Dermal IL-17-producing γδ T cells establish long-lived memory in the skin
See accompanying Commentary by Prinz and Sandrock.
See accompanying Commentary:
Abstract
Conventional αβ T cells have the ability to form a long-lasting resident memory T-cell (TRM) population in nonlymphoid tissues after encountering foreign antigen. Conversely, the concept of ‘innate memory’, where the ability of nonadaptive branches of the immune system to deliver a rapid, strengthened immune response upon reinfection or rechallenge, is just emerging. Using the αβ T-cell-independent Aldara psoriasis mouse model in combination with genetic fate-mapping and reporter systems, we identified a subset of γδ T cells in mice that is capable of establishing a long-lived memory population in the skin. IL-17A/F-producing Vγ4+Vδ4+ T cells populate and persist in the dermis for long periods of time after initial stimulation with Aldara. Experienced Vγ4+Vδ4+ cells show enhanced effector functions and mediate an exacerbated secondary inflammatory response. In addition to identifying a unique feature of γδ T cells during inflammation, our results have direct relevance to the human disease as this quasi-innate memory provides a mechanistic insight into relapses and chronification of psoriasis.
Introduction
Psoriasis is an immune-mediated skin disease, with a relapsing and remitting nature. It affects approximately 2–3% of the world's population 1, 2. Despite rarely being life-threatening, psoriasis has a negative effect on the life expectancy of the sufferers of approximately 3.5 years in males and 4.4 years in females 3, 4. Moreover, the high morbidity in psoriasis patients results in its burden on the economy being comparable to diabetes, cardiovascular diseases, and CNS illnesses 5, 6. Psoriasis is generally limited to inflammation of the skin, but as many as 30% of the patients also suffer from debilitating psoriatic arthritis, while the general population has a prevalence of around 1% 2. Psoriasis has many well-established comorbidities, in particular cardiovascular diseases 2, 7, Crohn's disease 1, 4, type II diabetes 3, 6, obesity 2, 5, metabolic syndrome 8, and lymphoma 9.
The exact mechanism of psoriasis pathogenesis is still poorly understood. Complicating matters further is the dynamic nature of psoriasis, which makes it likely that different cell types play different roles during initiation, progression, maintenance, and remission of the disease 2. Much of what we have learned about the pathogenesis of psoriasis stems from preclinical model systems 10. The Aldara model of psoriasis 11 has recently become increasingly popular 12. It is based on clinical observations that topical treatment with Aldara cream, for unrelated conditions, could trigger and/or cause relapses of psoriasis in patients 13-17. Moreover, it replicates the key features of the human disease and even the cytokine profile with abundant amounts of IL-17 and 18, 19 IL-22 19, 20. Accordingly, the production of IL-23 by DCs 18, 21 and efficacy of the antibody to IL-12/23p40 19 were also observed in Aldara-treated mice. In the Aldara model, the primary source of IL-17 and IL-22 was shown to be not TH17 cells but γδ T cells 18-20, 22. Moreover, mice deficient for γδ T cells developed a drastically reduced disease 18, 19.
