Comparison of volatile compounds of hot-pressed, cold-pressed and solvent-extracted flaxseed oils analyzed by SPME-GC/MS combined with electronic nose: Major volatiles can be used as markers to distinguish differently processed oils
Abstract
Analysis of volatile profiles from differently processed flaxseed oils (FSO) was performed by SPME-GC-MS, electronic nose (E-nose) and descriptive sensory analysis. A total of 61 volatiles were tentatively identified and then quantified. Among these components, 51 volatiles were obtained from the hot-pressed FSO, 47 from cold-pressed FSO, and 40 by solvent extraction. Principal component analysis (PCA) demonstrated that three FSO samples tested resulted in significant differences of three treatments (p<0.05) and the marker compounds that contributed to discrimination of different processed FSO were hexanal, (E, E)-2, 4-pentadienal, (E, E)-2, 4-heptadienal, 6-hydroxy-2-hexanone, 1-hexanol, methyl-pyrazine, nonanal, 2,3-pentanedione, 1-butanol, acetic acid, hexanoic acid, and ethyl acetate. In addition, there was good consistency among GS-MS, sensory evaluation and E-nose analysis results. These results indicated that the process method has a significant effect on the aroma quality of FSO and may be helpful in evaluating aroma quality and the detection of frauds.
Practical applications: The results showed that the major volatile components could be used as chemical markers to distinguish differently processed FSOs by the chemometric method. There was good consistency among GS-MS, sensory evaluation and E-nose analysis results, suggesting that E-nose technique combined with a PCA of the data provided by the sensor arrays has the good potential to evaluate FSO quality and the detection of frauds.
Sixty-one volatiles were identified and significant differences were found in different processed flaxseed oils. Twelve major volatile compounds contributed to discrimination of different processed flaxseed oils. Flaxseed oil samples were distinguished from each other through PCA of E-nose data, suggesting that the E-nose can successfully discriminate different processed flaxseed oils.
Abbreviations
-
- CPO
-
- Cold-pressed FSO
-
- CVD
-
- cardiovascular disease
-
- DVB/CAR/PDMS
-
- Divinylbenzene/Carboxen/Polydimethylsiloxane
-
- EO
-
- Solvent extracted FSO
-
- E-nose
-
- electronic nose
-
- FSO
-
- flaxseed oil
-
- HPO
-
- hot-pressed FSO
-
- HS-SPME
-
- head-space solid phase micro-extraction
-
- PCA
-
- principal component analysis
-
- RI
-
- retention index
1 Introduction
Flax (Linum usitatissimum) is an economically important oilseed crop in the world 1. Usually, flaxseed contains about 40% oil, 30% dietary fiber, 20% protein, 4% ash, and 6% moisture 2. Flaxseeds are also well-known sources of ALA and have an exceptionally high content of n-3 fatty acids, which have been linked to reduced triglycerides, blood pressure and cardiovascular disease (CVD) 3, 4. Flaxseed oils (FSO) are consumed in many countries, such as Canada, America, India and China, due to their nutritional and therapeutic properties.
Flax cultivation is widespread throughout the north-western region of China and is important for the rural economy, local heritage, and the environment. Xinjiang autonomous region is one of the largest producers of FSO in China, the production is approximately 200 000 tonnes/year. Raw flaxseeds are bitter and require processing to make them suitable for consumption. Worldwide interest exists in using alternative processing techniques for oil productivity and flavor 5. Flavor is closely connected with the qualitative and quantitative composition of volatile compounds and is considered to be the quality index of FSO 6, playing an important role in consumer acceptability. The flavor of plant oils depends on the variety, degree of fruit ripeness, environmental conditions, growing region, storage, and techniques of processing 7-9.
Processing technique affects the concentrations of major compounds and consequently causes chemical and physical changes, particularly oils volatile flavor. In China, there are many traditional styles of processing. Solvent extraction and mechanical pressing are the leading methods for commercial oil extraction 5. Hot-pressing technique is a conventional method used to extract essential oils from flaxseeds, it can be used in industry and has no chemical pollution, but the heat-sensitive compounds can be destroyed in a certain degree and affect the quality of the FSO 5. While cold-pressing technique can avoid damage to the heat-sensitive compounds but low in the oil rate and flavor quality 6, 10. While solvent extraction can process most flaxseeds, regardless of variety, is a rapid and high efficiency sample pretreatment technique. Compared with the pressing techniques, the solvent extraction has been used widely in vegetable oil extracts and pharmaceutical industry. From a commercial viewpoint, specific varieties are preferred because of superior technological and organoleptic factors and consumer preference 11. The effect of processing equipment has also been studied as well as the effects of the processing variables of malaxation time and temperature 2, 6, 10, 12. However, the effects of these techniques on profile of aroma-related volatiles are still unknown. Only a few studies have addressed the volatile compounds attributed to FSO 8, 12, 13. For instance, Krist et al.8 reported that hexanol, trans-2-butenal and acetic acid could be identified as the main volatile compounds in the linseed oil samples, while trans-2-butenaland acetic acid, accompanied by trans, trans-3, 5-octadiene-2-one and trans, trans-2, 4-heptadienal dominated the headspace of the examined camelina oil samples. Similarly, Yalcin et al. 12 reported that the dominant volatile compound of linseed was limonene, 1-hexanol, styrene (aromatic hydrocarbon), and 1-hexanol content only decreased at 7.0 kGy, and benzaldehyde, p-cymene, and nonanol were not determined at irradiation doses above 4.0 kGy. Such studies have shown that there are cultivars and storage techniques differences on the volatile compounds of FSO. While Mildner-Szkudlarz and Jeleń 14 have confirmed the effectiveness of SPME-GC/MS method of volatile compounds analysis with subsequent PCA treatment of data for differentiation between virgin olive oil samples adulterated with hazelnut oil. Zheng and Jun 15 has successfully classified sesame oils with different adulteration levels, and predict the percentage of adulteration by using an electronic nose based on 10 metal oxide semiconductor sensors. To the best of knowledge, the effect of processed technology on FSO volatile profile was rarely described in current literatures. Considering the direct impact of different processing techniques on the FSO flavor and aroma, one could expect that the volatile profile could be a valuable tool for the differentiation of different processed FSO.
