Correlation between thermal properties and chemical composition of Italian virgin olive oils
Abstract
Thirteen monovarietal extra virgin olive oils (EVOos) from two Italian regions were evaluated by means of differential scanning calorimetry (DSC) to study statistical correlations among major and minor chemical components and thermal properties obtained by cooling transitions and their deconvoluted peaks. The application of DSC for the discrimination of EVOos according to the cultivar and geographical origin was also considered. Thermal properties of the cooling transitions (except for the crystallization enthalpy) were almost all influenced by triolein content and fatty acid composition. Thermal properties (area, onset and offset temperatures of transition, transition range, and peak temperature) of the three deconvoluted peaks were found to be significantly correlated not only to major but also to minor components (diacylglycerols, free fatty acids) and oxidative stability indices. The analysis of thermal properties obtained by cooling transition did not lead to sample discrimination according to geographical provenience. However, Ton, Toff, and peak areas of the two deconvoluted transitions that peaked at the lowest temperatures, as well as Toff of deconvoluted peak at the highest temperature, significantly differentiated oil samples according to their geographical origin. These findings may be confirmed by the appliance of multivariate statistical analysis to a larger set of samples to select thermal parameters able to discriminate among EVOos of different geographical provenience.
Practical applications
DSC application exhibits some advantages upon the classical analytical methods as it does not require sample preparation and use of solvents, thus resulting in a reduced environmental impact. The results of this study suggest that DSC can be used to test quality and to determine geographical origin of EVOo.
Abbreviations:
1,2-OL, 1-oleoyl-2-linoleoyl-sn-glycerol; 1,2-OO, 1,2-diolein; 1,2-PL, 1-palmitoyl-2-linoleoyl-sn-glycerol; 1,2-PO, 1-palmitoyl-2-oleoyl-sn-glycerol; 1,3-OO, 1,3-diolein; 1,3-PO, 1-palmitoyl-3-linoleoyl-glycerol; DSC, differential scanning calorimetry; DSTAG, disaturated triacylglycerols; EC, European Community; EVOo, extra virgin olive oil; FA, fatty acids; FFA, free fatty acids; LLL, trilinolein; LLP, dilinoleoyl-palmitoyl-glycerol; LLPo, dilinoleoyl-palmitoleoyl-glycerol; MSTAG, monosaturated triacylglycerols; OLL, dilinoleoyl-oleoyl-glycerol; OLnO, dioleoyl-linolenoyl-glycerol; OLP, palmitoyl-oleoyl-linoleoyl-glycerol; OLPo, palmitoleoyl-oleoyl-linoleoyl-glycerol; OOO, triolein; OOPo, dioleoyl-palmitoleoyl-glycerol; OSI, oxidative stability index; POP, dipalmitoyl-oleoyl-glycerol; POPo, palmitoyl-palmitoleoyl-oleoyl-glycerol; POV, peroxide value; SFA, saturated fatty acids; SLO, stearoyl-oleoyl-linoleoyl-glycerol; TUTAG, triunsaturated triacylglycerols.
Introduction
Among vegetable oils, extra virgin olive oil (EVOo) can be considered the highest quality product, as it is obtained from olive fruit only by physical operations (milling, pressing, centrifugation, and filtration). Its peculiar chemical composition, rich in oleic acid (C18:1), and natural antioxidant molecules (especially phenolic compounds) provide recognized advantages for health and a favorable dietary contribution as a part of the “Mediterranean diet” 1, 2. The production and consumption of EVOo mostly concern the Mediterranean countries, where Italy is the second worldwide producer (average production of 632 000 t/year for the 2002–2009 period) 3, with an olive oil germoplasm that includes about 540 cultivars 4. The olive tree (Olea europaea L.) grows in a large part of the Italian territory, especially in the central and southern regions, where the EVOo production is mainly located.
The characterization of EVOo in terms of cultivar and geographical origin is a currently debated problem. In fact, the quality and the peculiarity of EVOo are well known to be largely influenced by cultivar, pedoclimatic conditions, and agricultural practices 5. Several analytical methods have been proposed for the EVOo classification according to geographical origin and/or cultivar, including both physical 6-9 or chemical techniques by means of complex multivariate statistical analyses 10, 11.
Differential scanning calorimetry (DSC) is a well-known calorimetric technique largely recognized as a valuable tool for the characterization of the thermal behavior of oils and fats 12. Its application presents several advantages as it does not require chemical treatments or time-consuming manipulation practices before each measurement. In addition, thermal parameters obtained by cooling and heating thermograms have been found to be statistically correlated with chemical parameters related to composition 13, 14 and oxidative stability of vegetable oils 15-17.
DSC has also been applied for studying EVOo. The analysis of crystallization and melting profiles and the relationship between thermal properties and main chemical components (triacylglycerols (TAG) and fatty acids (FA)) were employed to differentiate among Spanish monovarietal virgin olive oils 18. DSC was also able to efficiently discriminate between commercial and guaranteed origin EVOo, even though no characterization of the chemical macrocomponents was here performed 19.
More recently, the relationship between thermal properties and both major (i.e., TAG and FA) and minor chemical components (i.e., free fatty acids (FFA), diacylglycerols (DAG), primary and secondary oxidation products) were explored on cooling 20 and heating thermograms 21 of Italian monovarietal EVOos from Sicily. In these studies, statistically correlations were only considered and determined between percentages of TAG (grouped according to the type of FA bound to the glycerol structure) and areas of peaks obtained after deconvolution of cooling and heating transitions, as a small set of samples was analyzed 20, 21.
Relationships between thermal properties and chemical composition were subsequently applied to assess DSC employment for the discrimination of commercial categories of olive oil 22, the detection of adulteration of EVOo with cheaper vegetable oils 23, 24, and the evaluation of oxidative stability of different commercial categories of olive oil after microwave heating 25. In addition, Kotti et al. 26 preliminary evaluated DSC potentiality in discriminating EVOos on the basis of cultivar–environment interaction on Tunisian samples from two different cultivars, by means of cooling and heating profiles and related thermal properties. The authors found that onset and offset temperatures of crystallization, as well as heating profiles, were characteristic of samples from different cultivars and/or geographical provenience when chemical components (i.e., TAG and FA) exhibited a quite different degree of unsaturation 26.
To the authors best knowledge, deep evaluation of the statistical correlations existing among major and minor chemical components and all thermal properties obtained by cooling thermograms and their deconvoluted peaks was never reported in literature for EVOo. Thus, the first aim of this study was to verify the existence of statistical correlations among major (i.e., TAG and FA) and minor (i.e., DAG and FFA) chemical components, the oxidative stability index (OSI values) and the thermal properties (obtained by cooling transitions and their deconvoluted peaks) of monovarietal EVOos from two Italian regions.
