Supplementation of tocotrienol-rich fraction increases interferon-gamma production in ovalbumin-immunized mice
Abstract
Palm oil is a rich source of vitamin E. The tocotrienol-rich fraction (TRF) extracted from palm oil contain 70% tocotrienols and 30% tocopherols. The effect of TRF supplementation on the immune modulation was evaluated in 6-wk-old female BALB/c mice immunized with ovalbumin (OVA) adjuvanted with alum. Mice in control and experimental groups were immunized subcutaneously (s.c.) on days 14 and 28 with a single dose of 50 µg OVA. The mice in the experimental group were orally gavaged daily with 1 mg of TRF from palm oil while those in the control group received carrier oil. The results show that mice in the experimental group produced significantly (p<0.05) higher levels of interferon-gamma (IFN-γ) compared to the control group. There was no significant (p>0.05) difference in the levels of interleukin-4 (IL-4) produced between the control and experimental animals. Lymphocyte proliferation in response to mitogen or OVA stimulation was significantly (p<0.05) higher in splenocytes derived from the TRF supplemented mice compared to control mice. These findings show that daily supplementation of palm TRF can induce a strong cell-mediated immune response, i.e., T-helper-1 (Th1) response, which would be beneficial to fight viral infections and cancer.
Abbreviations:
α-T, alpha tocopherol; CTL, cytotoxic T-lymphocytes; IFN-γ, interferon-gamma; IL-4, interleukin-4; IL-12, interleukin-12; NK, natural killer; OVA, ovalbumin; s.c., subcutaneous; Th, T-helper cells; TRF, tocotrienol-rich fraction.
Introduction
Vitamin E is a generic term that refers to a class of compounds that are divided into two subgroups called tocopherols and tocotrienols. Palm oil is a rich source of tocotrienols consisting mainly of alpha-, gamma-, and delta-tocotrienols. Tocotrienols act as important lipid-soluble antioxidants, with powerful anticancer, neuroprotective, and cholesterol lowering properties that are often not exhibited by tocopherols 1. Previously it has been shown that tocotrienols, but not tocopherols, inhibit growth of human breast cancer cells in vitro 2, 3. Several studies have also reported that α-tocotrienol, in contrast to α-tocopherol (α-T), prevents neurodegeneration by regulating specific mediators of cell death 1, 4, 5. Other experimental models have shown that tocotrienols can suppress the activity of a hepatic enzyme responsible for cholesterol synthesis 6.
Improvement of cell-mediated immunity by vitamin E has been extensively documented in animal studies 7-10. There are also some clinical studies showing that supplementation with tocopherols can enhance the immune system of elderly human subjects 11. However, the vitamin E used in these animal and clinical studies was a tocopherol, namely α-T. To date there are only few studies that use tocotrienols to improve immunity. A recent study had shown that comparable effects on immune modulation were observed following daily supplementation with tocotrienol-rich fraction (TRF) or α-T in normal human volunteers 12. In a rat model it was shown that tocopherol and tocotrienol supplementation could induce immunomodulatory effects in rats as well as stimulate the proliferation of spleen and mesenteric lymph node lymphocytes 13. However, in both the clinical and animal study, the authors did not challenge the immune system of the volunteers or experimental animals with any immunogens.
Interferon-gamma (IFN-γ) is the major cytokine involved in T-helper-1 (Th1) type, i.e., cell-mediated immune response while interleukin-4 (IL-4) is the major cytokine involved in Th2 type or humoral immune response 14. IFN-γ is reported to exert antitumor or antiviral effects by directly inhibiting replication or through the stimulation of innate immune effector cells like natural killer (NK) cells, macrophages, and neutrophils 15. Production of IFN-γ by NK cells and various T-cell populations is triggered either directly, through the recognition of infected cells, or indirectly, involving other cytokines such as interleukin-12 (IL-12). The production of IL-12 is considered crucial for subsequent IFN-γ synthesis and provides help for the activation of cytotoxic T-lymphocytes (CTL) as well as T-cell growth and proliferation 16, 17 as well as class switching of B-lymphocytes to IgG producing plasma cells 18. Determining the levels of IFN-γ and IL-4 are essential in investigating the immunoregulatory effects of dietary palm TRF on the development of humoral and cell-mediated immunity against diseases as the decision to differentiate toward either Th1 or Th2 phenotype has been shown to be dictated by exogenous cytokines present at the time of antigen exposure 15.