The objectives of the present study were to compare the volatile profiles of cold-pressing FSO (CPO), solvent extraction FSO (EO) and hot-pressing FSO (HPO), followed by GC-MS and E-nose analysis, and to visually assess their significant differences by applying PCA 16. This research may be helpful in evaluating FSO quality and the detection of frauds.
2 Materials and methods
2.1 Flaxseeds
The flaxseed samples were grown in the same field located in Xinjiang Uygur Autonomous Region, China, a typical flax growing area. The flaxseeds were hand-selected to remove fruits with blemishes, defects, and insect damage and the remainder were washed with tap water under pressure to remove impurities.
2.2 Chemicals, reagents, standards, and reference materials
All chemicals used for sample preparation and GC-MS analysis were of analytical and HPLC grade unless otherwise stated, and water was obtained from a Milli-Q purification system (Millipore, North Ryde, NSW, Australia). The following compounds were obtained commercially: C6–C23 normal alkanes for calculating the retention indices (RI) were purchased from Shanghai Chemical Reagent Co. (Shanghai, China). The chemical standards of the volatile compounds including pentanal, hexanal, heptanal, benzaldehyde, 3-methyl-2-butenal, 2-hexanone, 1-hexanol, 1-octanol, 1-butanol, acetic acid, propanoic acid, hexanoic acid, pentanoic acid, butyrolactone, ethyl acetate, tridecane, tetradecane, hexadecane, 2-methylpyrazine, and cyclohexanol for SPME-GC-MS analysis were purchased from Sigma–Aldrich (Sigma Chemical Company, St. Louis, USA).
2.3 Sample preparation of FSO
Three samples of flaxseeds were randomly selected and each was divided into three lots (1 kg each). Basic quality parameters of the seeds used in preparing the oils are to be free from pests, ergot, poisonous seeds, mycotoxins, mould, mites, and any bacterial-related diseases. Two flaxseeds lots were subjected to the cold-pressed processing and hot-pressed processing, and the third lot was extracted by solvent extraction methods. The experiments were conducted in triplicate during 3 months at RT (20–25°C).
2.3.1 HPO
FSO was obtained by mechanical pressing using a homemade pressing unit. Prior to pressing, the screw was first allowed to heat for 20 min via an electrical resistance heating ring attached around the press head to raise its temperature to 95°C. Flax seeds were roasted in a roller pan at a temperature of 200°C and stirred continuously until to get a desirable flavor and the samples were then pressed by the screw press to produce corresponding flax seed oil samples. The pressed oil was then centrifuged and collected in 25-mL brown glass volumetric flask under nitrogen for protection from light prior to storage at −25°C.
2.3.2 CPO
Flax seeds were pressed directly by the screw press to produce corresponding flax seed oil samples. Temperature of extracted oil was measured during the whole experiment with digital thermometer and it was continuously at below 40°C. The other conditions were carried out as described above.
2.3.3 EO
Solvent extraction using normal hexane (74%)-cyclohexane (16%) (boiling range 60–90°C), was done in a laboratory oil extraction apparatus following a standard method 2, 17. Prior to extraction, seeds were prepared by grinding them in two 15-s cycles in a rotary mill (Stein Mill, Model DFY-2000). During normal hexane-cyclohexane extraction, the oil temperature was maintained at 40 ± 1°C. The lipid extracts were filtered through lipid-free filter paper and the solvent was removed in vacuo at temperatures below 40°C. The extracted oil storage was carried out as described above.
2.4 Head space-solid phase microextraction (HS-SPME)
HS-SPME is a rapid and convenient method for the pre-concentration and analysis of the volatile compounds from aromatic plants 18-21. In our preliminary experiment, a fiber coated with Divinylbenzene/Carboxen/Polydimethylsiloxane (DVB/CAR/PDMS) was used to analyze the volatile components with satisfactory results 13. Before use, the fiber was conditioned by introducing it into the injector of the GC system set at 270°C for 2 h in a stream of helium. Then the FSO sample (10 g) was placed in a 125 mL headspace vial fitted with a silicone septum. After an equilibration time of at least 5 min, SPME sampling was performed by exposing the fiber for 40 min in the headspace of the sampling at 50°C under magnetic stirring. After sampling, the SPME device was placed into a GC system equipped with a trace mass spectrometer (Finnigan, San Jose, California, USA).
2.5 GC-MS analysis of volatile compounds
Volatiles were separated by a DB-WAX silica capillary column (30 m × 0.25 mm, i.d.0.25 µm film thickness, Supelco Co., USA), with helium as the carrier gas (flow rate of 0.8 mL/min). The injector and detector temperatures were at 250°C. The following column temperature program sequence was used: the initial temperature of 40°C was held for 3 min and then increased at 6°C/min to 120°C. From this point, the temperature was increased at 10°C/min to a temperature of 230°C, and was held for 8 min. Mass spectra were recorded in the electron impact mode at an ionization voltage of 70 eV in the 33–373 amu scan range. The ion source temperature was 200°C.