Materials and methods
Samples
Thirteen samples of monovarietal EVOos, obtained from drupe cultivars that are commonly grown in two different Italian regions (Abruzzo and Apulia), were analyzed (see Fig. 1). Seven oil samples were produced from olives grown in Abruzzo, whereas the other six were obtained from drupes collected in Apulia. Olives of nine different cultivars were utilized, five of which were from Leccino cultivar.
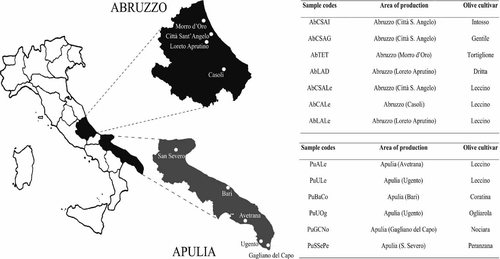
Map of Italy showing the two regions (Abruzzo and Apulia) where drupe cultivars were grown and monovarietal EVOos were produced. Sample codes and area of production are also reported for all samples.
Drupes used for oil production were handpicked in 2007 and processed in continuous industrial mills. All samples were stored in dark bottles without headspace at room temperature before analysis.
Reagents, solvents, and standards
All solvents used were analytical or HPLC grade (Merck, Darmstadt, Germany). Reagents and commercial standards of TAG (triolein (OOO) and trilinolein (LLL)), DAG (dimyristin, dipalmitin, distearin, and diolein), tridecanoic acid methyl ester, and dihydrocholesterol (94.8% purity) were purchased from Sigma–Aldrich (St. Louis, MO). The standard mixture of FA methyl esters (GLC 463) was supplied by Nu-Chek (Elysian, MN). Silica SPE cartridges (500 mg stationary phase/3 mL Strata cartridges) were purchased from Phenomenex (Torrance, CA).
Chemical analysis
HPLC analysis of TAG was performed according to Chiavaro et al. 23. TAG were tentatively identified based on their UV–Vis and mass spectra obtained by HPLC-APCI-MSD and literature data 27. The LOQ was 0.01 g/100 g of TAG. TAG were grouped according to the type of FA bound to the glycerol structure as monosaturated triacylglycerols (MSTAG), disaturated triacylglycerols (DSTAG) and triunsaturated triacylglycerols (TUTAG). The following TAG were identified: LLL, dilinoleoyl-palmitoleoyl-glycerol (LLPo), oleoyl-linoleoyl-linolenoyl-glycerol (OLLn), dilinoleoyl-oleoyl-glycerol (OLL), palmitoleoyl-oleoyl-linoleoyl-glycerol (OLPo), dilinoleoyl-palmitoyl-glycerol (LLP), dioleoyl-linolenoyl-glycerol (OLnO), dioleoyl-linoleoyl-glycerol (OLO), palmitoyl-oleoyl-linoleoyl-glycerol (OLP), dioleoyl-palmitoleoyl-glycerol (OOPo), palmitoyl-palmitoleoyl-oleoyl-glycerol (POPo),OOO, stearoyl-oleoyl-linoleoyl-glycerol (SLO), palmitoyl-dioleoyl-glycerol (POO), dipalmitoyl-oleoyl-glycerol (POP), dioleoyl-stearoyl-glycerol (SOO), and palmitoyl-stearoyl-oleoyl-glycerol (SOP).
GC determination of the FA composition (as methyl esters) was performed according to Cercaci et al. 28. Tridecanoic acid methyl ester was used as internal standard for FA quantification and peak identification was carried out by comparing the peak retention times with those of the GLC 463 FAME standard mixture. The GC response factor of each FA was calculated by using the GLC 463 FAME standard mixture and the internal standard (C13:0). LOQ was 0.01 g/100 g of FA. FA were expressed according to their unsaturation degree as saturated (SFA), monounsaturated (MUFA), and polyunsaturated fatty acids (PUFA).
DAG were determined according to a modified version of the method suggested by Bonoli et al. 29, where dihydrocholesterol was used as internal standard. Seventy microliters of a solution of dihydrocholesterol (1 mg of dihydrocholesterol in 1 mL of n-hexane/isopropanol (4:1 v/v)) was added to 100 mg oil and dissolved in 500 µL of n-hexane before loading into the SPE cartridge. The rest of the DAG purification by SPE elution was the same as reported by Bonoli et al. 29. Identification of DAG was carried out by comparing the peak retention times and the GC traces with those of the DAG standards and chromatograms reported in literature 30, respectively. The following DAG were identified: 1-palmitoyl-2-oleoyl-sn-glycerol (1,2-PO), 1-palmitoyl-2-linoleoyl-sn-glycerol (1,2-PL), 1-palmitoyl-3-linoleoyl-glycerol (1,3-PO), 1,2-diolein (1,2-OO), 1-oleoyl-2-linoleoyl-sn-glycerol (1,2-OL), and 1,3-diolein (1,3-OO) and they were all expressed as percentage with respect to the total DAG content. LOQ was 0.04 g/100 g of DAG. DAG were also grouped according to the type of FA bonded to the glycerol structure as 1,2-DAG and 1,3 DAG and expressed as the sum of the percentages of the identified single DAG.
Free acidity value (expressed as % oleic acid) and peroxide value (POV) (expressed as meq O2/kg lipids) were evaluated according to the official methods described in annex III of EEC Regulation 2568/91 31.
The evaluation of the oxidative stability was carried out by using an eight-channel OSI (Omnion, Decatur, IL), according to Jebe et al. 32. The conductibility was measured in polycarbonate tubes using twice distilled water. The air flow was set at 120 mL/min. The OSI were run at 110 ± 0.1°C. Results were expressed as induction time (h).
For each chemical determination, three replicates were prepared and analyzed per sample.
DSC analysis
DSC analysis was performed according to Chiavaro et al. 20. Samples of oil (8–10 mg) were weighed into aluminum pans, sealed into place and analyzed with a DSC Q100 (TA Instruments, New Castle, DE, USA). Indium (melting temperature 156.6°C, ΔHf = 28.45 J/g) and n-dodecane (melting temperature −9.65°C, ΔHf = 216.73 J/g) were used to calibrate the instrument and an empty pan was used as reference. Oil samples were equilibrated at 30°C for 3 min and then cooled at −80°C at a rate of 2°C/min. Dry nitrogen was purged in the DSC cell at 50 cm3/min. Cooling thermograms were analyzed with Universal Analysis Software (Version 3.9A, TA Instruments) to obtain enthalpy (ΔH, J/g), onset temperature (Ton, °C) and offset temperature (Toff, °C) of the transitions (intersection of baseline and tangent at the transition). Range of the transitions was calculated as temperature difference between Ton and Toff. Overlapping transitions of the cooling thermograms were deconvoluted into individual constituent peaks using PeakFitTM software (Jandel Scientific, San Rafael, CA). The following parameters were considered for each deconvoluted peak: Ton, Toff, and peak temperatures (Tp), % peak area (percentage area of the total peak area) and range of the transitions. Five replicates were analyzed per sample.