The present study has been set up to investigate the immunoregulatory effects of dietary tocotrienols on humoral and cell-mediated immune response by examining the cytokine productions in BALB/c mice that were immunized subcutaneously (s.c.) with ovalbumin (OVA) adjuvanted with alum.
Materials and methods
Materials
Plastic tissue culture dishes were purchased from Costar (Corning, USA); growth medium, FBS, and antibiotics were obtained from Gibco BRL (Paisley, UK). TRF was obtained from Golden Hope Plantations (Selangor, Malaysia). The TRF contains 70% tocotrienol (113 mg/g α-tocotrienol, 91 mg/g γ-tocotrienol, 36 mg/g δ-tocotrienol, 10 mg/g β-tocotrienol) and 30% tocopherol (78 mg/g α-T, 0.5 mg/g β-T) 12. Con A, OVA, and α-T was purchased from Sigma Chemical Company (Croydon, UK).
Mice
Six-wk-old BALB/c female mice were purchased from the Animal Breeding Unit, Institute of Medical Research (IMR), Kuala Lumpur, Malaysia. All animal experiments complied with the Guidelines for Care and Use of Laboratory Animals. This study was approved by the Animal Ethics Committee of IMR, Malaysia.
Study design
Twenty BALB/c mice were divided into two groups (control and experimental) of ten mice each. Experimental animals were orally gavaged with 50 µL (1 mg) of the prepared TRF emulsion (20 mg/mL) daily throughout the study while the controls were supplemented with corn oil (vehicle), which was the material used to make up the TRF supplement. Mice in both groups were immunized s.c. with a single dose of 100 µL (50 µg) OVA adjuvanted with alum on day 14. Five of the mice in each group were sacrificed on day 28 (V1) and their spleens were recovered for splenocytes culture. The remaining mice in both groups received a booster immunization s.c. with 100 µL (50 µg) OVA adjuvanted with alum on days 14 and 28. Following the booster vaccination, the remaining five mice in each group were sacrificed on day 42 (V2) to perform splenocyte cultures. Crystallized OVA (from chicken) was diluted in 0.9% saline and mixed with alum at 1:1 ratio to give a final concentration of 0.5 mg/mL. Prior to the vaccinations, mice were lightly anesthetized with diethyl ether. The summary of the immunization protocol is shown in Fig. 1.
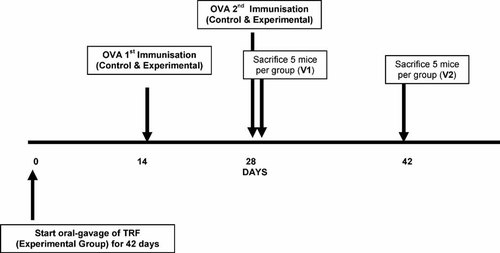
A schematic diagram showing the animal study design. The control and experimental groups contained ten mice each. The control and experimental mice were divided into two groups, i.e., V1 and V2 of five mice each. All mice received a single dose of 50 µg OVA subcutaneously (s.c.) on day 14. The mice in V2 of the control and experimental groups also received a booster dose of 50 µg OVA s.c. on day 28. Throughout the period of this study, mice in the experimental groups received daily supplementation of TRF until the mice were sacrificed.
Splenocyte proliferation assay
Five of the mice in control and experimental mice were sacrificed 2 wk after the first OVA immunization on day 28 (V1). The remaining five mice in each group were sacrificed after the second OVA immunization on day 42 (V2). The spleen of each mouse was removed aseptically into individual culture medium (complete RPMI-1640 containing 10% v/v FBS, 300 µg/mL L-glutamine, 100 IU/mL penicillin, and 100 µg/mL streptomycin). Single cell suspension was made by teasing out the splenocytes from the splenic capsule. Cells were counted using a hemocytometer. Splenocytes were seeded in quadruplicate at a density of 5 × 105 cells/well in flat-bottomed 96-well plates and cultured in the presence or absence of a mitogen (10 µg/mL Con A), the specific antigen (OVA at 25 or 50 µg/mL), or both in a humidified 5% CO2 incubator at 37°C for 72 h. After 72 h, cell proliferation was measured using a commercially available methyl thiazole tetrazolium (MTT) assay (Chemicon, USA) 19.
Quantification of cytokines by ELISA
After 72 h of incubation, supernatants from quadruplicate wells of the splenocytes culture were pooled, centrifuged to remove cell debris, and stored at −80°C. The supernatants will be thawed just prior to perform ELISA. Concentrations of IFN-γ or IL-4 in cell-free culture supernatants were determined using commercial mouse ELISA kits according to the manufacturers' recommended protocol (BD, PharMingen). Cytokine levels are expressed as pg/mL of protein. The detection limit of the each assay was 12 pg/mL.
