Anionic long-circulating liposomes for delivery of radioiodinated antisense oligonucleotides
Abstract
Long-circulating liposomes have been widely used to enhance efficacy of gene therapy. Antisense therapy might increase the efficacy of radiation or chemotherapy; therefore, we undertook to optimize the composition of the liposomal delivery vehicles. The radiolabeling efficiency, radiochemistry purity, and specific radioactivity of radioiodinated antisense oligonucleotides (ASON) were 71.66 ± 7.73, 98.33 ± 0.39%, 4.09 ± 0.11 MBq/nmol. Radioiodinated ASON remained stable in 0.01 M HEPES buffer and human serum even after incubation for 4 hours. Mean diameter of the anionic long-circulating liposomes (ALCL) was 504 ± 31.76 nm with a polydispersity index (PDI) of 0.107 ± 0.008 before extrusion, 115 ± 8.5 nm with a PDI of 0.103 ± 0.002 after extrusion. The zeta potential of ALCL was −29.23 ± 0.45 mV. ALCL prepared for this study provided 70.28 ± 1.84% encapsulation efficiency. Compared with other liposome formulations, the ALCL mediated enhanced cellular uptake (32.51 ± 1.44%) by MCF-7 breast cancer cells (p<0.05).
Practical applications: Drug delivery systems can in principle provide enhanced efficacy and/or reduced toxicity for anticancer agents. LCL have become a commonly used carrier for gene therapy recently. ALCL are promising for the mediated radiation and antisense therapy for breast cancer which is the leading cause for women.
Abbreviations:
ALCL, anionic long-circulating liposomes; ASON, antisense oligonucleotide; CH, cholesterol; CLCL, cationic long-circulating liposomes; DDAB, dimethyldioctadecyl-ammonium bromide; DPPG, dipalmitoylphosphatidylglycerol; DSPE–PEG2000, distearyl phosphatidylethanolamine–polyethylene glycol; EE, encapsulation efficiency; FA, 125I bcl-2/bcl-xl ASON; LCL, long-circulating liposomes; NLCL, neutral long-circulating liposomes; PC, egg-phosphatidylcholine.
Introduction
The revolution in molecular biology over the past 20 years has made the concept of genetically based treatment possible. The use of antisense oligonucleotide (ASON) as a therapeutic agent in oncology has been proposed more than 10 years ago. Inhibition of gene expression by ASON has become a realistic possibility for gene-targeted therapy 1. Breast cancer cells have an inherent resistance to chemotherapy due to the abnormal expression of the Bcl-2 gene (an anti-apoptosis gene) in tumor cells. It has been the aim of Bcl-2/bcl-xL ASON therapy to reduce Bcl-2 protein in order to overcome this resistance and to induce tumor apoptosis.
Drug delivery systems can in principle provide enhanced efficacy and/or reduced toxicity for anticancer agents. Long-circulating liposomes (LCL) have recently become a commonly used carrier for gene therapy because they can exploit the “enhanced permeability and retention” effect for preferential extravasation from tumor vessels 2. There is a high level of association of ASONs with cationic LCL, and the lipoplexes have good, although nonspecific cell binding, and high transfection efficiencies in vitro. However, their large size and excess positive charge leads to their internalization by cells of the mononuclear phagocytic system. Little literature is available on anionic long-circulating liposomes (ALCL) for ASON. To improve the antitumor effects, antisense therapy combined with other treatments such as radiation or chemotherapy was also suggested. This commentary will discuss ALCL mediated radiation and antisense therapy for breast cancer cells in vitro. The preparation of LCL was previously reported in the 9th Congress of the World Federation of Nuclear Medicine & Biology (WFNMB) 3.
Materials and methods
Materials
Sodium 125I was purchased from Chengdu Nuclear Research Institute. Thallium trichloride, sodium sulfite, sephadex G25, n-methyl nitrosoure (MNU), and potassium iodide were from Sigma (USA). Sephadex 10, 50, and 75 were from Pharmacia Fine Chemicals (Uppsala, Sweden). Egg-phosphatidylcholine (PC), cholesterol (CH), distearyl phosphatidylethanolamine-polyethylene glycol (DSPE–PEG2000; Molecular weight 2000), dipalmitoylphosphatidylglycerol (DPPG) were purchased from Shearwater Polymers USA. dimethyldioctadecyl-ammonium bromide (DDAB) was obtained from Sigma.