2.6 Identification of volatile constituents
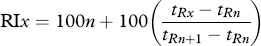
| Volatiles compounds with CAS-No | RIa) | Volatile compounds (%) | IDb) | ||
|---|---|---|---|---|---|
| CPO | EO | HPO | |||
| Aldehydes | |||||
| Pentanal [110-62-3]c) | 950 | NDd) | ND | 1.877 ± 0.007a | A, B, C |
| Hexanal [66-25-1] | 1078 | 2.363 ± 0.231b | 0.164 ± 0.002c | 5.290 ± 0.002a | A, B, C |
| (E)-2-Butenal [123-73-9] | 1096 | 1.120 ± 0.014c | 1.853 ± 0.003b | 3.542 ± 0.005a | B, C |
| (E, E)-2,4-Pentadienal [764-40-9] | 1146 | 3.038 ± 0.024b | 1.107 ± 0.007c | 6.290 ± 0.004a | B, C |
| Heptanal [111-71-7] | 1185 | 1.117 ± 0.016c | 2.752 ± 0.055a | 1.585 ± 0.003b | A, B, C |
| (E)-2-Hexenal [6728-26-3] | 1206 | 1.313 ± 0.015c | 2.328 ± 0.005a | 1.674 ± 0.002b | B, C |
| (E)-2-Heptenal [57266-86-1] | 1434 | 2.435 ± 0.011a | 1.216 ± 0.007b | 1.085 ± 0.003c | B, C |
| Nonanal [124-19-6] | 1394 | 1.822 ± 0.006b | 2.046 ± 0.002a | ND | B, C |
| (E)-2-Octenal [2548-87-0] | 1411 | ND | 0.553 ± 0.009a | 0.405 ± 0.008b | B, C |
| (E, E)-2, 4-Heptadienal [4313-03-5] | 1488 | 2.546 ± 0.031b | 1.023 ± 0.004c | 6.958 ± 0.003a | B, C |
| Benzaldehyde [100-52-7] | 1518 | ND | ND | 0.206 ± 0.007a | A, B, C |
| (E, E)-2, 4-Nonadienal [4313-03-5] | 1690 | 1.756 ± 0.010b | ND | 1.995 ± 0.002a | B, C |
| (E, E)-2, 4-Decadienal [25152-84-5] | 1776 | ND | 0.121 ± 0.002b | 0.475 ± 0.001a | B, C |
| 2,3-Dimethyl-pentanal [32749-94-3] | 997 | ND | 0.104 ± 0.003a | ND | C |
| 3-Methyl-2-butenal [107-86-8] (new)e) | 1377 | 1.224 ± 0.007a | 0.876 ± 0.021b | ND | A, C |
| Ketones | |||||
| 2-Hexanone [591-78-6] | 1145 | 2.757 ± 0.164a | ND | 2.263 ± 0.001b | A, C |
| 6-Hydroxy-2-hexanone [21856-89-3] (new) | 1196 | 1.650 ± 0.047b | ND | 3.653 ± 0.002a | C |
| 2-Heptanone [110-43-0] | 1199 | 0.451 ± 0.010b | ND | 0.547 ± 0.002a | B, C |
| (E)-3-Octen-2-one [18402-82-9] | 1298 | ND | 0.581 ± 0.002a | 0.577 ± 0.009b | C |
| (E, E)-3, 5-Octadien-2-one [38284-27-4] | 1458 | ND | 0.802 ± 0.003a | 0.141 ± 0.002b | C |
| 5-Ethyldihydro-2(3H)-furan-one [695-06-7] | 1752 | 0.135 ± 0.006c | 0.894 ± 0.004a | 0.539 ± 0.035b | C |
| 2,3-Pentanedione [600-14-6] | 1057 | 0.328 ± 0.002c | 3.784 ± 0.002a | 2.177 ± 0.009b | B, C |
| 3-Hydroxy-2-butanone [513-86-0] | 1231 | 1.486 ± 0.002a | ND | 1.328 ± 0.025b | C |
| Alcohols | |||||
| 1-Hexanol [111-27-3] | 1351 | 2.282 ± 0.004b | 1.430 ± 0.003c | 5.722 ± 0.005a | A, B, C |
| 1-Octanol [111-87-5] | 1545 | 0.525 ± 0.035b | ND | 1.221 ± 0.019a | A, B, C |
| 1-Pentanol [71-41-0] | 1240 | 2.027 ± 0.008b | 0.527 ± 0.008c | 2.292 ± 0.017a | B, C |
| 1-Nonanol [143-08-8] | 1656 | ND | 0.835 ± 0.004a | 0.789 ± 0.020b | B, C |
| 1-Butanol [71-36-3] (new) | 1228 | 0.574 ± 0.016c | 2.432 ± 0.004a | 0.785 ± 0.004b | A, C |
| 3-Hexen-1-ol [544-12-7] | 1290 | 0.752 ± 0.054a | 0.234 ± 0.004c | 0.440 ± 0.002b | C |
| 1-Heptanol [111-70-6] | 1465 | 1.123 ± 0.003a | ND | ND | B, C |
| (E)-1, 3-Butadienol [70411-98-2] | 1080 | ND | ND | 0.242 ± 0.010a | C |
| 1-Penten-3-ol [616-25-1] | 1160 | 0.784 ± 0.017b | ND | 1.849 ± 0.003a | B, C |
| 2-Methyl-1-butanol [137-32-6] | 1236 | 1.474 ± 0.016a | ND | ND | C |
| 1,5-Hexadien-3-ol [924-41-4] | 1283 | ND | ND | 0.742 ± 0.038a | C |