Extraction of vitamin E from mice subcutaneous adipose tissue
Approximately 0.5 g of mice s.c. adipose tissue was placed into a 15 mL centrifuge tube and homogenized with a mixture of 4:1:1 of hexane, ethanol, and 0.9% sodium chloride at 10 000 rpm for 5 min or until the tissue was reduced to a liquid form using a tissue homogenizer. The homogenate was then centrifuged at 2000 rpm for 10 min. The lipid-containing supernatant phase was transferred to 5 mL vials and dried down under nitrogen gas. The obtained sample was resuspended just before use in an appropriate amount of hexane (500 µL–2 mL) for HPLC.
Extraction of vitamin E from mice blood plasma
Approximately 0.3 mL of plasma from mice was placed into a 15 mL centrifuge tube and homogenized with a mixture of 4:1:1 of hexane, ethanol, and 0.9% sodium chloride at 10 000 rpm for 5 min. The homogenate was then centrifuged at 2000 rpm for 10 min. The lipid-containing supernatant phase was transferred to 5 mL vials and dried down under nitrogen gas. The obtained sample was resuspended just before use in an appropriate amount of hexane (500 µL–2 mL) for HPLC.
HPLC analysis
Analytical HPLC was performed using the LC-10AT HPLC system which consisted a Shimadzu Model RF-10AXL fluorescence spectrophotometer, a column chamber, and Shimadzu Class VP data acquisition software. The HPLC column was a YMC A-012, 5 µm, 150 mm × 6 mm silica column. The excitation wavelength and emission wavelength of the fluorescence detector were set at 295 and 325 nm, respectively. The mobile phase was hexane-isopropyl alcohol (99.5/0.5 v/v) with a flow rate of 2 mL/min. Sample injection volume was set at 10 µL and a standard solution of mixture of α-T, α-, γ-, and δ-tocotrienols was also injected accordingly into the system. The peak areas of the components in the sample were compared with those of the standards and were used for quantitative calculation.
Statistical analysis
Statistical analysis was performed using the SPSS software. The data are shown as the mean ± SD. Vitamin E levels and cytokine concentrations from the TRF treated groups were compared with those of the untreated control mice by using the two-tailed Student's t-test for independent samples. Results were considered significant at p<0.05. Statistical comparisons for splenocyte proliferation stimulated in vitro with Con A and different concentrations of OVA (25 or 50 µg/mL) were performed using one-way ANOVA, followed by post hoc pair-wise comparisons with p<0.05 set as the level of acceptable statistically significant difference.
Results
High levels of vitamin E in the subcutaneous adipose tissues of TRF supplemented mice
Experimental mice fed with palm TRF showed significantly (p<0.05) higher levels of vitamin E accumulation in adipose tissues compared to the control animals (Fig. 2a). The vitamin E levels were higher in adipose tissues of experimental animals from the V2 group because these mice were orally gavaged with palm TRF for a longer period (42 days). The mice in the V1 group were only supplemented with TRF for 28 days. In contrast, supplementation of TRF did not produce any significant changes in plasma levels of total vitamin E (Fig. 2b) of both control and experimental animals.
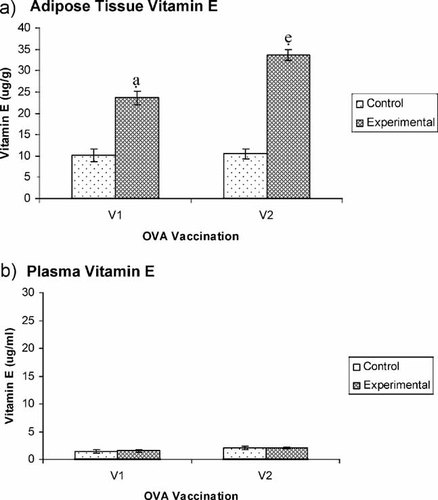
HPLC analysis of total vitamin E level in (a) subcutaneous adipose tissues and (b) plasma of BALB/c mice supplemented with 1 mg of TRF daily (experimental). Control mice did not receive any TRF supplementation. The mice in the V1 and V2 groups received a dose of 50 µg OVA s.c. on day 14. The mice in the V2 group received a booster dose of 50 µg OVA on day 28.
TRF supplementation enhances proliferation of splenocytes
The splenocytes from the TRF supplemented group had significantly (p<0.05) higher proliferation when cultured in the presence of Con A or Ova compared to control group (Fig. 3). Cell proliferation significantly increased after second OVA immunization in the TRF supplemented animals following stimulation with Con A or OVA compared to the control animals (data not shown).