The 20-mer phosphorothioate bcl-2 and bcl-xL ASON (5′-AATCCTCCCCCAGTTCACCC-3′) which was complementary to the translation start region of the bcl-2 oncogene mRNA was used. The scrambled oligonucleotide (SON, 5′-AAAGCATCCCAGCCTCCGTT-3′,) and nonsense oligonucleotide (NON, 5′-AAAGTATCCCAGCCGCCGTT-3′) was used as controls. All oligodeoxynucleotides used in the study according to reference 4, and were purchased from the same source (Shanghai Biotech, China) as the uniform phosphorothioates.
Methods
Preparation of antisense radiopharmaceutical
The ASONs were labeled with 125I according to the previously method 5 with modifications. The reaction conditions were optimized after systematic variation of pH, reaction time, and reaction temperature. DNA (20 µg) was prepared in 20 µL of 0.1 M ammonium acetate, pH 5.0. In a separate reaction vial, 10 µL of 250 µM potassium iodide, 10 µL of 30 mM thallium trichloride, and 100 µCi (8 µL) of sodium 125I in 0.01 M sodium hydroxide were combined. The reaction was initiated by transferring the ASON into the reaction vial followed by heating at 60–70°C for 30 min to 1 h in a heating block. After cooling, the reaction was stopped by the addition of 5 µL of 0.1 M sodium sulfite and the pH was adjusted to 7.0 by the addition of 20 µL of 1 M ammonium acetate, pH 7.0. The reaction was then heated at 60°C for 60 min to dissociate unstable iodide adducts from the ASON.
Purification of iodinated ASON
The radioiodinated oligodeoxynucleotide mixture was chromatographed on sephadex G-25 (Sigma), eluting with HEPES buffer (0.01 mol/L, pH 7.4). The yield and specific activity of iodinated ASON were determined from the absorbance at 260 nm by UV-2100 spectrophotometer (Beckman and Com, USA) and gamma counting. The radiolabeling efficiency was obtained using the SA-2128 fraction collection (BioRad, USA).
Stability of iodinated ASON
The stability of 125I-ASON after incubation in 0.01 M HEPES buffer and human serum for 1–4 h was observed. The 125I-ASON was incubated in HEPES buffer and human serum at 37°C for 1 , 2 , 4 h with occasional shaking. After the desired time period an aliquot was taken for measurement of radiochemistry by paper chromatography.
Preparation and characteristics of ASON liposome formulations
Preparation of liposomes
For comparison, we prepared different kinds of liposomes. Briefly, 125I-Bcl-2/bcl-xl ASON (FA) ALCL, FA cationic long-circulating liposomes (CLCL), FA neutral long-circulating liposomes (NLCL), 125I-SON-anionic LCL (SLCL); 125I-NON-anionic long-circulating liposomes (NONLCL), 125I-anionic anionic long-circulating liposomes (IALCL), bcl-2/bcl-xl ASON-anionic long-circulating liposomes (BLCL), FA conventional liposomes (CL) were prepared by hydration of a dried lipid film followed by extrusion, as described before 6, 7 with modifications. PC, CH, DSPE–PEG, and DPPG in the respective molar ratios of 0.50:0.35:0.06:0.10, 10:4:1:3.3, 2:1:0.2:0.66, and 0.50:0.29:0.06:0.12 (see Table 1) were dissolved in different organic solvents with varying ratios of volume to volume, organic solvent (isopropyl ether–chloroform and chloroform–methanol), and rotary speeds. Then the solvent was evaporated to form a dry film, which was hydrated with isotonic 0.01 M HEPES buffer (pH 7.4) containing 125I-ASON, and extruded through a polycarbonate filter (LiposoFast™ Avestin) to reduce the liposomal size.