| (z)-3-Hexen-1-ol [928-96-1] | 1288 | 0.316 ± 0.008b | 0.446 ± 0.050a | ND | C |
| Carboxylic acids | |||||
| Acetic acid [64-19-7] | 1422 | 2.086 ± 0.002b | 3.562 ± 0.008a | 2.059 ± 0.004c | A, B, C |
| Propanoic acid [79-09-4] | 1466 | 0.340 ± 0.006c | 0.772 ± 0.004a | 0.359 ± 0.004b | A, B, C |
| Hexanoic acid [142-62-1] | 1817 | 1.381 ± 0.007b | 3.700 ± 0.002a | 0.147 ± 0.007c | A, B, C |
| Pentanoic acid [109-52-4] | 1735 | ND | ND | 0.230 ± 0.006a | A, B, C |
| Octanoic acid [124-07-2] | 2082 | 0.437 ± 0.012b | ND | 0.454 ± 0.003a | B, C |
| Nonanoic acid [112-05-0] | 2163 | 0.276 ± 0.001b | 0.174 ± 0.006c | 0.295 ± 0.003a | B, C |
| Esters | |||||
| Butyrolactone [96-48-0] | 1621 | 0.921 ± 0.003c | 2.190 ± 0.005a | 1.944 ± 0.025b | A, B, C |
| γ-Hexalactone [695-06-7] | 2456 | 0.485 ± 0.003c | 0.562 ± 0.004b | 1.055 ± 0.004a | C |
| Isobutyl phthalate [84-69-5] | 2480 | 0.172 ± 0.004c | 0.544 ± 0.001a | 0.382 ± 0.005b | C |
| Hexyl formate [629-33-4] (new) | 1388 | 8.221 ± 0.007a | 3.990 ± 0.007c | 6.949 ± 0.005b | C |
| Ethyl acetate [141-78-6] | 980 | 3.334 ± 0.005c | 5.692 ± 0.008a | 3.769 ± 0.003b | A, B, C |
| Acetic acid butyl ester [123-86-4] (new) | 1743 | 0.349 ± 0.007a | ND | ND | B, C |
| Alkanes | |||||
| Tridecane [629-50-5] | 1298 | 0.346 ± 0.002b | ND | 0.714 ± 0.014a | A, B, C |
| Tetradecane [629-59-4] (new) | 1388 | ND | ND | 0.641 ± 0.004a | A, B, C |
| Hexadecane [544-76-3] | 1494 | 0.134 ± 0.005b | ND | 0.738 ± 0.034a | A, B, C |
| Heptadecane [629-78-7] | 1683 | ND | 0.114 ± 0.002a | ND | B, C |
| Heterocyclic compounds | |||||
| 2-Ethyl-furan [3208-16-0] | 1024 | 0.940 ± 0.008b | 0.138 ± 0.002c | 1.373 ± 0.003a | C |
| 2-Methylpyrazine [109-08-0] | 1263 | 1.363 ± 0.004b | 0.562 ± 0.002c | 5.194 ± 0.003a | A, B, C |
| 2-Ethyl-3,5-dimethylpyrazine [27043-05-6] (new) | 1450 | 1.879 ± 0.003a | 0.994 ± 0.003b | ND | C |
| 5-Methyl-2-furaldehyde [620-02-0] (new) | 1558 | 0.853 ± 0.001a | ND | 0.844 ± 0.003b | B, C |
| Naphthalene [91-20-3] (new) | 1723 | 0.503 ± 0.005a | ND | ND | B, C |
| 2-Methyl-naphthalene [91-57-6] (new) | 1783 | 0.691 ± 0.005b | 0.591 ± 0.005c | 1.014 ± 0.002a | C |
| 1-Methyl-naphthalene [90-12-0] (new) | 1799 | 0.448 ± 0.001c | 0.864 ± 0.002b | 0.957 ± 0.058a | C |
| Azulene [275-51-4] (new) | 1659 | ND | 1.137 ± 0.002a | 0.320 ± 0.002b | C |
| Other compounds | |||||
| Methyl-benzene [108-88-3] | 1038 | 1.910 ± 0.010c | 2.164 ± 0.003b | 2.932 ± 0.003a | C |
| Alpha-pinene [7785-70-8] | 1056 | 2.494 ± 0.060b | 1.413 ± 0.012c | 2.792 ± 0.006a | B, C |
- a) RI: retention indices calculated on DB-WAX column using C6-C23 alkanes.
- b) Identification: the identification was indicated by the following symbols: A = mass spectrum and RI agree with that of the authentic compound run under similar GC-MS conditions; B = mass spectrum and LRI agree with literature data; C = tentative identification based on interpretation of mass spectrum and comparison with similar compounds. Literature RI 20, 36-38.
- c) Data are means of three replicates. Values expressed as mean ± SD.
- d) ND: Not detected or percentage of the component is lower than 0.01%.
- e) The substances have not been reported by previous studies in the volatiles of FSO.