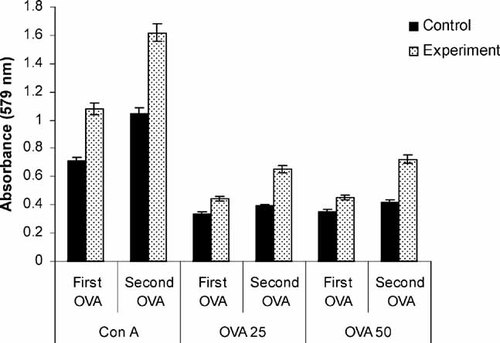
Proliferative responses of splenocytes from BALB/c mice after the first (First OVA) and second (Second OVA) immunization on days 14 and 28, respectively. Splenocytes were cultured for 72 h in a humidified 5% CO2 incubator at 37°C in the presence of 25 µg/mL (OVA 25) or 50 µg/mL (OVA 50) ovalbumin was quantified using a commercial MTT assay kit. Con A (10 µg/mL) was used as a positive control. Experimental animals were supplemented with 1 mg of TRF daily until they were sacrificed while the control mice did not receive any TRF supplementation.
Effects of TRF supplementation on production of IFN-γ and IL-4 production
To assess the effects of palm TRF supplementation on immune cell activity, we compared the levels of IFN-γ produced by Con A- or OVA-stimulated splenocytes from both control and experimental animals. Significantly (p<0.05) higher levels of IFN-γ can be detected in the supernatants from OVA-stimulated splenocytes from experimental compared to control mice (Fig. 4a). In addition, the amount of IFN-γ produced also increased significantly (p<0.05) after the second OVA immunization in the experimental mice (Fig. 4a).
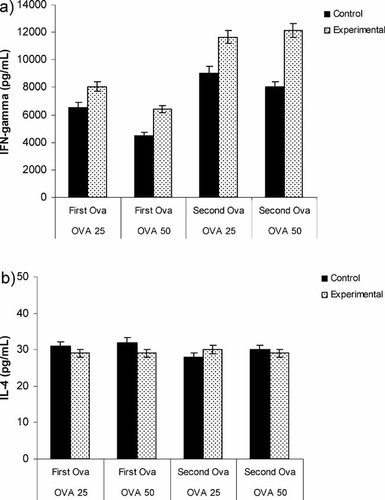
The amount of (a) IFN-γ and (b) IL-4 produced by splenocytes from BALB/c mice cultured for 72 h in a humidified 5% CO2 incubator at 37°C in the presence of 25 µg/mL (OVA 25) or 50 µg/mL (OVA 50) OVA after the first (First OVA) and second (Second OVA) OVA vaccination was quantified using ELISA. Experimental animals were supplemented with 1 mg of TRF daily until they were sacrificed while the control mice did not receive any TRF supplementation.
Supernatants from splenocytes stimulated with OVA (25 or 50 µg/mL) did not exhibit any significant (p>0.05) difference in the amount of IL-4 produced between experimental or control mice after 72 h of culture (Fig. 4b). There were also no detectable differences in IL-4 production between the first and second OVA vaccination in experimental or control animals.
Discussion
We have previously demonstrated that palm TRF is effective in inhibiting the growth of human breast cancer cells in nude mice 2, 3. The supplementation of TRF from palm oil might be involved in modulating the immune response as it was able to support the host in fighting tumor growth and spread in the nude mice. However, as nude mice have a deficient immune system, it was difficult to determine whether the protective effect observed was due to an effect on the host immune system or due to the inherent ability of the TRF to directly fight tumor growth and spread 3. In addition, we have previously shown that TRF and tocopherols have comparable immunomodulatory effects in healthy young human volunteers 12. A similar finding was also observed in the rat 13. So, these studies suggest that while the subjects are in good health, supplementation with either TRF or tocopherol does not appear to produce any significant immunomodulatory effects. However, the immune systems of the subjects of both these studies 12, 13 were not challenged with any form of immunogens. Thus in this study, we used an immunocompetent mice model where the mice were immunized with a specific antigen (OVA) and compared the changes in various immune parameters in the absence and presence of TRF supplementation.