| Liposome | Liposome composition | Molar ratios of liposome composition | |||
|---|---|---|---|---|---|
| ALCL | PC/CH/DSPE–PEG2000/DPPG | 0.50:0.35:0.06:0.10 | 10:4:1:3 | 12:1:0.2:0.66 | 0.50:0.29:0.06:0.12 |
| CLCL | PC/CH/DSPE–PEG2000/DDAB | 0.50:0.35:0.06:0.10 | 10:4:1:3 | 2:1:0.2:0.66 | 0.50:0.29:0.06:0.12 |
| CL | PC/CH | 0.50:0.35 | 10:4: | 2:1 | 0.50:0.29 |
| NLCL | PC/CH/DSPE–PEG2000 | 0.50:0.35:0.06 | 10:4:1 | 2:1:0.2 | 0.50:0.29:0.06 |
- ALCL, anionic long-circulating liposomes; CLCL, cationic long-circulating liposomes; CL, conventional liposomes, NLCL, neutral long-circulating liposomes.
Characterization of liposomes
The quality of liposomes was evaluated after the crude liposomes were repeatedly extruded through 400, 200, 100 nm polycarbonate membranes consecutively.
Dynamic light scattering
The sizes of all kinds of liposomes, the zeta potential of ALCL and CLCL were determined by NanoZS90 quasi-elastic light scattering (Malvern, UK).
Transmission electron microscopy (TEM)
Samples were stained with phosphotungstic acid and examined using TEM (HITACHI H-600, Japan).
Stability studies
After storage at 4°C for 3 months, the ALCL was examined by TEM (HITACHI H-600, Japan).
Encapsulation efficiency (EE)
To separate 125I-ASON from 125I-ASON entrapped ALCL, protamine sulfate (Sigma) sedimentation, sephadex G-10, 50,and 75, dialysis filter (molecular weight, 8000–15 000 Da), and ultracentrifugation were used.
The radioactivity content and the labeling efficiency were determined by counting the radioactivity of each fraction collected, along with radioactivity remaining in the column, using γ-counter, and calculating the percentage of radiolabel present in form of 125I-ASON-LCL and 125I-ASON versus the total radioactivity after ultracentrifugation.
Tumor cell binding
Cell culture
Human breast cancer MCF-7 cell line was obtained from Internal Peptide Laboratory of West China Hospital and the cells were grown in RPM1640 medium containing penicillin at 100 U/mL, streptomycin at 100 U/mL, and supplemented with 10% FCS. Cells were maintained as monolayers in a humidified 5% carbon dioxide atmosphere.
Time-dependent nonspecific tumor binding
A suspension of approximately 1 × 105 cells in 2 mL growth medium was deposited per well in six-well Falcon plates. Cells were allowed to attach to the plates and grow for no longer than 3 days at 37°C. Spent growth medium was replaced with 2 mL fresh serum-containing medium per well. Liposomes encapsulated with 2 µCi radioiodinated ASON, FA were added to the wells, and the cells were incubated for increasing times (0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, and 7 h) at 37°C. Each well was rinsed four times with 1 mL ice-cold PBS to remove noncell associated material. Final results were expressed as percentage uptake by tumor cells.
Results
Radioiodination of ASON
The reaction conditions were optimized after systematic variation of pH, reaction time, and reaction temperature for radioiodination of ASON. It was found that the conditions of reaction under 30 min at 60°C (pH 5.0), then 45 min at 60°C (pH 5.0), and 60 min at 60°C (pH 7.0) gave satisfactory results. The radiolabeling efficiency (RE), radiochemistry purity (RP), and specific radioactivity (SR) of 125I-ASON were 71.66 ± 7.73, 98.33 ± 0.39%, and 4.09 ± 0.11 MBq/nmol, respectively (see Table 2). 125I-ASON remained stable in 0.01 M HEPES buffer and human serum even after incubation for 4 h (see Tables 3 and 4, respectively).
| Oligonucleotides | Rf | RE | RP | SP |
|---|---|---|---|---|
| 125I-ASON | 0.1 | 72.80 ± 0.68 | 98.33 ± 0.39 | 0.63 ± 0.11 |
| 125I-NON | 0.1 | 72.77 ± 0.81 | 98.42 ± 0.40 | 0.62 ± 0.11 |
| 125I-SON | 0.1 | 72.21 ± 0.60 | 98.28 ± 0.36 | 0.63 ± 0.14 |
- ASON, antisense oligonucleotide; NON, nonsense oligonucleotide; SON, scrambled oligonucleotide.