2.7 Electronic nose/MOS analysis of FSO
The flavor of FSO was analyzed using a FOX-3000 electronic nose (Alpha MOS, Toulouse, France), which consisted of a sampling apparatus, array of sensors, HS-100 auto sampler, air generator equipment and software (Alpha Soft V11) for data recording and analyzing 23. The sensory array was composed of 18 metal oxide sensors divided into three chambers: LY, T, and P. Total of 2 g of FSO was placed in a 10-mL bottle, sealed and equilibrated for 30 min at RT. Then the vials were loaded into the auto-sampler tray, and equilibrated for 10 min at 50°C using purity air as the carrier gas at a flow rate of 200 mL/min. The injection volume, time and stirring rate were 2500 µL, 3 s and 250 rpm, respectively. The durations for acquisition and delay between injections were 50 and 180 s, respectively. The maximum response points of the electronic nose, automatically recorded for each of the 18 sensors, were used for analysis. Four replicates were measured for each sample.
2.8 Sensory analysis of FSO
The odor of the three different processed FSO was evaluated by a panel of 10 trained nonsmoker assessors (six women and four men, between 25 and 40 years old), all of them belonging to the State Key Laboratory of Food Science and Technology, Jiangnan University. The sensory evaluation of FSO was performed according to GB/T 8325-2008, vocabulary and attributes of oils as well as their references were selected.
To get a more specific training on the aroma of FSO, the panelists were instructed to gently swirl the covered sensory glass and to subsequently uncap the glass and sniff the headspace of the sample with short sniffs and to replace the cover of the glass quickly 24. Then they evaluated an array of different commercial oils (HPO, EO, and CPO), describing sample odor qualities on the basis of their personal criterion. A number of discussion sessions with panelists were needed to remove possible semantic differences, after a final consensus-building discussion, the common descriptors were carefully defined and inserted on the profile sheet. In that way, multiple odor qualities were achieved, panelists finally agreed on a common list of seven descriptors for FSO: “almond,” “green,” “sweet,” “herbaceous,” “oily,” “roasted,” and “cereal-like.” The scale intensity ranged from 0 to 8 according to the GB/T 8325-2008 method.
After suitable training, tasters were requested to evaluate the sensory characteristics of FSO to verify whether the new profile sheet was able to differentiate FSO in relation to different processing technologies. The oils were presented according to an experimental design which minimized possible biases and carry-over effects. All samples were evaluated in triplicate.
2.9 Statistical analysis
The data were statistically analyzed by ANOVA and Duncan's multiple range test using commercially available software package SPSS software program (SPSS, Inc., Chicago, Ill., USA). Statistical significance was inferred at p<0.05. The data obtained from the sensor array of the electronic nose were analyzed by the principal component analysis (PCA) performed by the Unscramble software (V.9.7, CAMO ASA, and Oslo, Norway).
3 Results and discussion
3.1 Profiles of volatile compounds in different processed FSO
As was expected, ANOVA for volatiles of FSO, which were produced from the same cultivar and growing in the same climatic conditions, processed by different techniques showed that were highly significant (Table 1, Fig. 1). A total of 61 volatile compounds were tentatively identified and then semi-quantified. Of all, 51 volatile components were obtained by the hot-pressing technology, 47 components by cold-pressing technology, and 40 by solvent extraction. Most of the volatile compounds identified in present study have already been identified in other plant oils 7, 9, 11, 22. While the following substances, to our best knowledge, have not been reported by previous studies to be present in FSO profiles 8, 12, 13: 3-methyl-2-butenal, 6-hydroxy-2-hexanone, 1-butanol, hexyl formate, acetic acid butyl ester, tetradecane, 2-ethyl-3, 5-dimethyl pyrazine, 5-methyl-2-furaldehyde, naphthalene, 2-methyl-naphthalene, 1-methyl-naphthalene, and azulene. Since these compounds were not reported before, some of their identity confirmation should be pursued in further studies as newly unconfirmed compounds in FSO space volatile profile.
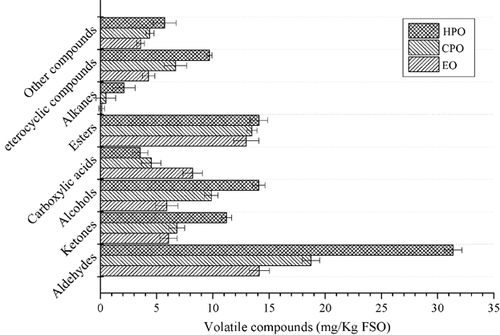
The compounds isolated and identified in three FSO samples were mainly aldehydes (14.14–31.38%), ketones (6.06–11.23%), alcohols (5.90–14.08%), carboxylic acids (3.54–8.21%), esters (12.98–14.10%), alkanes (0.11–2.09%), heterocyclic compounds (4.30–9.75%), and other compounds (3.58–5.72%) (Table 1, Fig. 1). The percentages of total ketones, alcohols, carboxylic acids, alkanes and heterocyclic compounds differed according to the FSO processed techniques (Fig. 1). Furthermore, the percentages of total volatiles increased from EO, CPO to HPO except for carboxylic acids, which showed inconsistent changes (Fig. 1). Apparently, pentanal (1.877%), benzaldehyde (0.206%), (E)-1, 3-butadienol (0.242%), 1, 5-hexadien-3-ol (0.742%), pentanoic acid (0.230%) and tetradecane (0.641%) were only found in HPO. 1-Heptanol (1.123%), 2-methyl -1-butanol (1.474%), acetic acid butyl ester (0.349%) and naphthalene (0.503%) have been identified solely in CPO, whereas 2, 3-dimethyl-pentanal (0.104%), heptadecane (0.114%) were detected only in EO (Table 1). In addition, when comparing with each other, some new compounds appeared (Table 1). All these results indicated the techniques related to FSO processing are one of the most important factors influencing volatile composition.