Statistical analysis
Means and SDs were calculated with SPSS (Version 15.0, SPSS, Chicago, IL, USA) statistical software. One-way-analysis of variance and Tukey's honest significant difference test at a 95% confidence level (p<0.05) were performed to identify differences among groups. Pearson correlation coefficients were calculated among the measured variables at a 95% confidence level (p<0.05).
Results and discussion
Chemical composition of EVOo samples
General information about samples is reported in Fig. 1, where the abbreviations used for the different samples are shown. Seven oil samples were produced from drupes grown in Abruzzo, region located in Middle Italy, whereas the other six were obtained from olives collected in Apulia, a Southern region which accounts for the highest Italian production of EVOo (200 000 t) 3. The olive groves in both regions are present along the whole coastline and a large part of inland. Monovarietal oil samples were obtained from olives of nine different cultivars, five of which were from Leccino cultivar (three from Abruzzo and two from Apulia regions); in fact, Leccino is one of the more widespread Italian cultivars as it presents a large adaptation capacity to environments with different pedoclimatic conditions 33 The other samples originated from other representative cultivars of both regions.
All oil samples exhibited free acidity (0.27 ± 0.10% oleic acid) and POVs (7.96 ± 1.00 meq O2/kg) below than the limits set by the European Community (EC) Regulation for EVOo (free acidity <0.8% oleic acid, POV<20 meq O2/kg) 34.
The other chemical parameters (TAG, DAG, FA, and oxidative stability expressed as OSI time) are summarized in Table 2. Seventeen TAGs were identified in all samples; seven of them were separately quantified and the others were quantified as pairs (LLL + LLPo, OLL + OLPo, LLP + OLnO, OLP + OOPo, and SLO + POO). OOO was the main TAG in all samples, as expected; two samples of Leccino (AbCALe and PuULe) contained the highest OOO amount. EVOo samples from Leccino also presented higher DSTAG content than other oil samples, except for AbCSALe whose disaturated TAG content was significantly lower. Among all samples, EVOo from Coratina cultivar (PuBaCo) had the lowest and highest content of MSTAG and TUTAG, respectively.
FA percentages were within the range indicated by the Commission Regulation for EVOo category 34. In general, EVOo samples from Leccino showed the highest and the lowest levels of oleic and linoleic acids, respectively. Similar amounts of linolenic acid were detected in all samples. Relatively high MUFA and low PUFA contents were also found in Leccino samples. It must be noted, however, that AbCSALe sample displayed a different FA distribution as compared to the other Leccino samples, though. Mean values of oleic and linoleic acid ratio of Leccino monovarietal oils was about 10, which agrees with the results of a previous study carried out on monovarietal oils from Abruzzo region 35. In the AbCSAG sample, the value of this ratio was similar to that previously found in EVOo from Gentile cultivar 35.
Total DAG slightly varied among samples, where AbTET exhibited the highest content. DAG were mostly present as 1,2-DAG (mainly1,2-OO and 1,2-OL) in all samples, confirming the oils' freshness 36. Two samples from Leccino (AbLAle and PuULe) presented the highest amount of 1,2-OO and the lowest levels of 1,2-OL and 1,3-OO, respectively. Similar profiles were also observed in other two Apulian oils obtained from Coratina (PuBaCo) and Ogliarola (PuUOg) cultivars. Total percentages of 1,2-DAG and 1,3-DAG are also reported in Table 1. AbLAle, PuULe, and PuBaCo exhibited the highest amount of 1,2-DAG and the lowest level of 1,3-DAG, thus suggesting that these oils may have been obtained from better quality fruits as compared to the other samples here analyzed 36.
| AbCSAI | AbCSAG | AbTET | AbLAD | AbCSALe | AbCALe | AbLALe | PuALe | PuULe | PuBaCo | PuUOg | PuGCNo | PuSSePe | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TAG (% of total TAG) | |||||||||||||
| LLL + LLPo | 1.6 bc | 1.5 bc | 1.4 c | 1.8 ab | 2.9 a | 1.3 c | 0.9 d | 1.4 c | 1.2 cd | 1.3 cd | 1.2 cd | 2.4 a | 2.2 a |
| OLLn | 0.7 b | 0.5 bc | 0.6 bc | 0.8 b | 1.0 a | 0.9 a | 0.4 c | 0.7 b | 0.8 b | 0.5 c | 0.8 b | 0.5 bc | 0.7 b |
| OLL + OLPo | 11.8 a | 11.3 ab | 11.2 ab | 11.0 bc | 12.5 a | 10.8 bc | 9.3 c | 10.6 bc | 9.6 c | 11.8 a | 9.8 c | 12.1 a | 12.1 a |
| LLP + OLnO | 7.8 b | 7.5 b | 6.4 c | 7.7 b | 9.5 a | 5.0 de | 4.4 e | 4.3 e | 4.8 de | 4.3 e | 4.6 e | 4.7 de | 6.4 c |
