCF)]. The chemical composition, thermal stability, antioxidant properties, and physicochemical properties of the oils were determined. The main ingredient in seed and cake was fat, fiber, total sugar, and considerable amounts of amino, 74.59 and 93.25 g/100 g protein, respectively were found. The results also indicated that oleic acid and linoleic acid were the dominant unsaturated fatty acids in tea seed oils, irrespective of extraction methods and sources. The TSO(SCF) contained the highest levels of squalene, lupeol, and total sterols whereas TSCO(SCF) and TSO(CP) had higher contents of total phenolics and α-tocopherol. The TG/DTG curves showed TSO(CP) and TSO(SCF) were more thermally stable than TSCO(SCF), and the antioxidant activity assays demonstrated that TSO(CP) and TSCO(SCF) exhibited stronger radical scavenging ability. The overall physicochemical properties of the tested oils indicated that TSO(SCF) had the best oil quality. This work may be useful to food oil industry to produce high quality tea seed oils through utilization of suitable oil extraction methods and raw materials.
Practical applications: The growing consumption of green tea promoted the increased production of tea seed, which has totaled as much as 1.6 million tons annually since 2011. As an important source of edible oil, the exploitation rate of tea seed is insufficient (≤1%) in China. This study indicates that oil extracted from tea seed as well as cold-pressing cake can be exploited for high quality edible oil because of similar fatty acid composition and better bioactive compound composition (e.g., α-tocopherol, total phytoterols, and lupeol) compared to olive oil. Further, the evaluation of thermal stability provides basis for oil's refining to prepare salad oil, mayonnaise, etc. TSO(SCF) and TSCO(SCF) both had good antioxidant activity, which is applicable in the industry of skincare.
A comparison study has been performed for tea seed oils (TSO) extracted from different methods and sources, which were oil from cold-pressing of tea seeds [TSO(CP)], oil from supercritical fluid extraction of seeds [TSO(SCF)], and oil from supercritical fluid extraction of tea seed cake [TSCO(SCF)]. The chemical composition, thermal stability, antioxidant properties, and physicochemical properties of the oils were determined.
Abbreviations
-
- CP
-
- cold pressing
-
- GAE
-
- gallic acid equivalents
-
- SCF
-
- supercritical fluid
-
- TG
-
- thermo-gravimetric
-
- TPC
-
- total phenolic content
-
- TSO
-
- tea seed oils
1 Introduction
In addition to leaves, tea seeds are another product of tea plant (Camellia sinensis L.), which is different from oil-tea plant (Camellia oleifera Abel.) with respect to species, although they both originated from Theaceae, Camellia. Generally, the seed or oil from the latter is also called tea seed or tea seed oil, but its leaves can't be used for processing tea except Camellia nitidissima.
In recent years, due to the growing demand of teas, the production of tea seeds has also increased. It was reported that there were about 1.6 million tons of tea seeds produced annually in China 1. In practice, tea seeds are considered as a by-product of tea leave production and discarded as waste. Recent research indicated that tea seeds are a good source of edible oils 1. Tea seed oils are rich in unsaturated fatty acids and the contents of oleic and linoleic are as high as 80%, which are comparable with olive oil 2. Moreover, some other important bioactive compounds such as polyphenols, tocopherols, phytosterols, and β-carotene compounds have also been reported in tea seed oils 3, 4. A number of studies have shown that tea seed oils were a good source of emollients for skin care, and possess antioxidant activity and anti-obesity activity 3, 5, 6.
Tea seed cake is the residual after press extraction of oil from tea seeds in the traditional oil processing industry. Practically, oils in the seed cake can be further extracted using organic solvents. However, this method has distinct drawbacks such as potential hazardous solvent residue, time-consuming, and labor intensive. Thus, it is important to research and apply alternative methods in extracting oils from low oil content materials (e.g., tea cake) with less pollution and better quality. Supercritical fluid (SCF) extraction is a novel oil extraction method developed in recent years. Carbon dioxide is the most commonly used solvent in SCF because it is non-toxic, inert, non-flammable, economic, and can be easily eliminated from the extracts. More importantly, SCF-CO2 has a relatively low critical temperature (31.1 °C) and moderate critical pressure (7.39 MPa) that can be conveniently operated in industrial conditions. Currently, SCF-CO2 has been applied in extracting oils from green tea seed, linseed 7, 8, and also extracting natural substances such as pigments, flavonoid, flavors, and fragrances from plants 9-11.
Extraction of oils from cottonseed flakes, rapeseed cake, and wheat bran using SCF-CO2 method has been reported previously 12-14. However, no information is available in the extraction of oils from tea seed cake, which is still rich in oils 1. The objective of this study was to extract oil from tea seed using the traditional cold-pressing [TSO(CP)] and SCF-CO2 methods [TSO(SCF)]. The oils in the tea seed cake after cold-pressing were further extracted by the SCF-CO2 method [TSCO(SCF)]. A comparison study was conducted among the three oils with respect to chemical composition, thermal stability, and physicochemical characteristics. In addition, the antioxidant capacities of the oils were evaluated by 1-diphenyl-2-picrylhydrazyl (DPPH) and diammonium salt (ABTS) radical-scavenging assays. The data obtained in this work could be useful to the food oil industry to produce high quality tea seed oil and expand its potential use as functional food ingredients.