Total vitamin E levels in plasma and adipose tissue from control and experimental animals were analyzed using HPLC. The concentration of vitamin E in the adipose tissue was analyzed because vitamin E is a fat-soluble compound and so it is usually stored in s.c. adipose tissue 5, 20. The total vitamin E levels in adipose tissues of BALB/c mice were significantly enhanced in experimental animals compared to unsupplemented control mice following both primary and secondary immunization. In contrast, there were no significant differences in the total vitamin E in the plasma between control and experimental mice. The results from the HPLC analysis of the fat adipose tissue would be a more reliable indicator of the availability of vitamin E than plasma levels over a relatively long time, because the former is not affected by rapid changes in the level of plasma lipoproteins or by acute changes in its intake 20.
Cell-mediated immunity plays an important role in resistance to infectious diseases. The lymphokine IFN-γ is thought to be a principal mediator of cell-mediated immune response, i.e., activation of CTL, NK cells, and macrophage activation and resistance to intracellular pathogens 21, 22. Being the signature cytokine for Th1 response, IFN-γ increases expression of MHC class II molecules on antigen-presenting cells and thus promotes presentation of antigens to the Th cells 14. Increased levels of IFN-γ not only activate macrophages but also CTL and NK cells, which can work together to enhance the functions of the immune system. IFN-γ, however, works inversely with IL-4, which promotes humoral responses by activating Th2 cytokine and B-lymphocytes 18.
Many experimental studies indicate that phytonutrients such as vitamin E and carotenoids are able to boost the immune system and enhance the response of T- and B-lymphocytes 14, 23. In our study we were able to demonstrate that daily supplementation of palm TRF can increase the levels of IFN-γ and proliferation of T-lymphocytes in the presence of a specific antigen, i.e., OVA. We show here that T-cell proliferation took place when the splenocytes from the OVA-immunized animals were cultured in the presence of either Con A or OVA. Strong T-cell proliferation is often influenced by cytokines in the microenvironment following the induction of Th1 stimulatory activity 24. These Th1 cells respond to IL-12 to increase production of IFN-γ. Stimulation of the primed T-lymphocytes with a mitogen-like Con A or a specific antigen (e.g., OVA) will induce the clonal expansion of these cells, which could subsequently result in increased IFN-γ production from these cultures. Daily supplementation of TRF enhanced the proliferative response of splenic cells to Con A (10 µg/mL) or OVA (25 or 50 µg/mL) in experimental compared to in control mice. The proliferative response was significantly enhanced after the second OVA immunization in Con A-stimulated cultures.
The enhancement of IFN-γ production is often associated with the stimulation of CD4+ T-lymphocytes of the Th1 subset 14. In this study, our findings revealed that the production of IFN-γ was higher in experimental than the control mice in both groups after the first and second OVA vaccinations. In experimental mice, TRF supplementation resulted in a significant increase of IFN-γ production, especially after the second OVA immunization. In contrast, there was no significant difference observed in the production of IL-4 between the control and experimental animals after the first or second OVA vaccination. Thus, it appears that the effects of TRF supplementation on the levels of IFN-γ and IL-4 produced are consistent with the induction of a Th1 type immune response.
The findings in this paper show that oral administration of TRF from palm oil stimulated a greater Th1 type immune response in mice following immunization with OVA. This response is observed as elevated levels of IFN-γ and increased T-cell proliferation in TRF supplemented mice compared to control mice, which were not given the TRF supplementation. These results demonstrate the usefulness of tocotrienols from palm oil for modulating an immune response in both the intensity (lymphocyte proliferation) of the response and the polarity (IFN-γ>IL-4) of such response toward a Th1 type upon immunogenic challenge. The induction of cytotoxic and Th1 cellular responses is it is highly desirable and essential for targeting either viral diseases or cancers 25, 26. From this study, we were able to demonstrate that supplementation of palm TRF can enhance the cellular immunity following an antigenic challenge. This suggests that TRF can act as a potent adjuvant to promote activation and clonal expansion of primed T-lymphocytes in the presence of specific antigen. It appears from our study that TRF supplementation promotes development of Th1 type of immune response as evidenced by the enhance production of IFN-γ, the signature cytokine for Th1 response in experimental animals.
Supplementation of palm TRF enhances Th1 immune response in mice following an immunogenic challenge. These findings could be useful in controlling infectious diseases. It may also open the door to successful therapeutic or preventive approaches, for example, against cancer, infectious, or autoimmune diseases. Thus, up-regulation of Th1 responses following TRF supplementation is a logical approach for immunotherapy against infectious diseases.
Acknowledgements
The authors would like to thank the Malaysian Palm Oil Board, Bangi, Malaysia for proving the grant to support this study.
The authors have declared no conflict of interest.