| OLO | 19.2 b | 20.6 ab | 18.3 cd | 18.0 cd | 18.1 d | 15.8 e | 16.5 de | 15.8 e | 14.8 f | 21.9 a | 16.8 d | 17.1 d | 19.2 b |
| OLP + OOPo | 14.5 ab | 15.7 a | 14.2 bc | 14.6 ab | 15.7 a | 13.8 b | 13.1 c | 13.7 b | 12.8 d | 12.9 d | 13.9 b | 13.2 de | 14.3 bc |
| POPo | 2.8 c | 3.1 bc | 2.7 c | 2.5 cd | 3.8 a | 3.0 bc | 2.8 cd | 3.5 ab | 2.3 d | 1.6 e | 2.6 cd | 2.4 d | 2.9 c |
| OOO | 21.8 c | 22.0 c | 22.9 c | 20.8 de | 19.0 e | 22.3 c | 22.2 c | 22.7 c | 25.6 a | 24.7 b | 22.9 c | 21.3 d | 25.6 a |
| SLO + POO | 11.4 d | 12.3 d | 15.7 bc | 15.7 bc | 12.2 d | 19.0 a | 19.0 a | 18.4 a | 19.3 a | 15.2 bc | 19.0 a | 17.5 ab | 14.0 c |
| POP | 1.7 d | 1.8 d | 2.7 b | 2.4 bc | 2.0 c | 3.8 a | 3.7 a | 3.8 a | 3.2 b | 2.1 c | 2.8 b | 2.8 b | 2.1 c |
| SOO | 3.8 c | 3.4 cd | 3.2 d | 3.8 c | 2.5 e | 3.4 cd | 3.5 cd | 4.2 b | 4.7 ab | 3.0 d | 4.0 bc | 5.1 a | 3.3 d |
| SOP | 0.8 b | 0.3 d | 0.8 b | 0.5 c | 0.7 bc | 0.9 ab | 0.6 bc | 0.8 b | 0.9 ab | 1.0 a | 0.8 b | 0.8 b | 0.7 b |
| DSTAG | 2.5 de | 2.1 e | 3.5 bc | 2.8 c | 2.7 c | 4.7 a | 4.3 ab | 4.6 a | 4.1 b | 3.1 bc | 3.6 b | 3.6 b | 2.8 c |
| MSTAG | 40.4 bc | 42.0 ab | 42.1 ab | 44.3 a | 43.8 a | 44.3 a | 42.9 a | 44.2 a | 43.8 a | 36.8 c | 44.2 a | 43.0 a | 40.8 bc |
| TUTAG | 57.1 ab | 55.9 cd | 54.4 cd | 52.9 de | 53.4 d | 51.1 e | 52.7 de | 51.2 e | 52.1 e | 60.1 a | 51.5 e | 53.4 d | 56.4 bc |
| FA (%) | |||||||||||||
| Palmitic acid | 14.2 d | 15.1 bc | 15.2 bc | 15.1 bc | 16.7 a | 15.6 b | 14.7 c | 14.8 c | 14.0 d | 12.7 e | 14.8 c | 13.9 d | 14.7 c |
| Palmitoleic acid | 0.8 d | 0.8 d | 1.0 c | 1.0 c | 1.1 c | 1.2 c | 1.1 c | 1.7 a | 1.4 b | 0.4 e | 1.7 a | 1.3 b | 0.9 d |
| Stearic acid | 3.2 a | 3.0 ab | 2.1 c | 3.1 a | 2.0 c | 2.0 c | 1.7 d | 1.7 d | 1.8 d | 2.2 c | 2.3 c | 2.8 b | 2.3 c |
| Oleic acid | 66.1 e | 66.1 e | 70.1 c | 69.4 d | 63.9 f | 71.9 bc | 74.5 ab | 72.8 b | 75.3 a | 75.2 a | 72.4 b | 69.9 d | 69.0 d |
| Linoleic acid | 13.2 a | 12.8 b | 9.6 e | 9.7 e | 14.0 a | 7.4 f | 6.2 g | 7.2 f | 5.7 g | 7.4 f | 7.1 f | 10.0 d | 11.0 c |
| Linolenic acid | 0.7 a | 0.7 a | 0.8 a | 0.6 a | 0.8 a | 0.8 a | 0.6 a | 0.7 a | 0.7 a | 0.8 a | 0.7 a | 0.8 a | 0.8 a |
| SFA | 18.4 b | 19.0 a | 16.5 e | 18.9 ab | 19.6 a | 18.2 b | 17.0 c | 17.1 c | 16.3 d | 15.5 f | 17.7 c | 17.5 c | 17.7 c |
| MUFA | 67.7 h | 67.6 h | 73.1 e | 70.8 g | 65.6 i | 73.6 d | 76.1 b | 75.0 c | 77.3 a | 76.3 b | 74.5 d | 71.7 f | 70.4 g |
| PUFA | 13.9 a | 13.4 a | 10.4 c | 10.3 c | 14.8 a | 8.2 d | 6.9 e | 7.9 d | 6.4 e | 8.2 d | 7.8 d | 10.8 c | 11.9 b |
| Total DAG (g/100 g oil) | 1.0 d | 1.0 d | 1.6 a | 1.1 d | 1.0 d | 1.3 c | 1.0 d | 1.1 e | 1.3 c | 1.0 d | 1.2 c | 1.3 c | 1.4 b |
| DAG (% of total DAG) | |||||||||||||
| 1,2-PO | 7.5 bc | 7.6 bc | 5.7 e | 7.4 bc | 7.8 ab | 5.0 e | 8.1 a | 8.0 a | 7.9 ab | 7.0 cd | 8.2 a | 8.0 a | 6.7 d |
| 1,2-PL | 5.2 bc | 5.0 bc | 7.0 a | 5.0 bc | 5.6 b | 7.2 a | 3.4 f | 4.4 de | 4.2 e | 3.3 f | 5.2 bc | 4.4 de | 5.1 bc |
| 1,3-PO | 1.0 bc | 0.9 cd | 1.6 a | 1.0 bc | 0.7 f | 1.6 a | 0.5 g | 0.8 ef | 0.7 f | 0.4 g | 1.1 b | 0.4 g | 0.9 de |
| 1,2-OO | 31.0 de | 31.3 de | 22.4 f | 30.6 de | 31.3 de | 22.0 f | 42.4 a | 34.7 c | 38.3 b | 38.8 b | 34.7 c | 33.8 cd | 22.4 f |
| 1,2-OL | 18.9 d | 18.7 d | 23.7 b | 19.9 cd | 18.3 de | 28.4 a | 14.0 f | 15.8 ef | 16.1 ef | 17.8 de | 18.5 d | 18.3 de | 22.1 bc |
| 1,3-OO | 4.8 cd | 4.5 de | 7.1 a | 5.1 c | 3.9 e | 6.0 b | 2.2 g | 6.6 a | 2.9 f | 3.9 e | 3.9 e | 4.5 de | 6.0 b |
| 1,2-DAG | 62.6 ab | 62.6 ab | 58.8 b | 62.9 ab | 62.8 ab | 62.6 ab | 67.9 a | 62.8 ab | 66.6 a | 66.9 a | 67.2 a | 64.7 ab | 63.6 ab |
| 1,3-DAG | 5.8 de | 5.4 ef | 8.3 a | 5.8 de | 4.6 g | 7.5 b | 2.7 i | 7.6 b | 3.6 h | 3.5 h | 5.0 fg | 4.9 fg | 6.9 c |
| OSI (h) | 19.2 g | 19.3 g | 39.1 b | 31.9 d | 18.1 g | 28.0 e | 35.5 c | 25.4 d | 32.2 d | 45.2 a | 24.7 d | 22.1 f | 26.4 d |
- a) Same letters within each row do not significantly differ (n = 3, p<0.05). RSD of TAG, FA, DAG, and OSI are ≤8.0, ≤2.5, ≤4.5, and ≤3.5%, respectively.
The oxidative stability of the oils was assessed by OSI values, which are related to both TAG composition and antioxidant molecule contents 2. EVOo samples exhibited quite different OSI values. In particular, PuBaCo showed the highest oxidative stability (high OSI values) followed by ABTET, whereas other samples from Abruzzo (AbCSAI, AbCSAG, and AbCSALe) were less stable. The high OSI value (45.2 h) of Coratina (PuBaCo), which is in agreement with results of a previous study 37, might be related to the characteristic high phenol content of oils produced from this cultivar.