2 Materials and methods
2.1 Materials
Tea seeds (C. sinensis (L.) O. Kuntze, Theaceae) were kindly supplied by Liu Jiaxiang Food Co., Ltd. (Quzhou, China). Seeds were dried at 45 °C for 24 h in an oven and then pulverized to homogeneous size by a Chinese medicine disintegrator (DJ-04B, Shanghai Dianjiu Traditional Medicine Machinery Manufacture Co., Ltd., China). Virgin olive oil was purchased from a local supermarket (Tesco®, Hangzhou, China) and used as a control in quality evaluation.
2.2 Chemicals
Hexane, methanol, potassium hydroxide, isopropanol were purchased from Shanghai Lingfeng Medicine Chemical Reagent Co., Ltd. (Shanghai, China). Tea saponin and α-tocopherol were provided by Guizhou Di Technology Co., Ltd. (Guiyang, China). Isooctane, potassium iodide, gallic acid, iodine monochloride, glucose, vanillin, 1, DPPH, and 2,2'-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) ABTS were purchased from Aladdin Industrial Corporation (Shanghai, China). Folin–Ciocalteu's reagent was supplied by Shanghai La Baide Biotechnology Co., Ltd. (Shanghai, China) and carbon dioxide (99.9%) by Hangzhou Jingong Specialty Gas Co., Ltd. (Hangzhou, China).
2.3 Extraction procedures
2.3.1 Cold-press extraction
Prior to extraction, the moisture content of seed was adjusted to 8.0% in an oven 15. Cold-pressing extraction was performed using a Dragon screw press (Model D-1688, Zhengzhou Dragon Heavy Industry, Henan, China) and the temperature of oils was controlled at 40 °C according to Martíneza et al. 15. The residual cake after cold-pressing was used for SCF-CO2 extraction later.
2.3.2 Supercritical carbon dioxide (SCF-CO2) extraction
Oils were extracted from tea seed and cake using a supercritical fluid extraction device (SFE-prime, Applied Separations Inc., USA). For each experiment, the extraction pressure (30 MPa), temperature (50 °C) and CO2 flow rate (10 kg/h) were controlled by adjusting the regulating valves. When the scheduled time was achieved, the oils were collected and then kept at the temperature of 4 °C in a refrigerator for further analysis.
2.4 Analytical methods
2.4.1 Proximate composition of tea seed and cake
Proteins of seed and cake were analyzed according to Kjeldahl method, using the general factor (6.25) to calculate the total protein by AOAC 978.04 Method 16. Total sugar was determined according to the method described by Dubois et al. 17. Crude fat was determined using AOAC Method Ba 3–38 18. Moisture content of seed was determined according to AOAC Method 950.46 19, and crude ash by standard AOAC Method 942.05 20. Tea saponin was determined according to the method described by Yan et al. 21. Crude fiber was calculated by subtracting the sum of the percentages of fat, protein, ash, moisture, tea saponin, and total sugar from 100%.
2.4.2 Amino acid analysis of tea seed and cake
Defatted seed and cake (100–150 mg) were hydrolyzed by treatment with 10 mL of 6 M hydrogen chloride and 3–4 drops of freshly distilled phenol in a sealed tube at 110 °C for 24 h. Then the hydrolyzate was paper filtered and the filtrate was diluted to 250 mL with distilled water. Prior to injection into an automatic amino acid analyzer (S7130-Sykam, Germany) for amino acid determination, the preparation was filtered through an organic microporous filter (Φ13 mm × 0.45 µm, Zhenzhou Guoda Instruments & Equipment Co., Ltd., Zhenzhou, Henan, China).
2.4.3 Fatty acid composition of oils
Fatty acid composition was analyzed by gas chromatography-mass spectrometry (Thermo-ISQ, Thermo Fisher Scientific Inc., USA) equipped with a capillary column (HP-INNOWAX; 60 m, 0.25 mm ID, 0.25 µm film thickness). Prior to analysis, the oils were converted into the corresponding fatty acid methyl esters (FAME) via esterification reaction according to the method described by Long et al. 22. Oil (1 mg) was added into a 10 mL tube by mixing 2 mL 0.5 mol/L KOH-Me solution and 2 mL hexane, and then the mixture were shaken vigorously and placed in a water bath at 45 °C for 15 min. The organic phase was allowed to separate and 0.5 g of Na2SO4 was added. The supernatant was filtered and then used for GC-MS analysis. The injection volume was 1 µL at a split ratio of 10:1. The column temperature was initially maintained at 150 °C for 2 min, and then increased to 230 °C at 4 °C/min and maintained for 15 min.