The function and tissue tropism of γδ T-cell subsets is developmentally hard wired in the neonatal thymus. After functional rearrangement and expression of the γ- and δ-TCR chains, these cells become preprogrammed depending on the TCR signaling strength. Strong agonist signal drives γδ T cells into the IFN-γ-producing lineage, which can be defined by CD27 expression. In contrast, low TCR signaling leads to the development of IL-17-producing γδ T cells (Tγδ17) 23, 24. Although γδ T cells only make up 1–5% of all lymphocytes in secondary lymphoid tissues (SLTs) and blood, they are highly enriched in different epithelial tissues such as the reproductive tract, intestine, and skin. Therefore, they act as a first line of defense against pathogens including fungi, bacteria 25, viruses 26, and parasites 27 by providing a rapid cytokine response. The murine skin harbors different subsets of γδ T cells dependent on the skin layer. Within the epidermis, the vast majority of γδ T cells express the canonical Vγ5 and Vδ1 TCRs (TCRγδ high), whereas the dermis contains Vγ4+ and Vγ6+ T cells (TCRγδ intermediate) (Heilig and Tonegawa nomenclature is used) 28-31. A minor population of dermal Vγ4+ cells also expresses the Vδ4 chain 31, 32. Dermal Vγ4+ cells are strong producers of IL-17 cytokines (Tγδ17) and migrate from the skin to the skin draining lymph nodes (dLN) 31, 33. In addition, mouse studies have shown that Tγδ17 cells also play a pathogenic role in the development of autoimmune diseases such experimental autoimmune encephalomyelitis (EAE) 34, 35, inflammatory skin disease 19, and collagen-induced arthritis 37, 38. The contribution of γδ T cells in the context of relapses and chronicity in such diseases has not been addressed in great detail. Up to now, most attempts to study or harness antigen-specific ‘memory’ γδ T cells, by immunizing or infecting mice have not been very successful 39. Some studies have demonstrated that populations of γδ T cells respond to a reinfection with recall-like expansion 40-43. However, in those studies, the role of the responding γδ T-cell populations in the overall response is not easily addressed, and their contribution to protection is unclear or dispensable 43. Most recently, an imiquimod (IMQ)-induced skin inflammation model was used to describe memory-like γδ T cells in mice 44. They found that treatment with IMQ leads to an expansion of a population of Vγ4+Vδ4+ T cells, which also expresses IL-1R and spread to non-inflamed skin.
Using the same mouse model, we initially determined the fate and persistence of the γδ T cells, which caused the skin inflammation after primary Aldara challenge. Fate-mapping experiments show that the effector Vγ4+Vδ4+ T cells involved in the primary challenge survive in the skin for extended periods and exhibit clonality. Moreover, these cells confer stronger and more rapid responses to a secondary Aldara challenge. Finally, we show that these memory γδ T cells drive the memory response to Aldara and are able to spread to previously unchallenged distant areas of the skin where they provide TCR-dependent responses.
Results
Vγ4+Vδ4+ T cells accumulate in Aldara-induced inflamed skin and produce IL-17
RORγt+ dermal γδ T cells are responsible for mediating Aldara-induced inflammation in the mouse 19. The Vγ4+ subset of γδ T cells was significantly increased in the skin and produced high levels of pro-inflammatory cytokines. Given the diverse TCR specificities of γδ T cells in the skin, we further characterized infiltrating and resident γδ T-cell populations in the skin of Aldara-treated mice and compared them to naïve controls (Fig. 1A). As expected, a significant increase in total γδ T-cell cellularity was observed in challenged skin (Fig. 1B). We further analyzed the TCR specificities within the Vγ4+ population, and found that a population of Vγ4+Vδ4+ cells was preferentially expanded during Aldara challenge (Fig. 1C). In the inflamed skin, IL-17A production was restricted to dermal γδ T cells with no contribution from dendritic epidermal T cells (DETCs) (Fig. 1D). We investigated if these Vγ4+Vδ4+ cells express cytokines required for initiating psoriasiform plaque formation. In challenged skin, Vγ4+Vδ4+ cells produce high levels of IL-17A and IL-17F (Fig. 1E and F). Furthermore, we found increased CLA+ γδ T cells in the skin dLN of Aldara-treated mice, which mainly consisted of Vγ4+Vδ4+ (Supporting Information Fig. 1).
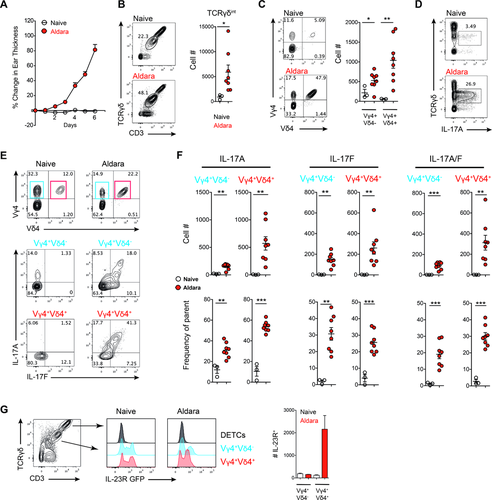
It has been shown by several studies that Aldara-induced psoriasiform plaque formation is mediated by cytokines such as IL-23 from DCs 21 and that γδ T cells constitutively express the IL-23 receptor (IL-23R) 45, 46. Hence, we used IL-23R-GFP reporter mice to further characterize IL-23R expression on different γδ T-cell subsets in the skin and found that Vγ4+Vδ4− as well as Vγ4+Vδ4+ cells constitutively express IL-23R at steady-state and challenged conditions. However, only Vγ4+Vδ4+ cells increased in numbers in this cytokine-driven skin disorder (Fig. 1G). Therefore, within the inflamed skin, γδ T cells with Vγ4+Vδ4+ TCR specificity expand and in response to IL-23 express high levels of pro-inflammatory cytokines required to drive skin inflammation.