| Oligonucleotides | 1 h | 2 h | 4 h |
|---|---|---|---|
| 125I-ASON | 96.8 ± 0.78 | 96.2 ± 0.74 | 95.6 ± 0.75 |
| 125I-NON | 95.5 ± 0.88 | 94.2 ± 0.69 | 93.4 ± 0.59 |
| 125I-SON | 96.0 ± 0.76 | 95.4 ± 0.85 | 94.6 ± 0.94 |
- ASON, antisense oligonucleotide; NON, nonsense oligonucleotide; SON, scrambled oligonucleotide.
| Oligonucleotides | 1 h | 2 h | 4 h | F | p |
|---|---|---|---|---|---|
| 125I-ASON | 92.3 ± 0.49 | 84.7 ± 0.58 | 82.6 ± 0.38 | 3.66 | 0.09 |
| 125I-NON | 90.2 ± 0.52 | 85.9 ± 0.68 | 80.4 ± 0.70 | 4.25 | 0.07 |
| 125I-SON | 91.0 ± 0.60 | 88.0 ± 0.52 | 82.0 ± 0.67 | 1.8 | 0.23 |
| p | 0.72 | 0.52 | 0.90 |
- ASON, antisense oligonucleotide; NON, nonsense oligonucleotide; SON, scrambled oligonucleotide.
Quality of liposomes
Choice of molar ratio of lipid components
Liposomes composed of PC/CH/DSPE–PEG2000/DPPG, PC/CH/DSPE–PEG2000/DDAB, PC/CH/DSPE–PEG2000 and PC/CH at the molar ratios of 0.50:0.35:0.06:0.10, 0.50:0.29:0.06:0.12, 0.50:0.35:0.06, and 0.50:0.35, respectively, prepared at 10 mg/mL in isopropyl ether–chloroform (1:1 v/v) and chloroform–methanol (2:1 v/v) using a rotary evaporator at 41–42°C and rotary speed at 200 rpm were of small size with satisfying polydispersity index (PDI) and zeta potential.
Liposome size
Mean diameter of liposomes was 504 ± 31.76 nm with a PDI of 0.107 ± 0.008 before extrusion, 115 ± 8.5 nm with a PDI of 0.103 ± 0.002 after extrusion (see Figs. 1 and 2, respectively). The zeta potential of ALCL and CLCL were −29.23 ± 0.45 mV (see Fig. 3), +41.91 ± 0.58 mV (see Fig. 4), respectively.
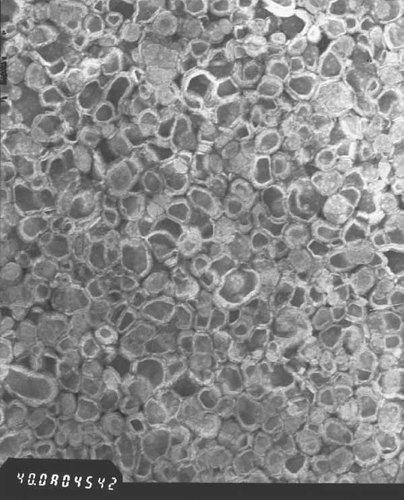
TEM of 115 nm ALCL.
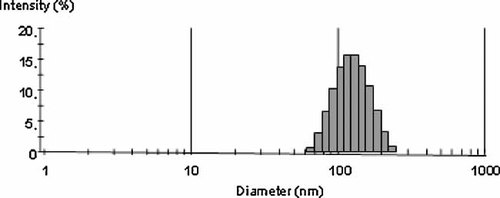
Size distribution profile of liposomes and polydispersity.
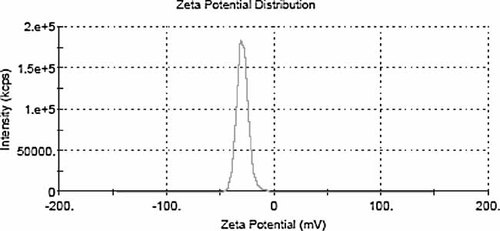
Zeta potential distribution of ALCL (−29.23 mV).