2.4.4 α-tocopherol and total phenolic content (TPC)
Alpha-tocopherol was analyzed according to Miraliakbari and Shahidi 23 on a high performance liquid chromatography system (1525-Waters, USA) with minor modifications. A plus C18 column (Syncronis-Thermo Scientific; 250 mm, 4.6 mm ID, 5 µm film thickness) was used with n-hexane/isopropanol (98.5/1.5, v/v) as eluting solvent at a flow rate of 1.2 mL/min. The eluent was monitored by a fluorescence detector at 295 nm. The oil sample (1.0 g) was dissolved in 10 mL n-hexane and then ultrasound treated for 10 min, filtered and 20 µL solution was injected. The calibration curve was prepared using α-tocopherol as standard.
The total phenolic content (TPC) values of the oils were determined using the Folin–Ciocalteu reagent according to the method described by Lutterodt et al. 24. Briefly, 2.5 mL Folin–Ciocalteu reagent and 0.5 mL oil sample were mixed and shaken for 5 min. Then 2 mL of 7.5% sodium carbonate was added. After 60 min of reaction at room temperature, the absorbance was read at 765 nm and used to calculate the TPC, using gallic acid as the standard. Distilled water was used as a control and results were expressed as mg gallic acid equivalents (GAE)/kg of oils.
2.4.5 Squalene, lupeol, and sterol content
The sterol fraction of oils was determined by GC-MS analysis of the complete unsaponifiable fraction according to the method described by Iafflice et al. 25. Oil sample was mixed with 15 mL of 1 mol/L KOH-ethanol and 1 mL internal standard solution (0.36 mg/mL of cholesterol in chloroform). Then the solution was refluxed for 2 h at 80 °C. After saponification, 40 mL of water was added and the non-saponifiable compounds were extracted with 45 mL mixture of diethyl ether: petroleum ether (2:1, v/v). The organic phase was transferred to a glass flask and the lower phase was washed twice. The three extracts were combined and washed with 50 mL distilled water until the pH of the washing water was neutral. The organic fractions were pooled and evaporated at 45 °C under vacuum using a rotary evaporator. The unsaponifiable matter was dissolved in 10 mL chloroform and then analyzed using the GC-MS. The determination program was as follows: the initial column temperature for the GC was 200 °C and kept for 1 min, then increased to 250 °C at 25 °C/min and finally reached 285 °C at 5 °C/min, and held for 15 min. The sample was analyzed in the splitless mode with the injection volume of 1 µL. MS data were acquired in EI mode, with a scan range of 30–550 m/z. Solvent delay was 3 min. The analysis was performed in triplicate.
2.4.6 Thermal stability of oils analysis
Thermal stability of the oils were evaluated by thermo-gravimetric (TG) technique on a thermo-gravimetric analyzer (Q-5000, TA Instruments Inc., USA) under nitrogen and normal air atmosphere (25 mL/min) conditions, respectively. The weighed samples (10 mg) were put into open alumina crucibles and heated in 40–750 °C temperature range at a heating rate of 10 °C/min. TG and DTG (the first derivative of TG and represents the rate of weight loss of the sample as a function of temperature and time) curves, were measured to predict thermal behaviors of the oils.
2.4.7 DPPH radical scavenging activity assay

2.4.8 ABTS radical scavenging activity
Radical scavenging capacity of the tea seed oils against ABTS•+ was measured as described by Rebolleda et al. 14. Briefly, ABTS•+ was freshly prepared by mixing 14 mM ABTS with an equal volume of 4.95 mM potassium persulfate and kept for 16 h in the dark at room temperature. This ABTS•+ solution was used for the assay after dilution in ethanol with an absorbance of 0.7 ± 0.02 at 734 nm. To 0.1 mL of various concentration of oil sample, 5 mL ABTS radical solution was added. The absorbance at 734 nm was determined after 20 min of the reaction. The inhibition ratio of ABTS was calculated using the same equation as described in DPPH radical assay.
2.4.9 HunterLab color measurements and chemical properties
The colors of oils were evaluated using a HunterLab colorimeter (HunterLab ColorQ, Shanghai Shanion Creative Electronic Ltd., Shanghai, China). Four milliliters of each sample was pipetted into a cuvette and color values of L* (lightness), a* (redness), and b* (yellowness) were determined using a D65/10° (daylight 65/10° illuminant observer) setting.
International Organization for Standardization (ISO) methods were used for the determination of acid value, peroxide value, iodine value, and saponification value of the oils according to ISO 660: 2005 26, ISO 3960: 2005 27, ISO 3961: 2008 28, ISO 3657: 2008 29, respectively.
2.5 Statistical analysis
All experiments were performed in triplicate and the results were the average of three independent experiments. Statistical analysis was achieved by using Origin Pro 8.5 software (OriginLab, Northampton, MA). Data were subjected to one way analysis of variance (ANOVA). Significant differences between two mean values were determined by Tukey's multiple range test with a confidence level of 95% (p < 0.05).