Cooling thermograms and thermal properties: Statistical correlations with chemical parameters
Figure 2 reports the cooling thermograms of EVOo samples from different cultivars of Abruzzo (Figure 2A) and Apulia regions (Figure 2B), as well as those of samples from Leccino variety belonging to both regions (Figure 2C). A quite similar phase transition profile was exhibited by all samples, having mainly two exothermic events; the major peaked at the lowest temperature and was previously attributed to the crystallization of highly unsaturated TAG (OOO, in particular), whereas the minor displayed its peak maximum at the highest temperature and was formerly associated to the crystallization of more saturated TAG fractions 18-20, 22, 25, 26, 38. However, some small differences were observed among samples from different cultivars and/or geographical provenience. The major peak was higher in all Apulian samples (Fig. 2B) than in Abruzzo oils (Fig. 2A). In addition, it is also be noticeable that, among samples from Leccino (Fig. 2C), AbCSALe showed a shift of the major peak toward lower temperature, exhibiting a shorter and broader profile as compared to the other Leccino samples.

Representative DSC cooling thermograms of EVOo samples obtained from Dritta, Tortiglione, Gentile, and Intosso in the Abruzzo region (A); Coratina, Ogliarola, Nociara, and Peranzana in the Apulia region (B); and from Leccino (C) in both Italian regions.
Table 2 reports the characterizing thermal properties (enthalpy, Ton, Toff, and transition range) of the cooling thermograms of all samples. In general, lipids in Abruzzo's oils required lower energy to crystallize than in Apulia's samples, except for sample AbLAD. However, net, significant differences among all oils from the two regions were not observed. This is in agreement with previous results, as these thermal properties were unable to differentiate monovarietal EVOo samples with dissimilar chemical composition 20, 26. Among samples from Leccino, AbCALe, and PuULe showed significantly different crystallization enthalpy with respect to the other three oils from the same cultivar; furthermore, they exhibited the highest values among all EVOos.
| Sample | ΔH (J/g) | Ton (°C) | Toff (°C) | Rangeb) (°C) |
|---|---|---|---|---|
| AbCSAI | 63.6 e | −10.4 ab | −50.5 f | 40.2 a |
| AbCSAG | 63.1 ef | −9.9 a | −50.2 f | 40.3 a |
| AbTET | 65.2 de | −12.0 d | −48.9 de | 36.9 c |
| AbLAD | 61.1 f | −10.4 ab | −48.5 de | 38.2 bc |
| AbCSALe | 63.8 e | −10.7 bc | −51.1 g | 40.4 a |
| AbCALe | 69.7 a | −11.4 c | −46.9 bc | 35.5 d |
| AbLALe | 63.5 e | −11.3 c | −49.9 f | 38.6 bc |
| PuALe | 65.9 de | −13.0 f | −45.3 b | 32.3 fg |
| PuULe | 68.2 ab | −13.4 f | −45.7 b | 32.3 fg |
| PuBaCo | 66.8 cd | −14.1 g | −44.3 a | 30.2 g |
| PuUOg | 66.3 cd | −12.5 d | −47.2 cd | 35.1 d |
| PuGCNo | 67.5 bc | −11.1 c | −50.2 f | 39.1 ab |
| PuSSePe | 65.7 de | −13.2 f | −46.0 bc | 33.4 ef |
- a) Data are expressed as mean of five determinations. Same letters within each column are not significantly different (n = 5, p<0.05). RSD ≤ 3%.
- b) Temperature difference between Ton and Toff.
Some differences were also noted among the other thermal properties (Ton, Toff, and transition range), even though the statistical differences were not able to clearly discriminate samples from the two regions. Crystallization in most Abruzzo oils occurred over a larger temperature range as compared to Apulia EVOos, leading to onset and offset shift toward higher and lower temperatures, respectively. On the contrary, mean differences for such thermal properties were found to be significant for samples of the same cultivar (Leccino) and different geographical provenience; in fact, Leccino oils from Abruzzo exhibited significantly higher Ton and larger range of transition as compared to Apulian samples of the same cultivar.
Statistical correlations were calculated among major (TAG and FA) and minor (DAG and lipid oxidation products) chemical components and thermal parameters to further characterize the nature of their relationships 20. Enthalpy of crystallization was not influenced by chemical composition of EVOo, as none of the chemical components considered in this study was correlated with this property. On the contrary, all the other thermal properties obtained from the cooling thermograms were statistically correlated with TAG, FA, and lipid oxidation products. In particular, OOO was highly correlated with both Ton (negatively, R = −0.746, p<0.01) and Toff (positively, R = 0.652, p<0.05) of crystallization, leading to a negative correlation (R = −0.724, p<0.01) with range of transition. A narrower range of crystallization was previously found at higher OOO content in EVOo samples from different varieties; it was suggested that a more uniform and ordered crystal structure of TAG (i.e., more compactly associated chains) could have lead to a more highly cooperative phase transition for these samples 20, 26. In addition, onset and offset temperatures of transition (and range of crystallization, as consequence) were largely influenced by other TAG in the present study. All these thermal properties were statistically correlated with LLP + OLnO (R = 0.827, p<0.01; R = −0.766, p<0.01; R = 0.829, p<0.01 with Ton, Toff, and range, respectively), OLP + OOPo (R = 0.791, p<0.01; R = −0.714, p<0.01; R = 0.781, p<0.01 with Ton, Toff, and range, respectively) and POPo (R = 0.571, p<0.05; R = −0.602, p<0.05; R = 0.636, p<0.05 with Ton, Toff, and range, respectively) contents, displaying an opposite trend as compared to OOO.
Main FA (such as palmitic, stearic, oleic, and linoleic acid), as well as FA grouped according to their unsaturation degree (SFA, MUFA, and PUFA), also exhibited high statistical correlation values with Ton, Toff, and range of transition. In particular, crystallization shifted toward higher temperature occurring in a larger range of temperature, when palmitic acid (R = 0.619, p<0.05 and R = 0.631, p<0.05 with Ton and range, respectively), stearic acid (R = 0.709, p<0.01 and R = 0.731, p<0.01 with Ton and range, respectively), and SFA (R = 0.886, p<0.01 and R = 0.836, p<0.01 with Ton and range, respectively) increased. Crystallization temperature onset was previously reported to raise with increasing FA saturation degree in EVOo from different drupe varieties 26. On the contrary, a shift of transition toward lower temperature and a narrower range of crystallization were highly statistically correlated with oleic acid (R = −0.890, p<0.01 and R = −0.846, p<0.01 with Ton and range, respectively) and MUFA contents (R = −0.880, p<0.01 and R = −0.938, p<0.01 with Ton and range, respectively). A lower Ton and a narrower range of transition were previously associated to high oleic EVOo monovarietal samples 18, 26. Crystallization also shifted toward lower temperature and occurred in a narrower range with increasing linoleic acid (R = −0.889, p<0.01 and R = −0.899, p<0.01 with Toff and range, respectively) and PUFA contents (R = −0.899, p<0.01 and R = −0.885, p<0.01 with Toff and range, respectively), which is in agreement with former studies on EVOo samples produced from different varieties 18, 20, 26.