Vγ4+Vδ4+ T cells are long-lived and persist in the skin long after inflammation subsided
Among the cytokines required to mediate skin inflammation is IL-17F. We have previously demonstrated that Il17f−/− mice show significantly reduced skin inflammation upon challenge with Aldara 19. Having observed the high expression of IL-17 cytokines in these cells (Fig. 1E and F) and the recently reported ability of γδ T cells to persist after infection 43, we investigated if IL-17-expressing Vγ4+Vδ4+ T cells remain in the skin after an initial inflammation. We crossed IL-17F-Cre mice to the ROSA-STOP-EYFP fate mapping strain to generate a mouse (IL-17FEYFP) that would irreversibly label IL-17F-expressing cells in the skin 70. In addition, the similar expression kinetics of IL-17A and IL-17F in γδ T cells makes these mice an ideal tool to study the fate of IL-17-expressing cells in vivo. Initial characterization of Aldara-treated mice revealed that EYFP+ cells were in fact positive for both IL-17A and IL-17F production, showing the fidelity of this reporter strain for γδ T cells (Supporting Information Fig. 2A and B). IL-17FEYFP mice were challenged with Aldara for 6 days, and left to recover for a period of 2 months (Fig. 2A).
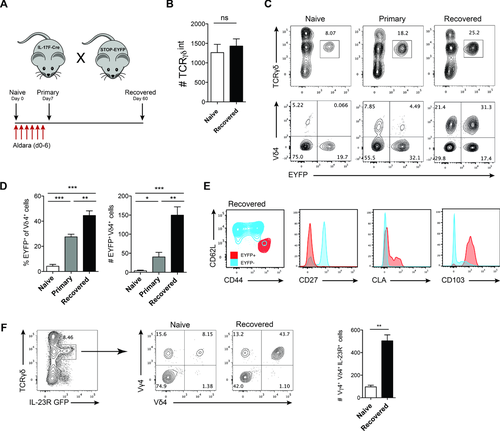
Sixty days after initial challenge we found the inflammation was macroscopically and microscopically completely resolved. Furthermore, upon quantification of total skin lymphocytes, we found no difference between untreated WT and Aldara-treated mice, which were left to recover for 60 days (Fig. 2B). While there was no overt change in the overall cellularity, we observed a strikingly higher proportion of EYFP+ cells, which mainly consisted of Vγ4+Vδ4+ cells (Fig. 2C and D). This contributed to a relative shift in the overall composition of different γδ T-cell subsets in favor of IL-17-expressing Vγ4+Vδ4+ cells after resolution of inflammation. Further characterization of isolated γδ T cells from the skin dLN revealed that EYFP+ cells phenotypically differ from EYFP− cells. EYFP+ cells were CD27− and additionally CD44hiCD62Llo, previously associated with ‘memory’ γδ T cells after infection with Listeria monocytogenes 43. Additionally, these cells expressed skin homing markers such as CD103 and CLA (Fig. 2E). Memory-like Vγ4+Vδ4+ cells also revealed enhanced capability of cytokine production in steady-state conditions compared to naïve mice (Supporting Information Fig. 3). To test if the overall numbers of IL-23-responsive cells from naïve and recovered mouse skin are changed, we again used the IL-23R-GFP reporter mice. When gating on IL-23R-expressing dermal γδ T cells, we observed a significant increase of Vγ4+Vδ4+IL-23R+ cell numbers in recovered mice, whereas the total number of skin γδ T cells was not significantly different between untreated and recovered mice (Fig. 2F). Thus, psoriasiform lesion formation results in a relative change of the dermal γδ T-cell pool toward clonally expanded, long-lived Vγ4+Vδ4+ T cells exhibiting enhanced effector capabilities and their persistence in the skin long after the initial inflammatory insult has been removed and skin returns to steady-state conditions.