3 Results and discussion
3.1 Proximate composition of tea seed and cake
The proximate compositions of tea seed and cake are shown in Table 1. The oil content in seed was 45.22%, which was higher than that in an Iran variety tea seed (30.3%) 30 and groundnut kernel seed (40.83%), but lower than that in palm kernel seed (54.18%) and gingerbread plum seed (47.28%) 31, 32. The tea cake after cold-pressing of the seeds contained 18.61% of oil, which indicated the cold-pressing extraction efficiency was only about 60%, and the residual oil in tea cake was comparable to that in the cake of Meliaceae seed after hexane extraction 33. A relatively low level of protein was determined, 7.12% in seed and 10.53% in cake, respectively, which was far lower than that in gingerbread plum seed 32.
| Constituents | Seed | Cake |
|---|---|---|
| Crude fat | 45.22 ± 0.29a | 18.61 ± 0.23b |
| Crude protein | 7.12 ± 0.15b | 10.53 ± 0.18a |
| Total sugar | 9.84 ± 0.07b | 18.26 ± 0.18a |
| Crude fiber | 19.08 | 32.07 |
| Tea saponin | 7.52 ± 0.10b | 9.65 ± 0.08a |
| Moisture | 8.08 ± 0.10a | 6.79 ± 0.07b |
| Ash | 3.14 ± 0.12b | 4.09 ± 0.13a |
- The results represent the mean of three replications (Mean ± SD); same superscripts in the same row do not differ significantly (p > 0.05).
Total sugar in the tea cake was almost twice as much to that in the seed, which was 18.26% and 9.84%, respectively (Table 1). Wei et al. 34 reported that the polysaccharide from tea seeds has inhibition activity against K562 tumor cells and immunological activity on lymphocytes.
The crude fiber was the major component in tea cake, which accounts for about one third of the cake while this was about one fifth in the seed (Table 1). This phenomenon is understandable as after cold-pressing, most of the oil and oil soluble compounds have been extracted and the residues contained more oil insoluble compounds including crude fiber, sugars, and proteins. Falcón et al. 35 reported that crude fiber has health beneficial effects that can stimulate the growth of intestinal flora and promote slower absorption of nutrients. The results implied that tea cake may be a good source of crude fiber.
Tea saponin is an important triterpenoids in tea seeds. Morikawa et al. 36 demonstrated that tea saponin has protective effect on gastric mucosal lesions induced by ethanol in rats and its activity is more potent than that of omeprazole, a known drug in treatment of dyspepsia, peptic ulcer disease, and gastroesophageal reflux disease. In addition, saponin isolated from tea root extract also showed anti-inflammatory and in vitro antioxidant activity 37. Comparing with the Thai and Japanese tea seeds (5.2% and 7.2%, respectively) 38, the saponin content in the present studied tea seed were higher, which suggested that the tea cake could be a good raw material for the extraction of saponin for potential drug development.
The moisture content in seeds and cake was 8.08% and 6.79%, respectively, suggesting the cake would be more stable during storage because of lower moisture content 39.
3.2 Amino acid composition of tea seed and cake
The amino acid compositions of tea seed and cake are presented in Table 2. Fifteen amino acids were detected and the most abundant one was glutamic acid, followed by arginine then cystine in both samples. Except threonine, proline, and alanine, values for other essential amino acids were not significantly different (p > 0.05). The content of glutamic acid in seed was similar to that in wheat (13.76 g/100 g), corn (14 g/100 g), sorghum (13.98 g/100 g), and soybean (13.71 g/100 g) 40. Essential amino acids (EAA) including histidine were 20.57 and 27.01 g/100 g protein for seed and cake, respectively, and the values were lower than that in gingerbread plum seed flour and defatted gingerbread plum seed flour 32. Tryptophan was not detected in tea seed, which has been completely destroyed under the condition of acid hydrolysis 41. In terms of phenylalanine and leucine, they could have been degraded partially and the residual content was below the detection level. Moreover, the ratio of essential to total amino acids in seed and cake was 27.6% and 29.0%, respectively, which were both lower than that recommended by WHO (≥36%). The least content of amino acid in both samples was proline which was less than 2 g/100 g protein.
| Content of amino acids | Seed | Cake |
|---|---|---|
| Aspartic acid | 6.32 ± 0.03b | 7.40 ± 0.08a |
| Threonine | 2.18 ± 0.10a | 2.83 ± 0.08a |
| Serine | 3.49 ± 0.13b | 4.21 ± 0.09a |
| Glutamic acid | 14.60 ± 0.11b | 21.51 ± 0.12a |
| Proline | 1.93 ± 0.03a | 1.83 ± 0.01a |
| Glycine | 2.62 ± 0.02b | 3.47 ± 0.05a |
| Alanine | 2.47 ± 0.02a | 2.47 ± 0.04a |
| Cystine | 6.90 ± 0.10b | 8.63 ± 0.12a |
| Valine | 1.31 ± 0.00b | 3.34 ± 0.06a |
| Methionine | 2.69 ± 0.02b | 4.57 ± 0.10a |
| Isoleucine | 4.87 ± 0.03b | 7.95 ± 0.12a |
| Tyrosine | 4.36 ± 0.08b | 6.17 ± 0.07a |
| Histidine | 5.38 ± 0.01a | 4.66 ± 0.12b |
| Lysine | 4.14 ± 0.03a | 3.66 ± 0.02b |
| Arginine | 11.33 ± 0.32a | 10.55 ± 0.08b |
| Total | 74.59 | 93.25 |
- The results represent the mean of three replications (Mean ± SD); same superscripts in the same row do not differ significantly (p > 0.05).