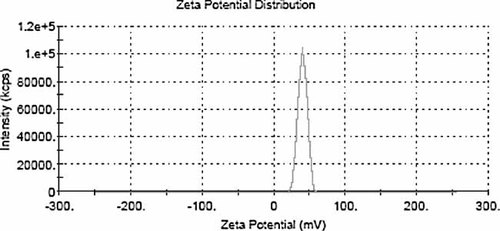
Zeta potential distribution of CLCL (41.91 mV).
Entrapment efficiency
The successful separation of radioiodinated ASON from radioiodinated ASON-entrapped ALCL was achieved using ultracentrifugation. The liposomal formulations prepared for this study provided 70.28 ± 1.84 and 71.57 ± 2.21% EE in cases of ALCL and CLCL, and 41.66 ± 2.03% in case of NLCL and 34.33 ± 3.17% in case of CL (p<0.05), respectively (see Table 5).
| Liposomes | EE (%) |
|---|---|
| ALCL | 70.28 ± 1.84 |
| NLCL | 41.66 ± 2.03 |
| CL | 34.33 ± 3.17 |
| CLCL | 71.57 ± 2.21 |
| p | <0.001 |
- ALCL, anionic long-circulating liposomes; CLCL cationic long-circulating liposomes; NLCL, neutral long-circulating liposomes; CL, conventional liposomes.
Morphology
As displayed in Fig. 4, LCLs were mostly round or elliptical with small unilamellar vesicles (SUV) in outright majority.
Stability studies
After 3 months, no visible flocculation or stratification was observed in ALCL which was confirmed by TEM.
Nonspecific tumor binding
Compared with groups of CLCL (21.92 ± 2.07%), NLCL (16.26 ± 1.01%), CL (13.24 ± 2.06%), and FA (9.55 ± 1.43%), the enhanced cellular uptake (32.51 ± 1.44%) was mediated by ALCL (p<0.05, see Table 6). The peak uptake time was at 3 h.
| Liposomes | Peak cell uptake (%) | Peak time |
|---|---|---|
| ALCL | 32.51 ± 1.44a) | 2.50 ± 0.50a) |
| CLCL | 21.92 ± 2.07a) | 3.16 ± 0.796a) |
| NLCL | 16.26 ± 1.01a) | 6.33 ± 0.57a) |
| CL | 13.24 ± 2.06 | 6.00 ± 1.77 |
| FA | 9.55 ± 1.43 | 3.16 ± 0.76 |
- ALCL, anionic long-circulating liposomes; CLCL cationic long-circulating liposomes; NLCL, neutral long-circulating liposomes; CL, conventional liposomes; FA, 125I-bcl-2/bcl-xl ASON.
- a) Significant difference as compared with FA.
Discussion
Antisense therapeutics are finally close to fulfilling their promise in the clinic 8. Therapeutic interventions that inhibit bcl-2/bcl-xL expression have been extensively investigated, indicating that ASONs have high specificity and potential clinical application 9-12. However, free ASON is short lived and easily be degraded by RNAase in blood before reaching the target site. Of nonviral delivery systems, liposomes, especially LCL, are recognized as the most promising ASON carrier systems for intravenous injection. Due to the unsatisfactory results of antisense therapy alone, combination treatments with chemotherapy or radiation have been proposed 13, 14. Therapeutic radionuclide-labeled bcl-2/bcl-xL ASON can efficiently inhibit the expression of anti-apoptotic genes and induce apoptosis. Therefore, the objective of this study was to investigate the preparation and use of ALCL for the delivery of radioiodinated bcl-2 ASON to human breast cancer cells in vitro. Up to now, there is no literature on ALCL.
Radiolabeled probes could be informative tools for studying the cellular up take of ASON encapsulated LCL. Owing to many contributing factors for the labeling efficiency of 125I, the reaction conditions were optimized in the study by careful selection of oxidizing agent and adjustment of reaction volume, pH changes, time, and temperature. TlCl3, a mild oxidizing agent, was adopted to oxidize 125I into 125I or 125I+ which replaced the H+ of pyrimidine under heated and acid conditions 5. Preliminary experiments established that standard Commerford conditions of heating at 60°C at pH 6 for 45 min and then 80°C at pH 9 for 60 min led to the degradation of DNA and to the formation of open circular and fused DNA that remained in the loading well. The labeling artifacts were the result of both the high pH and heating temperature of the second reaction which was used by Commerford to eliminate iodide from the less stable 6 position of the cytidine ring as opposed to the more stable 5-iodocytidine product. To overcome this limitation, ASON stability was studied while varying the pH and the temperature of the second reaction. Although the stability of DNA was improved, the 50% yield was not satisfactory 15. In this experiment, to establish the influence of temperature, the pH was held constant at 5.0, 7.0 and the reaction temperature of the first, second, and third reaction was maintained up to 60°C. Consequently, the reaction condition of pH 5.0, 7.0, temperature 60°C for 30, 45, and 60 min was determined to be optimal for the first, second, and third reaction since iodine replacement is able to achieve high labeling efficiency and minimize the radiolabeling injury to ASON.