Psoriasiform lesion formation is enhanced upon rechallenging murine skin with Aldara
Given the ability of Vγ4+Vδ4+ cells to produce pro-inflammatory cytokines and their relative increase in the recovered skin, we asked if a stronger inflammation would be elicited upon rechallenge with the initial inflammatory stimulus. Secondary challenge with Aldara after 60 days of recovery resulted in stronger, more robust inflammation, both clinically as assessed by skin swelling, as well as microscopically by histopathological analysis (Fig. 3A and B). The analysis of TCRγδ+ and CD4+ cells in the skin showed elevated numbers in primary and secondary challenge, but no significant differences for DETCs (TCRγδhi cells) (Fig. 3C). IL-17A/F co-expressing dermal Vγ4+Vδ4+ T cells were significantly increased in secondary challenge compared to primary challenge (Fig. 3D). Memory-like Vγ4+Vδ4+ T cells also showed strong proliferation in rechallenged compared to primary challenged mice (Supporting Information Fig. 4A). IL-17 cytokines are known to facilitate the recruitment of neutrophils to the site of inflammation. Consequently, we found elevated numbers of CD11b+ Ly6G+ neutrophils in the inflamed skin (Supporting Information Fig. 4B). Moreover, additional challenges with Aldara promote further expansion of Vγ4+Vδ4+ T cells, resulting in a dermal γδ T-cell compartment predominantly composed of Vγ4+Vδ4+ T cells (Supporting Information Fig. 5A, B and C). This is in accordance with conventional memory αβ T cell boosting following repeated challenges. Taken together, memory Vγ4+Vδ4+ T cells preferentially expand and produce high levels of IL-17A/F upon recall with Aldara, which leads to exacerbated secondary inflammation.
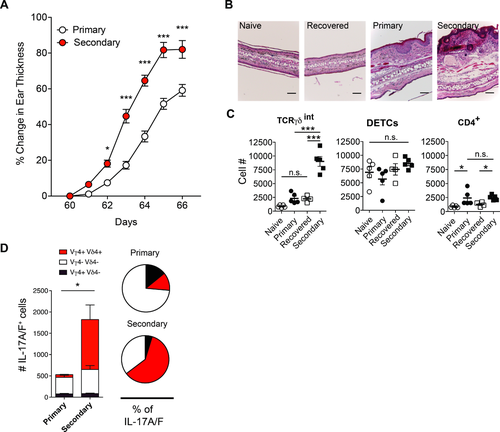
αβ T cells and skin stromal cells do not convey memory-driven skin inflammation
The conventional memory T-cell populations are characterized by at least three different subpopulations: central memory (CM), effector memory (EM), or resident memory (RM) T cells 47. Previous studies on CD8+ cells have shown that after an initial cutaneous challenge the TRM cells were able to populate the entire mouse skin and protect even previously uninfected sites more efficiently than TEM and TCM 48. Hence, it was plausible that in our model Vγ4+Vδ4+ T cells were able to populate the skin after the initial challenge with Aldara and form a TRM population. For this, IL-17FEYFP mice were treated only on the right ear and the composition of EYFP+ cells was compared to the left ear after the recovery phase. Surprisingly, the numbers of Vγ4+ EYFP+ cells in the previously treated (right) ear were equal to the unchallenged (left) ear among each individual animal (Fig. 4A). In addition, treatment of the ear skin alone resulted in a drastic increase of Vγ4+Vδ4+ EYFP+ cells in dorsal and ventral abdominal skin after prolonged recovery of 180 days (Fig. 4B). This effect was also present after repeated challenges with Aldara. We found that mice recovered from two challenges on the ear skin alone showed this expanded Vγ4+Vδ4+ T-cell subpopulation in distant skin areas (Supporting Information Fig. 5D and E). IL-7R expression was also selectively up-regulated in Vγ4+Vδ4+ T cells (Supporting Information Fig. 5F and G).