The total amino acids in cake were higher than those in seed, which accounted for 93.25 and 74.59 g/100 g protein, respectively. The rest of the nitrogen could be undetected amino acids and non-protein nitrogen such as alkaloids, ammonia, purines, pyrimidines, vitamins, and amino sugars 42. In addition, the total basic amino acids (aspartic acid and glutamic acid) (20.85 g/100 g protein) were found to be equal to the total acidic amino acids (arginine, lysine, and histidine), which were 20.85 to 20.92 g/100 g protein, respectively, suggesting protein in tea seed was probably neutral in nature.
3.3 Fatty acid composition
The fatty acid profile of tea oils and olive oil 2 is shown in Table 3. The oils contained palmitic, stearic, oleic, linoleic, linolenic, and 11-eicosenoic acid. There was significant difference (p < 0.05) in fatty acid composition among three kinds of oils. The total unsaturated fatty acids were about 76–80%, which was in consistent with Lahijan tea seed oils 2 and was similar to that in olive oil. However, 11-eicosenoic acid was not detected in Lahijan tea seed oils but found in the tea seed oil by Wang et al. 7. The discrepancy could be owing to the varieties of tea seeds. Similar phenomenon was observed by Sahari et al. 2 that eicosanoic (C20:0) was found in Iranian and Turkish tea seed oil (0.533% and 1.23%) but it was undetected in Southern Indian and Korean tea seed oil. The content of polyunsaturated fatty acid (PUFA) in TSO(SCF) was similar to that in TSCO-SCF but was higher than that in TSO(CP), indicating the SCF method was more efficient in extracting of PUFA. But its content in all the tea seed oils was far higher than that in olive oil and the ratio of S/M/P (SAFA/MUFA/PUFA) of tea seed oil is more reasonable. In addition, the content of linolenic acid in TSO(SCF) was markedly higher than that in TSCO(SCF) and TSO(CP), which was due to the linolenic acid is more susceptible to oxidation in the oil cold-pressing process (40 °C) and the oxidation rate of linolenic acid is 2–4 times higher than that of linoleic acid 43.
| Fatty acid | TSO(CP) | TSO(SCF) | TSCO(SCF) | Olive oil |
|---|---|---|---|---|
| C 16:0 | 18.00 ± 0.08c | 19.45 ± 0.03b | 19.81 ± 0.10a | 10.23 ± 0.05 |
| C 18:0 | 2.95 ± 0.06c | 3.20 ± 0.05b | 3.33 ± 0.02a | 3.233 ± 0.056 |
| C 18:1 | 57.60 ± 0.12a | 53.49 ± 0.08b | 53.53 ± 0.11b | 76.20 ± 0.2 |
| C 18:2 | 20.29 ± 0.06b | 22.28 ± 0.07a | 22.3 ± 0.05a | 8.567 ± 0.114 |
| C 18:3 | 0.38 ± 0.01b | 0.56 ± 0.03a | 0.30 ± 0.01c | 0.1967 ± 0.0056 |
| C 20:1 | 0.78 ± 0.01b | 1.02 ± 0.04a | 0.73 ± 0.04b | 0.45 ± 0.045 |
| SAFA | 20.95 ± 0.14c | 22.65 ± 0.03b | 23.14 ± 0.07a | 13.463 |
| MUFA | 58.38 ± 0.13a | 54.51 ± 0.05b | 54.26 ± 0.10b | 76.65 |
| PUFA | 20.67 ± 0.05c | 22.84 ± 0.08a | 22.60 ± 0.05b | 8.7637 |
- The results represent the mean of three replications (Mean ± SD); same superscripts in the same row do not differ significantly (p > 0.05).
- SAFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
3.4 Bioactive substances in oils
In the present study, the bioactive substances include TPC, α-tocopherol, squalene, lupeol, and phytosterols in tea seed oils and olive oil 44, which are presented in Table 4. The TPC were found to be 65.9, 78.7, and 170.1 mg GAE/kg in the oils of TSO(CP), TSO(SCF), and TSCO(SCF), respectively, which was over two times higher than that in TSO(CP) and TSO(SCF). This might be the concentration effect of TPC in the tea seed cake after the oils have been cold-pressingly extracted. The results also indicated that the total phenolic compounds in tea seed oils were higher than that in olive oils of 36.1 mg GAE/kg oil, suggesting tea seed oil is a good resource of phenolic compounds.