Regarding the minor components, no correlations were found when cooling thermal properties were compared with free FA values and primary lipid oxidation products (POV). Total DAG, specific DAG contents, and the total 1,2-DAG and 1,3-DAG percentages were not correlated to crystallization thermal properties as well, even though a previous study suggested that these minor components might influence onset temperature and crystallization range of EVOo when present at higher levels than those found in the samples here analyzed 20, 24. On the contrary, the oxidative stability measured by OSI, which depends on major (TAG) and minor (phenols) compounds and is negatively influenced by the presence of the oxidation products, affected thermal properties. In fact, OSI time showed high negative (R = −0.711, p<0.01) and positive (R = 0.698, p<0.05) correlations with Ton and Toff, respectively, leading to an enlargement of the crystallization range (R = −0.736, p<0.01) as well. These results suggest that lipid oxidation products can hinder TAG crystallization, leading to the formation of mixed forms of crystals, as previously suggested 20, 25.
Deconvoluted cooling thermograms and thermal properties: Statistical correlations with chemical parameters
Deconvolution of the overlapping transitions of cooling thermograms were previously applied by authors to better describe the complex nature of the crystallization process 20 and to evaluate the potentiality of DSC for the discrimination among commercial categories of olive oil 22 and/or the recognition of EVOo adulterants 24. In this study, deconvolution was applied on cooling thermograms to add more information about the relationships among chemical components and crystallization thermal properties, being further investigated by the analysis of the statistical correlations among chemical composition parameters and thermal properties of the deconvoluted peaks. The discrimination among EVOo samples from different cultivar and/or geographical provenience on the basis of thermal properties of the deconvoluted transitions was, for the first time, explored.
All cooling thermograms were best fitted with three peaks (R2 ≥ 0.98), as previously reported for EVOo 20, 22, 24. Area percentages, Tp, Ton, Toff, and temperature transition range of each deconvoluted peak are summarized in Table 3. The peaks area numbered starting from the lowest to the highest temperature and named as peaks 1, 2, and 3. The predominant peak (peak 1) was an asymmetric double Gaussian function, with a quite symmetrical lineshape and a narrow profile, while peaks 2 and 3 were asymmetric double sigmoid functions and exhibited a more complex, asymmetrical line shape, probably indicating a more complex crystallization pattern, as previously shown 20, 22, 23.
| AbCSAI | AbCSAG | AbTET | AbLAD | AbCSALe | AbCALe | AbLALe | PuALe | PuULe | PuBaCo | PuUOg | PuGCNo | PuSSePe | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak 1 | |||||||||||||
| Area (%) | 63.1 gh | 64.6 fg | 58.7 h | 62.3 gh | 55.9 m | 65.8 e | 64.5 fg | 72.5 c | 76.8 b | 79.3 a | 70.5 d | 67.5 e | 72.4 c |
| Tp (°C) | −42.0 gh | −42.0 gh | −40.4 ef | −39.6 de | −42.9 h | −38.9 d | −41.0 fg | −37.7 b | −35.5 a | −36.6 a | −38.8 c | −39.8 ef | −36.5 a |
| Ton (°C) | −36.6 g | −36.5 g | −34.8 ef | −34.5 e | −37.8 h | −32.5 c | −35.7 fg | −31.6 ab | −31.4 a | −33.5 cd | −34.6 e | −35.2 ef | −31.1 a |
| Toff (°C) | −50.6 h | −49.8 gh | −48.7 ef | −48.1 de | −50.9 h | −46.5 c | −49.3 fg | −44.7 a | −44.9 ab | −44.4 ab | −46.8 c | −49.8 gh | −45.7 b |
| Rangeb) (°C) | 14.0 b | 13.3 d | 13.9 b | 12.6 de | 12.2 e | 14.2 b | 13.6 cd | 13.1 d | 13.2 d | 11.9 e | 12.7 de | 13.9 b | 14.7 a |
| Peak 2 | |||||||||||||
| Area (%) | 23.3 c | 24.2 c | 26.7 b | 25.6 b | 29.5 a | 23.1 c | 24.0 c | 13.7 f | 11.7 g | 11.8 g | 17.5 de | 18.3 d | 16.3 e |
| Tp (°C) | −36.4 e | −36.2 e | −35.0 de | −34.2 cd | −36.4 e | −34.0 cd | −35.7 e | −32.5 ab | −31.7 a | −33.1 ab | −33.6 bc | −33.1 ab | −32.1 a |
| Ton (°C) | −16.8 ab | −16.6 a | −17.5 bc | −16.3 a | −18.4 d | −17.5 bc | −17.5 bc | −21.4 f | −19.8 e | −21.3 f | −17.4 b | −18.2 cd | −20.3 e |
| Toff (°C) | −38.9 e | −39.2 e | −37.7 d | −37.0 cd | −41.9 f | −37.0 cd | −38.7 e | −35.8 b | −33.4 a | −35.0 b | −35.5 b | −36.7 c | −34.0 a |
| Rangeb) (°C) | 22.2 b | 22.6 ab | 20.2 cd | 20.7 c | 23.6 a | 19.6 d | 21.2 c | 14.4 f | 13.7 f | 13.8 f | 18.1 e | 18.5 e | 13.8 f |
| Peak 3 | |||||||||||||
| Area (%) | 13.5 ab | 11.2 cd | 14.5 a | 12.1 bc | 14.6 a | 11.0 cd | 11.4 cd | 13.8 a | 11.2 cd | 8.9 e | 12.0 bc | 14.3 a | 11.5 cd |
| Tp (°C) | −13.4 b | −12.5 ab | −14.6 c | −12.4 a | −13.4 b | −15.3 c | −15.0 c | −14.9 c | −16.3 d | −16.9 e | −15.3 c | −12.9 ab | −15.3 cd |
| Ton (°C) | −10.0 b | −9.3 a | −11.5 cd | −10.1 b | −10.1 b | −11.4 cd | −12.2 d | −12.8 de | −13.0 e | −13.2 e | −11.9 d | −11.1 c | −13.2 e |
| Toff (°C) | −23.4 a | −23.5 a | −23.3 a | −23.6 a | −24.9 b | −25.8 c | −24.8 b | −27.0 ef | −26.4 de | −26.5 de | −26.3 de | −26.8 de | −27.3 ef |
| Rangeb) (°C) | 14.0 b | 14.1 b | 11.8 e | 13.9 c | 14.8 a | 14.4 ab | 14.6 ab | 14.2 ab | 13.1 cd | 13.3 cd | 14.5 ab | 15.7 a | 14.1 b |
- a) Data are expressed as mean of five determinations. Same letters within each column are not significantly different (n = 5, p<0.05). RSD ≤ 3%.