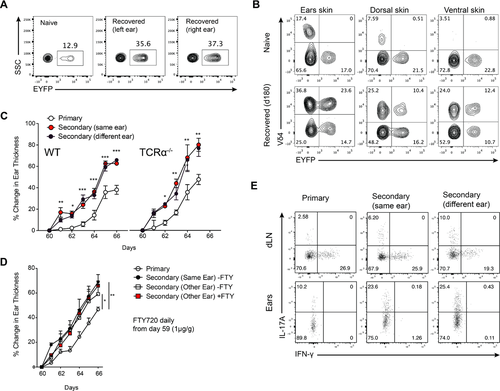
To further exclude that the phenomenon of the stronger secondary response is due to a scaring effect of the primary challenge, two groups of WT mice were treated on the right ear. One group received the rechallenge on the same ear and the other on the different (left) ear. Both groups of mice showed the same degree of rapid and enhanced inflammation, compared to mice freshly treated with Aldara (Fig. 4C). Studies have shown that αβ T cells can be important during Aldara skin inflammation under certain conditions 49, and that they can complement γδ T cells or vice versa during some immune responses 50, 51. We went on to exclude the contribution of αβ T cells to the memory response to Aldara. For this Tcra−/− mice were rechallenged with Aldara, and no difference was observed in the severity of inflammation between primary and secondary challenges, effectively ruling out that αβ T cells are involved in the memory response (Fig. 4C).
To further explore the origin of the memory response, we blocked the egress of lymphocytes from the SLTs by using FTY720 (fingolimod) 52. To test if FTY720 affects γδ T cells, we injected naïve WT mice with the compound daily for 6 days. Even a single injection resulted in the loss of αβ and γδ T cells in the blood circulation (data not shown). Therefore, we initially challenged two groups of mice with Aldara on one ear. Subsequently, both groups were rechallenged with Aldara on the different ear, with one of the groups receiving daily FTY720 starting on day 59. There was no significant difference between rechallenged groups of mice, suggesting that circulating γδ T cells from the SLTs are dispensable for the memory response (Fig. 4D).
Lastly, the quality of the accelerated γδ T-cell response upon secondary challenge is similar in terms of cytokines produced, regardless of the site of initial challenge (Fig. 4E). Interestingly, unlike previous report on intestinal memory Vγ6+ γδ T cells 43 we failed to observe co-production of IFN-γ and IL-17 by the memory γδ cells in the skin (Fig. 4E). Combined with the prolonged persistence of Vγ4+Vδ4+ in the skin, these γδ T cells generated in response to primary Aldara inflammation show hallmark characteristics of γδ TRM cells.
Persistent Vγ4+Vδ4+ T cells mediate severity of secondary challenge through the γδ TCR
The selective expansion of a specific population of Vγ4+ cells pointed to a possible role of the Vγ4Vδ4 TCR for the memory response. To address this during the responses to Aldara, we utilized an anti-Vγ4 monoclonal antibody (mAb). Some reports have shown that anti-TCRγδ antibodies cause internalization of the TCR complex without depletion of the cells, thus creating ‘stealth’ γδ T cells without functional γδ TCR on their surfaces 43, 53. We also addressed, whether the anti-Vγ4 mAb causes internalization of the TCRγδ complex or depletion of Vγ4+ cells. Given that internalization removes the required markers for identification of γδ T cells, we used the IL-17FEYFP fate-mapping strain, where EYFP+ cells are entirely restricted to dermal γδ T cells. Anti-Vγ4 treatment resulted in the loss of TCRγδ, CD3 and Vγ4 staining, yet the overall percentage of EYFP+ cells remained unchanged, suggesting that TCRγδ complex is internalized in Vγ4+ cells and thus generated ‘invisible’ γδ T cells (Supporting Information Fig. 6). The administration of the anti-Vγ4 mAb during Aldara treatment resulted in the loss of exacerbated disease and nearly identical degrees of inflammation in re-challenged and freshly challenged mice. Both of these were significantly lower than the isotype-treated freshly challenged mice and therefore very significantly lower than isotype-treated rechallenged mice (Fig. 5A). In line with this, we did not detect IL-17A expression of anti-Vγ4-treated mice (Supporting Information Fig. 7).