| Compounds | TSO(CP) | TSO(SCF) | TSCO(SCF) | Olive oil |
|---|---|---|---|---|
| TPC | 65.9 ± 2.1c | 78.7 ± 1.9b | 170.1 ± 4.8a | 36.1 |
| α-tocopherol | 707 ± 10a | 353 ± 3c | 470 ± 5b | 212.1 |
| Squalene | 314 ± 7a | 330 ± 16a | 194 ± 9b | 5990 |
| Lupeol | 470 ± 9b | 519 ± 12a | 469 ± 8b | nd |
| Phytoterols | ||||
| stigmasterol | 456 ± 10a | 196 ± 5b | 461 ± 9a | 15 |
| α-amyrin | 187 ± 3a | 117 ± 6c | 154 ± 2b | 16 |
| β-amyrin | 1376 ± 17b | 2033 ± 42a | 1127 ± 34c | nd |
| β-sitosterol | 147 ± 6b | 334 ± 8a | 149 ± 2b | 1200 |
| lanosterol | 1222 ± 25a | 1133 ± 28b | 1078 ± 31b | nd |
| others | nd | nd | nd | 1329 |
| Total | 3388 ± 25b | 3820 ± 32a | 2968 ± 12c | 2560 |
- nd, not detected: The results represent the mean of three replications (Mean ± SD); same superscripts in the same row do not differ significantly (p > 0.05).
- TPC, total phenolic content; expressed as mg of gallic acid equivalents (GAE)/kg of oils.
Interestingly, the content of α-tocopherol in TSO-CP was almost double to that in TSO-SCF and TSCO-SCF (Table 4), which suggested that the cold-press method was more effective in extracting of α-tocopherol from tea seed. Similar results were also reported by List and Friedrich 12, that the content of tocopherols in press extracted corn and cottonseed oil was higher than that in the SCF-CO2 oils. Zhu et al. 4 has studied the tocopherol content in tea seed oils from 13 different China provinces and the results indicated that the types and content of tocopherols were highly dependent on the regions and sources of tea seeds. And experimental parameters in the SCF-CO2 process could also affect the tocopherol content of oils 45.
However, the SCF-CO2 extracted tea seed oil, showed the highest content of squalene and lupeol, followed by TSO(CP) and TSCO(SCF) (Table 4). The difference could be explained that lupeol and squalene were weakly polar, and therefore can be extracted easily by the solvent of CO2 during the SCF process. Squalene is an unsaturated hydrocarbon and is distributed in shark liver oil and olive oil 46, which can act as an intermediate in the cholesterol biosynthesis pathway in the body and can effectively inhibit chemically induced skin, colon, and lung tumorigenesis in rodents 47. Obviously, its content in olive oil was about 20–30 times higher than that in tea seed oil. But the latter have a certain amount of lupeol, which is a triterpene and possesses some pharmacological activities, including anti- malarial, anti-carcinogenic, and anti-arthritic properties 48.
Research studies have shown that phytosterols can lead to a decrease in serum cholesterol levels 49. Five types of phytosterols were detected in tea seed oils including stigmasterol, β-amyrin, α-amyrin, β-sitosterol, lanosterol. Amyrin, especially β-amyrin was the majority, ranged from 1127 to 2033 mg/kg among oils and its content in TSO(SCF) was about two times higher than that in the other two oils. The lanosterol was the second most abundant phytosterol, which was in the range of 1078–1222 mg/kg. Stigmasterol and α-Amyrin in TSO(CP) and TSCO(SCF) were higher than that in TSO(SCF) while β-sitosterol was relatively low. The results were not in consistent with the finding of Zhu et al. 4, where β-amyrin was 100–700 mg/kg and ergosterol was detected instead of stigmasterol in the present study. These differences may come from different tea seed sources and different extraction methods (the authors employed ultrasonic method), and similar results have been reported by Rabrenović et al. 50. The total phytosterol contents were the highest in TSO(SCF), followed by TSO(CP) and TSCO(SCF), which were all higher than that in olive oil (2560 mg/kg) as well as coconut oil (1140 mg/kg) 51.
As discussed above, tea seed oil is better resource of total phenolic content, α-tocopherol, lupeol, and phytosterols (e.g., stigmasterol, amyrin, lanosterol) compared to olive oil. The high levels of bioactive compounds in tea seed oils implied that they could be used as healthy edible oils or developed as functional food ingredients.
3.5 Thermal stability analysis
Thermo-gravimetric curves of the tea seed oils in an inert N2 atmosphere (A) and in a normal oxidizing atmosphere (B) are shown in Fig. 1. Olive oil was used as a control in this analysis. The extrapolated onset temperature was about 374 °C for TSO(CP) and olive oil in nitrogen. And the highest was TSO(SCF), with a temperature of about 393 °C, which was over 30 °C higher than that of TSCO(SCF). However, when the oils were evaluated in air atmosphere, TSO(SCF) exhibited the best thermal stability, followed by TSCO(SCF) and TSO(CP), and olive oil was the most unstable oil that decomposed at the initial temperature of 237.5 °C. The final decomposition temperature of TSO(SCF) and TSO(CP) were around 590 °C, higher than that of TSCO(SCF) (553 °C) and olive oil (541 °C). In other words, the thermal degradation of oils was fully completed (oils disappeared) when the temperature was up to 600 °C.