These different oil processing techniques offer a number of individual advantages, but also suffer from specific limitations. In the HPO sample, volatile compounds such as pentanal, hexanal, (E)-2-butenal, (E, E)-2, 4-pentadienal, (E)-2-hexenal, (E)-2-heptenal, (E)-2-octenal, (E, E)-2, 4-heptadienal, (E, E)-2, 4-nonadienal, (E, E)-2, 4-decadienal, and 3-methyl-2-butenal were shown to be significantly correlated with the oxidative status of FSO 13, 25, 26. These aldehydes are widespread, as some of them have already been found in many other plant oils, such as olive oils 27, sesame oils 19, and peanut oils 22. 3-Methyl-2-butenal detected only in CPO and EO is one of the new reported volatile, which is primarily related with off-flavor. Hexanal is a typical oxidation volatile and has been commonly monitored for measuring lipid oxidation in plant oils and it has been related with green, oily, and fruity odors 25, 28. Furthermore, the presence of hexanal in three of them (CPO, EO, HPO) suggest that this compound are enzymatically produced in the lipid compartment of the fruit by PUFA through the LOX pathway as happens in the biosynthesis of olive oil volatile compounds 7, 28. On the contrary, (E)-2-hexenal, which is also derived from the LOX pathway and which is inversely related to the oxidation degree of virgin olive oil 29, was found to be present in lower amounts in CPO (1.313%) compared with EO (2.328%) and HPO (1.674%; Table 1). While most studies have used the ratio of hexanal/nonanal as a primary indicator of oxidation in olive oils 26, 29. Interestingly, nonanal detected in a relative higher amounts in CPO and EO, has not been identified in HPO at all. Its remarkable stability may be due to the presence of the endogenous antioxidants, lignans, and tocopherols 30. Thus, the observed differences in volatile profiles may signal a difference in the relative importance of these pathways in different processed FSO.
Regarding ketones, 6-hydroxy-2-hexanone (3.653%) and 2, 3-pentanedione (3.784%) were the only compounds detected at a higher percentage in HPO and EO, respectively, while 2-hexanone and 3-hydroxy-2-butanone were detected at relatively higher percentages in CPO and HPO (Table 1). The amount of 6-hydroxy-2-hexanone, as a new reported volatile, was not detected in EO at all. This was because purging with solvent might cause the low boiling point chemicals to evaporate thus reducing the concentration of 6-hydroxy-2-hexanone. Ketones might be formed by beta-oxidation of fatty acids, which generated a few important flavor compounds 31.
Most of the smaller ketones, with five to seven carbon atoms, such as 2-hexanone, 2-heptanone, which were only identified in CPO and HPO, were linked to positive sensory characteristics 8, 28. Although the flavor notes of ketones were generally desirable, most of their aroma contributions might have been minimal since low levels of these compounds were quantified.
Volatile compounds are also formed through fatty acid metabolism, producing alcohols, acids, and esters. Most alcohols were derived from bioremediation of unsaturated fatty acids, and were also a prerequisite for the formation of long-chain esters. Alcohols identified included mainly C5 and C6 compounds, such as 1-hexanol, 1-pentanol, 3-hexen-1-ol and 1-penten-3-ol deriving from the lipoxygenase pathway 7, were the major compounds detected in FSO samples. While alcohols amounts varied due to different processing techniques of FSO. As an example, 2-methyl-1-butanol (1.474%) was identified to be the main volatile component only in CPO samples. Markedly high amounts of 1-hexanol (5.722%) were identified in HPO. Further alcoholic compounds, like 1-pentanol, 1-butanol and 3-hexen-1-ol were identified in all FSO samples with different amounts. 1-Butanol was common in plant materials resulting from enzymatic deamination and decarboxylation of amino acids. Most of these alcohols were also reported in olive oils and soy sauce as important contributors of flavor 14, 28.
Carboxylic acids are linked to sour and pungent sensations synonymous with sensory defects in olive oil and peanut oil 7, 22, 28. While EO had the relative higher level of acetic acid (3.562%) and hexanoic acid (3.700%) compared with CPO and HPO. On the contrary, EO showed lower nonanoic acid (0.174%) contents than CPO and HPO. Among them, acetic acid was present in all analyzed FSO samples at comparable amounts (2.059–3.562%), and higher concentrations of corresponding ethyl acetate were found in all samples.
Esters are predominantly linked to the positive fruity aroma of FSO. Five compounds were common in all samples and butyrolactone, γ-hexalactone, hexyl formate and ethyl acetate were the major compounds detected. Most of the detected esters, derived from the esterification of free fatty acids and alcohols were previously found in various oils 7, 22. Interestingly, the hexyl formate levels in CPO were much higher than those in the other samples. Ethyl acetate, usually identified only in virgin olive oil 9, are also present in FSO with a relative high amounts (3.334–5.692%). Because acetic acid was present in all analyzed FSO samples at comparable amounts (2.059–3.562%), and corresponding ethyl acetate and acetic acid butyl ester were found in different FSO samples. While hexyl formate might produce from the esterification of formic and hexanol. The degradation of sugars to low molecular weight compounds such as formic, acetic, and pyruvic has been widely studied in aqueous alkaline solutions and in the presence of amines 32. Although esters are considered minor components compared to aldehydes, most esters have characteristic odors such as fruity, sweet, herbaceous, and honey odor.