Another helpful tool to address TCR signaling in T cells are mice expressing GFP from the early gene Nr4a1 (Nur77) locus 54. Nur77-GFP expression represents an activated T cell, predominantly due to TCR stimulation. In these mice GFP is most highly up-regulated upon TCR engagement reflecting the strength of TCR stimulation and less by pro-inflammatory cytokines 55, 44. Recent studies also used this mouse strain to investigate γδ T-cell responsiveness during the development 55. We sought to determine the GFP expression of dermal γδ T-cell populations upon primary and secondary challenge with Aldara after 5 days of treatment. γδ T cells from naïve animals did not show GFP expression. Challenged mice on the other hand up-regulated GFP only in the memory Vγ4+Vδ4+ but not in Vγ4+Vδ4− cells (Fig. 5B and C). Our data underline the importance of Vγ4+ T cells in both primary and secondary responses to Aldara and also indicate potential TCR stimulation of Vγ4+Vδ4+ cells. Taken together, we show that in a γδ T-cell-dependent setting, these cells themselves can mount a memory response characterized by expansion of long-lived Vγ4+Vδ4+ cytokine-producing T cells in response to an inflammatory stimulus.
Discussion
The Aldara model for psoriasis fulfills a number of important criteria for a good preclinical model 11, 12. Apart from the histopathology, which is strikingly reminiscent of human psoriasis vulgaris, the response to clinical therapeutic interventions of human psoriasis and the Aldara model significantly overlap 19. Two aspects of psoriasis not modeled by treatment with Aldara are the formation of relapses and the chronification of skin inflammation observed in psoriasis. Relapses of inflammation in chronic inflammatory disease are generally driven by Ag-experienced memory lymphocytes. Immunologists generally refer to memory cells only as Ag-experienced and persistent when talking about αβ T or B cells. The notion that innate immune cells can also form memory has only recently been introduced and is a matter of some debate 56. Our data demonstrate that in the Aldara model of psoriasis, innate Vγ4+Vδ4+ T cells are able to form skin-RM cells. These tissue-RM γδ T cells rapidly produce IL-17 cytokines and are able to mount an accelerated and exacerbated secondary response to Aldara even at previously unaffected/untreated sites.
The concept of quasi-innate memory has recently gained further momentum when γδ T cells were proposed to play an important role upon reinfection with L. monocytogenes. In that study, memory Vγ6+ T cells expand and persist in the intestine after initial challenge with Listeria and promote enhanced protection in a pathogen-specific manner 43. Also, using the same pathogen, Ryan-Payseur and colleagues previously demonstrated in rhesus macaques multifunctional effector Vγ2+Vδ2+ T cells, which accumulate in the intestine and undergo recall-like expansion 42. In our study, we add new evidence in the field of immunological memory by the innate immune system within the skin niche.
Dermal γδ T cells develop from the neonatal thymus and undergo homeostatic proliferation within peripheral tissues in adult animals without or with very little input from circulation, thymus, or bone marrow 29, 30. The development and survival of these cells is stringently dependent on IL-7 signaling 30. Furthermore, IL-7 together with TCR stimulation have been shown to trigger Vγ4+ T-cell proliferation 57. Here, we found that Ag-experienced Vγ4+Vδ4+ cells exhibit increased levels of the IL-7R expression, which could favor their maintenance within the skin. Even though IMQ is unstable and has a rapid pharmacokinetic, it is possible that other constituents of Aldara persist longer to stimulate γδ T cells and facilitate their survival.