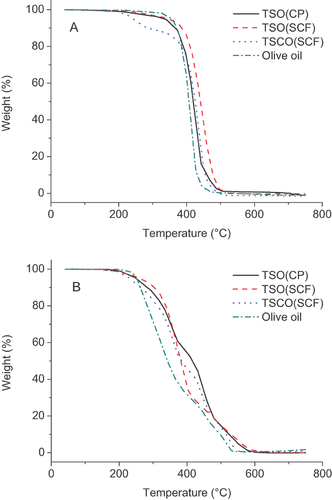
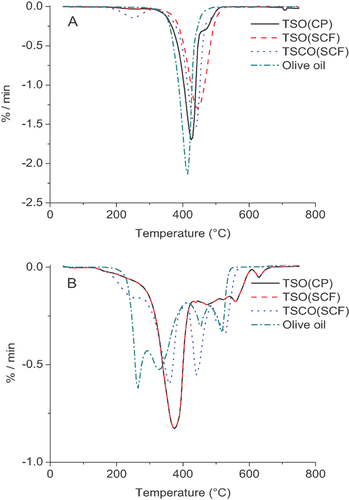
Figure 2 showed the DTG curves of oils in nitrogen (A) and in air atmosphere (B). Under nitrogen condition, there was only one stage of weight loss for all the oils and the decomposition occurred at the temperature around 415–445 °C, which can be attributed to the thermal decomposition of the oils 52. Under an oxidizing atmosphere, the process was complicated because of the reaction of oils and oxygen. The decomposition of all the oils occurred through four stages. TSO(CP) and TSO(SCF) had a very similar decomposition stages, with maximums at 375 °C in the first stage, at 470 °C in the second stage, and at 630 °C in the fourth stage, whereas TSO(CP) decomposed at 558 °C in the third stage, which was 10 °C lower than that of TSO(SCF). Surprisingly, the maximum decomposition temperature were far lower in the corresponding four steps, with a temperature at 259 °C, 360 °C, 441 °C, 525 °C for TSCO(SCF) and 265 °C, 328 °C, 453 °C, 516 °C for olive oil, respectively. The results indicated that TSCO(SCF) and olive oil may not be suitable to be used as frying oils. According to Bagoria et al. 53, the four distinct steps for oil degradation in normal air are decomposition of polyunsaturated, monounsaturated, saturated fatty acids, and the oxidation of carbonaceous residue, respectively. The principal behind vegetable oil decomposition is the oxidation of fatty acids components. Thus, the weight loss of oils from high temperature was compensated by the absorption and reaction of fatty acids with atmospheric oxygen 54.
3.6 DPPH radical scavenging activity
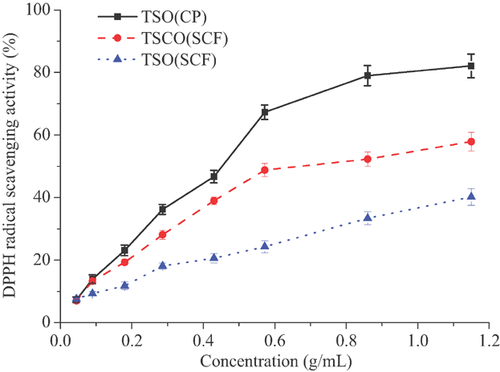
The DPPH radical scavenging activity assay was applied to determine the antioxidant activity of the tea seed oils. All the oils showed a concentration-dependent scavenging capacity under the concentration of 0.045–1.150 g/mL (Fig. 3). TSO(CP) exhibited the strongest radical scavenging activity assay (82.1%), while extracted by TSO(SCF) showed the lowest antioxidant activity (40.2%) at the concentration of 1.150 g/mL. The IC50 values of TSCO(SCF) was 0.739 g/mL, which was about two times more than that of TSO(CP) (0.399 g/mL), indicating a relatively low antioxidant activity of oils extracted from tea seed cake. Interestingly, the results from this work were not in inconsistent with the results of Wang et al. 7, in which the IC50 values of tea seed oil extracted with SCF-CO2 was the smallest. Fazel et al. 3 suggested that phenolic and tocopherol compounds were the main antioxidants of oils. As discussed in section 3.4, the TSO(CP) had the highest content of α-tocopherol (Table 4), which might have contributed its higher antioxidant activity in the present work. In addition, that can also be explained by the higher level of unsaturated fatty acids in cold-pressed tea seed oil 22.
3.7 ABTS radical scavenging activity
ABTS radical scavenging activity was employed to assess the lipid peroxidation inhibitory activity of tea seed oils. As shown in Fig. 4, the results were similar to those in the above DPPH assay. The ABTS radical scavenging activity of TSO(CP) ranged from 11.5% to 87.5% when the concentrations varied from 0.045 to 1.150 g/mL. However, compared with TSCO(SCF), TSO(CP) exhibited a higher activity only when its concentration was above 0.572 g/mL, indicating TSCO(SCF) exhibited a stronger activity in lower concentrations. In addition, the IC50 values of TSO(CP), TSCO(SCF), and TSO(SCF) were 0.401, 0.354, and 1.278 g/mL, respectively, suggesting TSCO (SCF) have better antioxidant activity in ABTS assay. The complex component and synergistic effects of antioxidant compounds in tea seed oil could be the reason for the different results of the tea seed oils in DPPH and ABTS assays. Parry et al. 55 also suggested that one substance may not necessarily have the best antioxidant activity in different assays and it should be evaluated comprehensively by different tests.