- b) Temperature difference between Ton and Toff.
In general, EVOos from Abruzzo showed significantly lower area percentages, Tp and Toff for peak 1 than Apulian samples; PuGCNo oil displayed a different behavior with respect to the other Abruzzo's oils, though. Among Leccino samples, discrimination between the two Italian regions was more evident, as Apulian oils exhibited significantly higher values for all thermal properties, except for temperature ranges (Table 3). Significant differences were more evident for peak 2; in fact, Abruzzo samples showed significantly both lower Tp and Toff and higher area percentages, Ton (except for AbCSALe) and range of transition than Apulian samples. The same behavior was observed among Leccino EVOos from the two different regions (Table 3).
Significant differences among thermal properties of peak 3 were only detectable for Toff of this transition, as EVOos from Abruzzo significantly peaked at higher temperature than Apulian samples. Leccino EVOos exhibited the same behavior, having peak 3 with significantly higher Toff in Abruzzo oils as compared to Apulian ones.
Correlations found among chemical components and thermal properties of the three deconvoluted peaks are summarized in Table 4. High correlation coefficients were previously found between the percent peak area (from deconvolution analysis) of peaks 1 and 2 and percent TUTAG and MSTAG, respectively, even though few samples were considered 20. In the present study, statistical correlations were calculated on a larger set of EVOos, considering major (TAG and FA) and minor (DAG and FFA) components, OSI values, and thermal parameters of deconvoluted peaks in an attempt to clarify the nature of the relationships formerly observed 20, 22, 24.
| Deconvoluted peak 1 | Deconvoluted peak 2 | Deconvoluted peak 3 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area (%) | Tp (°C) | Ton (°C) | Toff (°C) | Rangea) (°C) | Area (%) | Tp (°C) | Ton (°C) | Toff (°C) | Rangea) (°C) | Area (%) | Tp (°C)] | Ton (°C) | Toff (°C) | Rangea) (°C) | |
| TAG (% of total TAG) | |||||||||||||||
| LLL + LLPo | −0.488 | −0.589* | −0.704** | −0.596* | −0.308 | 0.483 | −0.483 | 0.209 | −0.428 | 0.484 | 0.504 | 0.383 | 0.401 | 0.157 | 0.274 |
| OLL + OLPo | −0.487 | −0.581* | −0.585* | −0.560 * | −0.336 | 0.255 | −0.388 | 0.120 | −0.429 | 0.315 | 0.287 | 0.167 | 0.181 | 0.216 | −0.078 |
| LLP + OLnO | −0.893** | −0.830** | −0.811** | −0.716** | −0.293 | 0.891** | −0.812** | 0.692** | −0.877** | 0.875** | 0.540 | 0.750** | 0.795** | 0.711** | −0.004 |
| OLP + OOPo | −0.896** | −0.810** | −0.724** | −0.627** | −0.278 | 0.875** | −0.784** | 0.714** | −0.852** | 0.873** | 0.510 | 0.756** | 0.772** | 0.664* | 0.037 |
| POPo | −0.743** | −0.492 | −0.412 | −0.647 * | −0.168 | 0.689* | −0.561* | 0.388 | −0.715** | 0.628* | 0.639* | 0.589* | 0.598* | 0.349 | 0.259 |
| OOO | 0.825** | 0.707** | 0.641* | 0.631* | 0.296 | −0.778** | 0.627* | −0.660* | 0.762** | −0.793** | −0.668* | −0.783** | −0.757** | −0.594* | −0.112 |
| FA (%) | |||||||||||||||
| Palmitic acid | −0.783** | −0.501 | −0.421 | −0.365 | −0.143 | 0.766** | −0.570* | 0.599* | −0.680** | 0.715** | 0.552* | 0.631* | 0.624** | 0.570* | 0.091 |
| Stearic acid | −0.532 | −0.527 | −0.699* | −0.722** | −0.063 | 0.586* | −0.643* | 0.534 | −0.509 | 0.575* | 0.430 | 0.627* | 0.673** | 0.548 | 0.063 |
| Oleic acid | 0.951** | 0.967** | 0.931** | 0.855** | 0.266 | −0.920** | 0.927** | −0.703** | 0.967** | −0.938** | −0.705** | −0.807** | −0.860** | −0.657* | −0.152 |
| Linoleic acid | −0.827** | −0.923** | −0.951** | −0.866** | −0.291 | 0.805** | −0.891** | 0.572* | −0.905** | 0.833** | 0.598** | 0.676** | 0.739** | 0.575* | 0.112 |
| SFA | −0.661* | −0.639* | −0.717** | −0.672** | −0.171 | 0.938** | −0.811** | 0.792** | −0.864** | 0.923** | 0.679** | 0.850** | 0.873** | 0.711** | 0.100 |
| MUFA | 0.940** | 0.966** | 0.944** | 0.864** | 0.274 | −0.917** | 0.932** | −0.697** | 0.963** | −0.932** | −0.669** | −0.788** | −0.842** | −0.671** | −0.110 |
| PUFA | −0.823** | −0.923** | −0.954** | −0.865** | −0.296 | 0.802** | −0.890** | 0.500 | −0.905** | 0.831** | 0.588* | 0.667** | 0.730** | 0.575* | 0.102 |
| DAG (% of total DAG) | |||||||||||||||
| 1,2-PO | 0.166 | 0.131 | 0.075 | −0.032 | 0.190 | −0.311 | 0.161 | −0.320 | 0.134 | −0.239 | 0.382 | 0.168 | 0.100 | −0.565* | 0.776** |
| 1,2-PL | −0.485 | −0.419 | −0.298 | −0.281 | −0.053 | 0.625** | −0.399 | 0.627** | −0.383 | 0.647* | 0.062 | 0.288 | 0.315 | 0.639** | −0.562* |
| 1,3-PO | −0.383 | −0.313 | −0.172 | −0.154 | −0.037 | 0.465 | −0.304 | 0.594* | −0.271 | 0.436 | −0.067 | 0.182 | 0.197 | 0.636** | −0.602* |
| 1,2-OO | 0.586* | 0.573* | 0.502 | 0.292 | 0.450 | −0.663** | 0.571* | 0.665** | 0.578* | −0.663 ** | −0.196 | −0.298 | −0.357 | −0.736** | 0.551* |
| 1,2-OL | −0.338 | −0.298 | −0.208 | −0.192 | −0.036 | 0.432 | −0.310 | 0.591* | −0.252 | 0.433 | −0.141 | 0.142 | 0.173 | 0.684* | −0.671* |
| 1,2-DAG | 0.559 * | 0.568* | 0.423 | 0.326 | 0.239 | −0.562* | −0.418 | −0.493 | −0.398 | −0.562* | −0.273 | −0.317 | −0.334 | −0.592* | −0.504 |
| OSI | 0.709** | 0.708** | 0.643* | 0.614* | 0.135 | −0.620* | 0.662* | −0.468 | 0.710** | −0.682* | −0.770** | −0.674* | −0.342 | −0.256 | −0.516 |
- a) Temperature difference between Ton and Toff.