Aldara-induced activation of dermal γδ T cells involves IL-23 and IL-1 induction 18, 29. Secondary exposure to Aldara resulted in an exacerbated skin inflammation, despite total numbers of IL-23-responsive cells not being altered in the recovered compared to naïve skin (data not shown). However, we found a relative increase of Vγ4+Vδ4+IL23R+ cells. The ability of memory Vγ4+Vδ4+ cells to produce IL-17 cytokines was highly enhanced compared to other dermal γδ T cells and to nonexperienced Vγ4+Vδ4+ cells. A possible explanation could arise from studies showing that neither TCR signaling nor cytokine stimulation alone mount and sustain a robust IL-17 response, indicating that cytokine or TCR signaling acts as second signal 58-63. Additionally, TCR signaling together with IL-7 stimulation enhanced functional responsiveness of γδ T cells 57. Finally, it is possible that activated γδ T cells undergo epigenetic changes, leading to enhanced effector functions upon rechallenge.
The expansion of a specific γδ T-cell population, namely Vγ4+Vδ4+ cells in primary and to an even greater extent in the secondary challenge with Aldara, pointed to the importance of the TCR specificity, which was underlined by the treatment with the anti-Vγ4 mAb. We also show TCR activation in Nur77-GFP mice only in dermal Vγ4+Vδ4+ cells, but not in Vγ4+Vδ4− cells. Interestingly, a recent study showed that in vitro stimulation with IL-1β and IL-23 could also induce a minimal GFP expression in Vγ4+ cells 44. However, given the current in vivo knowledge and our data (Fig. 1G), Vγ4+Vδ4− as well as Vγ4+Vδ4+ cells express quite similar levels of the IL-23R and both can produce IL-17 cytokines after Aldara treatment. Thus, their responsiveness to IL-23 and/or IL-1 stimulation should be quite similar. Hence, tight GFP-expression only present within the Vγ4+Vδ4+ population of Nur77-GFP mice indicates specific TCR engagement in these cells.
Nevertheless, some obvious questions arise: What antigen is recognized by memory γδ T cells and in what context is this antigen presented? One of the first identified ligands for γδ TCR was the nonclassical MHC Ib protein T22 64. More recently γδ TCRs have been shown to recognize a bacterial protein phycoerythrin (PE) 58, as well as lipid antigens in the context of CD1d 65, 66. It was also shown that isostearic acid, a component of Aldara cream, can cause inflammasome activation, which is necessary for the full inflammatory response to Aldara without the TLR7-dependent mechanism of IMQ 67. However, upon recovery it seems likely that the memory formation is aided by the up-regulation of an endogenous antigen as very similar expansion of Vγ4+Vδ4+ γδ T cells in the skin draining lymph nodes was caused by mycobacteria from complete Freud's adjuvant (CFA) 38.
Together with our previous findings that showed proliferation of Vγ4+ T cells both in the skin and in the draining lymph nodes 19, we propose that the initial treatment with Aldara causes a clonal expansion of Vγ4+Vδ4+ T cells. These activated cells are then able to seed throughout the skin due to their up-regulation of skin homing molecules, such as CLA and CD103. This migratory potential is underlined by recent reports, where Vγ4+ T cells are able to migrate from the skin to the skin dLN and vice versa after cutaneous challenge 31, 33. All these findings point toward a mechanism in which DCs recognize IMQ through TLR7 and produce IL-23, while keratinocytes produce IL-1 in response to isostearic acid in Aldara. Together with up-regulation of an endogenous stress ligand, these cytokines selectively activate dermal resident Vγ4+Vδ4+ γδ T cells. This activation leads to local proliferation of the Vγ4+Vδ4+ cells and production of pro-inflammatory IL-17 and IL-22, as well as migration of a fraction of these cells to the dLN where they undergo further proliferation. Following this, the ‘antigen’ experienced CD103 and CLA expressing Vγ4+Vδ4+ T cells enter circulation and seed the parts of the skin, which were not affected by Aldara, where they can provide a more rapid and robust inflammatory response upon rechallenge with Aldara. As most infections result in cell stress and death, this seems like an efficient mechanism for an enhanced early memory response to wide range of pathogens. However, many details of this pathway still need to be elucidated such as the nature and identity of the γδ TCR ligand, as well as the fine-tuning of the response to prevent undesirable overshooting inflammation upon every skin perturbation.