Alkanes are generally not considered to be important contributors to the flavors of fats, oils and lipid-containing foods because of their relative high odor threshold values 22. These compounds were mainly produced by decarboxylation of fatty acids from glycerides. A total of four alkanes were found in three FSO samples. Among them, the lower amount of heptadecane (0.114%) was only detected in the EO, whereas HPO had a medium amount of tetradecane (0.641%) and the highest amount of hexadecane (0.738%).
The relatively remarkable amounts of heterocyclic compounds seem to result from the Maillard reaction derived from the heating process resulting in furans, thiazoles, thiophenes, oxazoles, pyrroles, naphthalene, pyridines, and pyrazines 6. The amount of total pyrazines was highest in the HPO and lowest in the EO (Table 1). Most of the amounts of heterocyclic compounds with an increase in the HPO compared the other two, e.g., 2-ethyl-furan, 2-methyl-pyrazine, 2-methyl-naphthalene and 1-methyl-naphthalene showed a relevant increase. 5-Methyl-2-furaldehyde detected only in CPO and HPO, was less polar and more carbohydrate degradation products 7. Pyrazines, particularly alkyl pyrazines, which are considered to be important flavor components of olive oil and peanut oil, are responsible for the nutty, musty, or cocoa aroma odor, and are widely distributed in roasted foods 14, 33. The remarkable amounts of 2-methylpyrazine (5.194%) and 2-ethyl-furan (1.373%) in HPO compared to CPO and EO seem to result from previously heating flaxseeds, as both present Maillard compounds deriving from the heating process. Interestingly, 2-ethyl-3, 5-dimethyl-pyrazine was detected in CPO and EO with exception of HPO. Generally, the presence of pyrazines is not common in raw seeds as they are most commonly associated with heat processing (Maillard reactions, Strecker, and pyrolysis of amino acids). Therefore, the presence of 2-ethyl-3, 5-dimethylpyrazine in CPO and HPO might be a consequence of heat exposure during post-harvest processing. While Baltes and Bochmann 34 observed that the concentration of pyrazines during roasting depends on the ratio between the content of amino acids and sugars in a reaction system. An inappropriate ratio of amino acids in relation to the sugar influenced formation of heterocyclic compounds such as pyrazine, which might clarify the difference of 2-ethyl-3, 5-dimethylpyrazine among CPO, EO, and HPO. Although volatile compounds are also formed from amino acids 35, 36, the action of amino acids in FSO volatile generation has been paid little attention. It is established that glutamic acid, argentines, phenylalanine, and tyrosine are converted to volatile compounds, which have the potential to change the peanut oil sensory perception 22, 37. Naphthalene and its derivatives, which were identified in previous studies concerning the thermal degradation of carotenoids were also detected in different FSO samples 32. To our best knowledge, azulene, which can rearrange into naphthalene after being heated and have not been reported in the literatures, belongs to the product categories of aromatic hydrocarbons.
Two additional miscellaneous compounds were identified in the FSO. Alpha-pinene, which was detected as major volatile component in the hull of the fruits of Pistacia terebinthus 33, was also detected in all FSO samples with relative high amounts (Table 1). Methyl-benzene, which are considered environmental pollutants previously found in virgin oil fraction 9, was detected in a relative high amounts (1.910–2.932%) in all samples, but their origin has not yet been fully documented. It should also be noted that the volatile compounds present in high amounts are not always the main contributors to oil aroma 28, 38.
3.2 GC-MS multivariate analysis results
In order to obtain a comprehensive representation of the major volatiles that differentiated FSO, a PCA was carried out on the entire dataset. All analytical data were standardized as z-scores before the statistical procedures were applied 39. PCA plot gives graphical representations of inter-sample and inter-variable relationships and allows one to identify a relatively small number of factors, formed by linear combinations of the considered variables, without losing any relevant information 11.
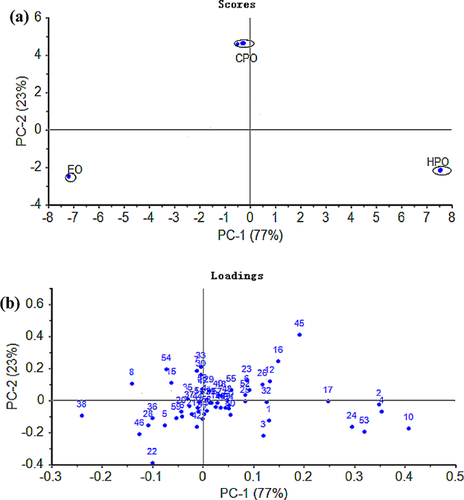
As shown in Fig. 2, the first two principal components accounted for 77 and 23% of the total variance, respectively. In the scores plot, a clear separation of EO, CPO, and HPO can be recognized (Fig. 2a). HPO and CPO had a positive value on principal component 1 (PC1) and component 2 (PC2), respectively, whereas EO had a negative value on PC1 and principal component 2 (PC2). This PCA of volatile compounds showed volatile compounds dissimilarity among the different FSO processing techniques. Moreover, the main volatiles positively correlated to PC1 are hexanal, (E, E)-2, 4-pentadienal, (E, E)-2, 4-heptadienal, 6-hydroxy-2-hexanone, 1-hexanol, methyl-pyrazine and negatively correlated volatiles to PC1 are nonanal, 2, 3-pentanedione, 1-butanol, acetic acid, hexanoic acid, ethyl acetate (Fig. 2b). Thus, FSO, processed by different technologies, could be easily distinguished by PCA analysis based on these volatile compounds variables.