- * Significance at the 0.05 level (p<0.05).
- ** Significance at the 0.01 level (p<0.01).
Among TUTAG, increasing content of LLL + LLPo and OLL + OLPo resulted into a shift of peak 1 toward lower temperature, as highly negative correlation values were found (Table 4). On the contrary, increasing amounts of OOO appeared to shift peaks 1 and 2 toward higher temperature and peak 3 toward lower temperature, narrowing the crystallization range. OOO percentage was also highly statistically correlated with the peak 1 area. Crystallization of MSTAG, such as LLP + OLnO and OLP + OOPo, seems to influence thermal properties of all the deconvoluted peaks, following the opposite trend observed for OOO; in fact, peaks 1 and 2 shifted toward lower temperature and peak 3 toward higher temperature, increasing the areas of both transitions. POPo percentage also correlated with thermal properties of peaks 2 and 3 (Table 4). Area percentages of peak 3 was found to be correlated only with OOO (negatively) and POPo (positively). A high correlation between the range of crystallization and TAG was only noted in the deconvoluted peak 2. Correlation values among TUTAG percentages and areas of peak 1 (R = 0.618, p<0.05) and MSTAG and area of peak 2 (R = 0.683, p<0.05) were slightly lower than those reported in a earlier study 20. On the other hand, no correlations were found among TAG grouped according to saturation degree and the other thermal properties, thus better correlations were established between single or pair TAGs and thermal properties of the deconvoluted peaks. This may be related to the presence of other minor chemical components, such as DAG, FFA, and lipid oxidation products, which could have interfered with the crystallization process of TAG in a different manner according to chemical structure and distribution of FA moieties on glycerol skeleton, thus affecting the size of the deconvoluted peaks.
FA greatly correlated with thermal properties after deconvolution. In particular, oleic and linoleic acids, which are the most representative for EVOo composition, exhibited high correlation values with all thermal properties of the deconvoluted peaks, having a similar trend to those displayed by OOO and both LLP + OLnO and OLP + OOPo, respectively. Palmitic and stearic acids highly correlated with thermal properties of peak 3, shifting the onset of this transition toward higher temperature. Consequently, SFA, MUFA, and PUFA percentages were also highly correlated with all thermal properties of the deconvoluted peaks.
Among minor components, DAG appeared to influence most thermal properties of peaks 2 and 3, even though these minor compounds were not correlated with thermal properties of the whole cooling transition reported in Table 2. In particular, area percentage of peak 2 was negatively correlated with 1,2-OO, which was the most representative DAG in all samples (Table 1), and positively correlated with 1,2-PL; on the other hand, 1,2-PL, 1,3-PO, 1,2-OO, and 1,2-OL retarded Ton, of this transition. In addition, increasing amount of these DAG and lowering content of 1,2-OO shifted Toff of peak 3 toward lower temperature, influencing range of transition and, thus, the peak profile, as well. The total 1,2-DAG percentage, which is an indicator of oil freshness was also found to be statistically correlated to the aforementioned thermal properties, such as area and Tp of peak 1 (both positively correlated as 1,2-OO does), area and range of peak 2 (both negatively correlated) and Toff of peak 3 (negatively correlated). DAG were reported to influence TAG crystallization by either accelerating or delaying crystallization in palm, coconut oils, and other fats 39, 40. It was also suggested that their incorporation into the crystal lattice of TAG might have altered the orientation of molecules at the crystal surfaces in EVOo samples with higher DAG contents than those found in this study 20, 22. The other minor compounds here evaluated were also correlated with thermal properties of deconvoluted peaks. In fact, area of peak 1 was positively correlated with high OSI values. A shift of this transition toward higher temperature was also evident with increasing OSI values. A shift of the peak temperature of the main exothermic event of crystallization toward lower temperature was previously reported for EVOo samples with increasing level of lipid oxidation products 25, 41. On the other hand, area of both transitions at lower temperature were augmented by decreasing oil stability (negative correlation values), which also shifted peaks 2 and 3 toward lower and higher temperatures, respectively; this led to a change of their crystallization profiles. Free acidity was positively correlated only with Toff of peak 3 (R = 0.546, p<0.05) and negatively with crystallization range of the same peak (R = −0.740, p<0.01). On the contrary, no correlations were found among POV and thermal properties of the deconvoluted peaks. Therefore, specific DAG, and FFA, as well as the oxidative stability, seem to influence crystallization pattern and process kinetics of the two minor transitions peaking at the highest temperature, as previously suggested 20.
Conclusions
The results of this study showed that the application of the deconvolution analysis could give a significant input to the knowledge of the nature of the relations between chemical composition of the oils and cooling thermal properties, since all thermal properties obtained by peak deconvolution were correlated to both major and minor components.
The minor compounds DAG, FFA, and the oxidative stability had a great influence on the cooling profiles and thermal properties of the deconvoluted peaks, particularly on the two highest temperature transitions. These compounds can interfere with the crystallization of TAG groups, making the attribution of each transition to single chemical species difficult; however, the analysis of the correlations evinced that differences in TAG and FA compositions (i.e., OOO, OOL + OLPo, LLP + OLnO, oleic, linoleic, and palmitic acids) of the oils may result in dissimilar profiles and thermal properties of both crystallization and deconvoluted transitions. The influence of minor compounds on cooling thermal properties needs to be further investigated by analyzing oil samples with a wide range of minor compounds contents.
Thermal properties obtained after deconvolution of cooling transitions could be also very useful for the discrimination of oil samples from different geographical origin, regardless of the olive cultivar, as some of these properties were found to significantly differ among samples. This aspect is of great interest for the potential application of DSC to EVOos discrimination according to geographical origin, even though these results require further validation by multivariate statistical analysis of a larger set of oil samples from several Italian regions, as well as other Mediterranean countries.
Acknowledgements
The authors very gratefully acknowledge Prof. Elena Vittadini for her scientific support and Irene Bellini for the practical assistance in performing part of the experiments. Stefano Savioli and Mara Mandrioli (University of Bologna) were also acknowledged for their technical support during sample analysis.
The authors have declared no conflict of interest.