The large difference of molecular weight between 125I and ASON allows them to be easily separated using sephadex G-25. Subsequently, the radiochemical purities were all above 98% with high specific activity (>0.60 MBq/µg). Being a special drug, ASONs must retain biological activity, stability in both vitro and vivo and be capable of penetrating cell membrane, a condition partially reached by using phosphorothioate ASON, in which one of the nonbridging oxygens at the phosphorus is replaced with a sulfur. More significantly, 125I-ASON remained stable at 4 h in 0.01 M HEPES buffer (pH 7.4) and human serum, indicating that 125I-ASON prepared in the study is promising for in vivo study.
It is assumed that lipid-based particles exhibiting a net surface negative charge bind more avidly to cells in culture than neutral particles. Cationic liposomes have proven to be useful tools for delivery of plasmid DNA and RNA into cells 16-18. There is a high level of association of ASONs with cationic lipoplexes, and the lipoplexes have good, although nonspecific, cell binding, and high transfection efficiencies in vitro as showed by our study. However, their large size and excess positive charge result in their clearance by cells of the mononuclear phagocytic system within a few minutes of their administration into the blood stream 1. Fortunately, data in this study showed that ALCL also rapidly associate with breast cancer MCF-7 cells, and saturation was typically reached within the first hour of incubation, presumably via electrostatic interactions with charged proteins and carbohydrates at the cell surface.
Traditionally, EE can be obtained by column chromatography, dialysis, and ultracentrifugation. In this study, ultracentrifugation with a centrifugal force 3.74 × 10E5 g for 6 h efficiently separated iodinated ASON from the liposomes with encapsulated iodinated ASON, while the former two methods failed. Since ASONs are hydrophilic, they can be passively entrapped within the aqueous space of liposomes 19, which made the high EE possible. ALCL with encapsulated ASONs enter the target cells by endocytosis.
In order for liposomes containing ASON to preferentially accumulate in tumor, they must exhibit a small size, stability, and long circulation time following intravenous injection as well as specific binding. These demands stem from behavior observed for liposomal systems that have proven clinical utility. Many researchers have shown that circulation time of liposomes depend on the particle size and surface characteristics of the carriers, such as hydrophobicity, charge, and fluidity. There is strong evidence that the size of LCL affected significantly their behavior in vivo 20-27. The optimal size of LCL to penetrate the capillary into tumor is 80–200 nm, and 100 nm LCL in particular exhibit a remarkable property termed “disease-site targeting” or “passive targeting”, which results in considerable improvements in the amounts of drug arriving at the disease site 28, 29. It is encouraging that the preparation of ALCL in the study achieved high loading efficiency, small particle size, and good stability.
We conclude that the preparation of ALCL in this study achieved high loading efficiency, small particle size, and good stability. Therefore, ALCL are promising as a vehicle for efficient uptake by tumor cells, and for use in drug delivery, and antitumor treatment for breast cancer cells in vitro.
Acknowledgements
We are grateful for patience and help from Tan Tianzhi, Liang Zhenglu, Li Yingchun, and all staff in Department of Nuclear Medicine, West China Hospital of Sichuan University. We thank all the staff from College of Pharmaceutical Sichuan University for their generosity of the experimental environment and instructions of liposome preparation. We are indebted to Dr. Al Driedger from Department of Nuclear Medicine/Oncology for his careful correction of the paper University of Western Ontario. We are also thankful to Miss Xie from animal center in Sichuan University for their help with the animal experiments. This study was financially supported by National Science Fund (no. 30700185).
The authors have declared no conflict of interest.