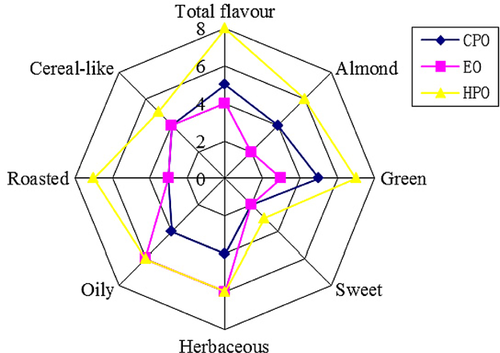
3.3 Sensory characteristics of differently prepared FSO
The sensory analysis of the three FSO samples revealed important differences for some of the descriptors evaluated, as can be seen in Fig. 3. Although both EO and HPO gave comparable intensity values for the “oily” and “herbaceous” descriptors, the odor profiles of three of them differed significantly with regard to the “green” and “almond” descriptors, while the HPO presented a strong intensity for “roasted,” “oily,” “herbaceous,” “green,” and “almond” descriptors. However, FSO processed under conditions of solvent extraction and cold-pressing produced a relative weak aroma. Indeed EO was considered to have less aroma intensity except for “oily and herbaceous” aroma 24. These results were in agreement with the multivariate analysis results above.
3.4 E-nose/MOS analysis
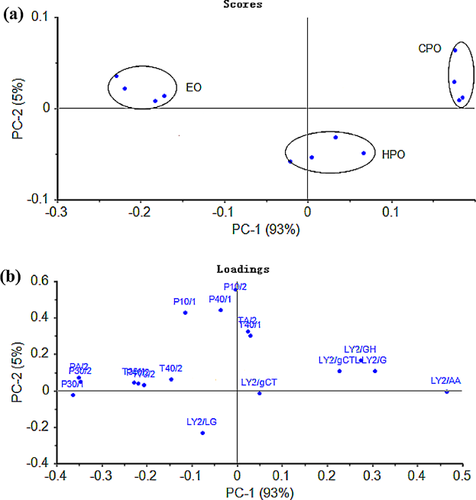
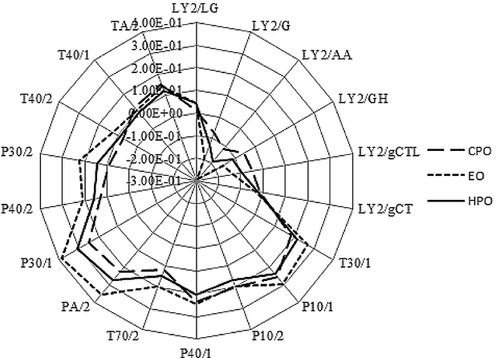
Volatile profiles of FSO with different processed techniques determined by electronic nose/MOS are shown in Fig. 4. PC1 and PC2 expressed 99.80 and 0.20% of the data variability of volatiles from sensor response data of E-nose. Three kind of FSO (HPO, EO, CPO) were clearly separated as indicated in Fig. 4a, illustrating the capability of the E-nose to discriminate the FSO with a PCA of the data provided by the sensor arrays. Figure 4b indicates that sensors LY2/AA, LY2/GH, LY2/G, LY2/gCTL, P30/1, P30/2, and PA/2 played a more important role in distinguishing the samples than the others in the vicinity of the origin. The radar chart showed the response values of different samples on the sensory array of the electronic nose. It can be seen from the figure that all of the samples exhibited a higher value on sensors P10/1, PA/2, P40/1, P30/1, LY2/GH, and LY2/AA (Fig. 5). Those results were also consistent with the PCA of the data. All the results suggest the potential capability of the E-nose to discriminate different isolated FSO with a PCA of the data provided by the sensor arrays.
4 Conclusions
In this study, a total of 61 compounds were identified and then semi-quantified in differently processed FSO samples by SPME-GC-MS. Among them, 12 volatile compounds have been first reported. While the three samples (CPO, EO, and HPO) tested resulted in significant differences of the FSO volatile profiles (p<0.05), the major constituents (hexanal, (E, E)-2, 4-pentadienal, (E, E)-2, 4-heptadienal, 6-hydroxy-2-hexanone, 1-hexanol, methyl-pyrazine, nonanal, 2,3-pentanedione, 1-butanol, acetic acid, hexanoic acid and ethyl acetate) could be used to distinguish differently processed FSO by using chemometric methods. Meanwhile there was good consistency among GS-MS, sensory evaluation and E-nose analysis results, suggesting that the E-nose technique has good potential to evaluate FSO quality. These results indicated that different processing technology has a significant effect on aroma quality of FSO and may be helpful in evaluating aroma quality and the detection of frauds.
This work was supported by the National Natural Science Foundation of China (31360389) and (31401617), the Fundamental Research Funds for the Central Universities (JUDCF12003) and the Ordinary University Graduate Students' Scientific Research Innovation Program of Jiangsu Province of China (CXLX12-0737).
The authors have declared no conflict of interest.