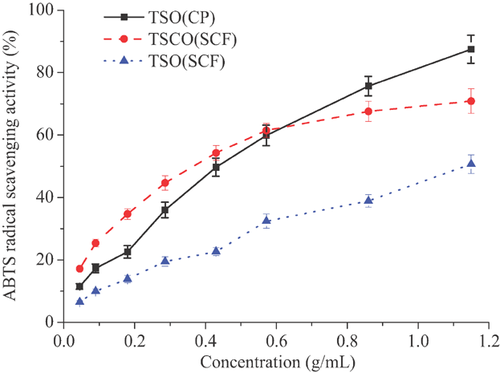
The antioxidant activity assays indicated that tea seed oils extracted by supercritical fluid tended to exhibit low antioxidant capacity than that by cold-pressing. Carbon dioxide is a non-polar medium and supercritical fluid is good at extracting non-polar and weakly polar compounds but has low extraction efficiency for polar and heavy phospholipids, which could be one reason for the lower antioxidant activity of the TSO(SCF). After cold-pressing, cell structure of tea seeds had been destroyed by squeezing and heating, and therefore more active substances could be extracted efficiently from the residual of cake, and in turn, relatively higher antioxidant activity of the TSCO(SCF) than TSO(SCF).
3.8 Physicochemical properties
The oil color is an important characteristic for its product market acceptability. The Hunter L*, a*, and b* values of the oils are summarized in Table 5. The TSO(SCF) was the lightest oil and TSO(CP) contained more yellow pigments. In comparison of the a-value, TSO(SCF) and TSCO(SCF) tended to be green while TSO(CP) had more redness. The L-value showed TSO(CP) was the darkest among all the oils tested, which might be due to the tiny dark seeds residues in the crude oil 56.
| Parameters | TSO(CP) | TSO(SCF) | TSCO(SCF) |
|---|---|---|---|
| L* | 5.42 ± 0.04b | 5.92 ± 0.15a | 5.52 ± 0.06b |
| a* | 2.75 ± 0.12a | 0.41 ± 0.06b | −0.75 ± 0.10c |
| b* | 5.88 ± 0.21a | 1.36 ± 0.21c | 5.01 ± 0.07b |
| Acid value (mg KOH/g) | 11.6 ± 1.1b | 4.9 ± 0.2c | 24.6 ± 0.1a |
| Peroxide value (meq/kg) | 17.18 ± 0.32a | 2.78 ± 0.04c | 12.48 ± 0.14b |
| Iodine value (g I2/100g) | 88.3 ± 0.48b | 93.4 ± 0.84a | 94.0 ± 0.56a |
| Saponification value (mg KOH/g) | 183.2 ± 0.75b | 187.5 ± 1.17a | 185.1 ± 0.79b |
- The results represent the mean of three replications (Mean ± SD); same superscripts in the same row do not differ significantly (p > 0.05).
The chemical properties of the tea seed oils were also evaluated (Table 5). The TSCO(SCF) had an acid value of 24.6 mg KOH/g, which was higher than that in TSO(SCF) (4.9 mg KOH/g) and TSO(CP) (11.6 mg KOH/g). These values were higher than 2.77 mg KOH/g reported in groundnut oil 31. The peroxide value of TSO(CP) was the highest, which was several times higher than that of oils by SCF-CO2, and one of the reasons was that the combined effect of CO2 and high pressure inhibited the activities of lipoxygenase and peroxidase 57. According to Ojeh 58, it can be concluded that TSO(CP) and TSCO(SCF) get rancid easily with high peroxide values. Importantly, TSO(SCF) reached the national standards of edible tea seed oil (≤10 meq/kg) 59 and was probably able to maintain for a long time without quality deterioration. However, the iodine and saponification values among oils were about 90g I2/100g and 185 mg KOH/g, respectively and the results were close to the values of Kenya tea seed oil using soxhlet extraction with hexane 60.
Overall, the TSO(SCF) had the lightest colors and lower acid value and peroxide value (Table 5), indicating it was the best quality oil among the tested samples.
4 Conclusions
The main composition in tea seed and cake was crude fat (45.22% and 18.61%), fiber (19.08% and 32.07%) and some amounts of protein, tea saponin, and total sugar. The major fatty acids were oleic, linoleic, palmitic acid, and the content of unsaturated fatty acids was about 76–80% of the total fatty acids. Oil extracted from TSO(SCF) was the richest in squalene, lupeol, and sterols but the highest levels of α-tocopherol and total phenolic content were found in TSO(CP) and oils from TSCO(SCF), respectively. Under an oxidizing atmosphere, TSO(CP) and TSO(SCF) showed higher thermal stability. The DPPH and ABTS antioxidant activity assay indicated that TSO(CP) possessed the strongest radical scavenging activity, followed by TSCO(SCF) and TSO(SCF). The physicochemical characteristics demonstrated that TSO(SCF) had the best oil quality.
The authors have declared no conflict of interest.