The development of psoriasis in humans depends on genetic as well as environmental factors with TH1 and TH17 cells thought to be the main producers of proinflammatory IFN-γ and IL-17 cytokines that drive the chronification of the disease. A recent report also suggested Vγ9+Vδ2+ T cells as potential players in the pathogenesis of psoriasis. According to their findings, these cells are rapidly recruited into perturbed skin and persist in lesional as well as in nonlesional skin of psoriasis patients 36. Together with case reports of relapses of psoriasis patients upon Aldara treatment and our data, we hypothesise that human γδ T cells could be involved in relapses and disease progression 13, 17, 68. Moreover, it could explain interesting features of the relapses of psoriasis such as Koebner phenomenon, the appearance of psoriatic lesions in the uninvolved skin of psoriatic patients as a consequence of trauma 69. Furthermore, these findings could also provide foundations for future immunization strategies, which would target skin protection against common pathogens.
In summary, our findings provide a conceptual advance for the understanding of γδ T-cell biology, not only in the context of skin inflammation and psoriasis, but also for the general understanding of cutaneous immune responses.
Materials and methods
Mice
C57BL/6 (WT) mice were obtained from Janvier. Tcra−/−, Nur77-GFP mice were purchased from The Jackson Laboratory. IL-23R-GFP mice were generously provided by M. Oukka. IL-17FCre mice were bred to ROSA26-STOP-EYFP mice.
Treatments
5 mg of Aldara (5% IMQ cream; 3M Pharmaceuticals) was applied on each mouse ear with the duration described in the text. For the back treatment the mouse back was shaved, and a daily dose of 50 mg of Aldara or control cream was applied.
Anti-Vγ4 treatment was performed by intraperitoneal injection (i.p.) of the mice with 200 μg/mouse hamster anti-mouse mAb (UC3-10A6) and respective hamster IgG isotype control antibody (BE0091, both Bio X Cell).
For FTY720 treatment, mice were injected (i.p.) with 1 mg/kg of FTY720 (AdipoGen) dissolved in water each day.
Cell preparation
Ears were cut into small pieces and digested with 1 mg/mL collagenase type IA and 100 μg/mL DNase (both Sigma) for 60 minutes at 37°C and filtered through 70 μm cell strainers. Leukocytes were harvested from cervical LNs followed by filtering through 70 μm cell strainers to obtain single-cell suspensions.
Histology
Samples were fixed using standard HOPE fixation protocol and stained with H&E.
Flow cytometry
We have used the Heilig and Tonegawa nomenclature for the murine γ and δ genes. The following monoclonal antibodies were used either from BD, eBioscience or BioLegend: anti-CD3-AF700 (17A2), anti-CD4-BV650 (RM4-5), anti-CD11b-allophycocyanin-Cy7 (M1/70), anti-CD27-allophycocyanin (LG.3A10), anti-CD44-allophycocyanin (IM7), anti-CD45-PacificBlue (30-F11), anti-CD62L-PE-Cy7 (MEL-14), anti-CD103-FITC (‘2E7), anti-CD127-PE-Cy7 (SB/199), anti-CLA-PacificBlue (HECA-452), anti-TCRγδ (GL3), anti-Vγ4 (UC3-10A6), anti-Vγ5 (536), anti-Vδ4-PE (GL2), anti-IL-17A-eFlour660 (17B7), anti-IL-17F-PECF594 (O79-289), anti-IL-22-PE, anti-IFN-γ-PE-Cy7 (XMG1.2). Cells were incubated with antibodies for 20 min at 4°C. For intracellular cytokine staining, cells were stimulated with PMA (Applichem) and ionomycin (Invitrogen) and treated with GolgiStop (BD) for 2 h. After surface staining, cells were permeabilized according to the manufacturer's (BD) recommendations and stained intracellularly. Stainings were analyzed with an FACS LSRII Fortessa (BD). Post-acquisition analysis was performed with FlowJo (Tree Star) software.
Statistics
For disease severity, differences between groups were evaluated by 2-way ANOVA with Bonferroni's post hoc test. For analysis of scatter plots of maximum thickness comparing ≥3 groups of mice, 1-way ANOVA with Bonferroni's post-test was used. Differences between two sets of data were evaluated by 2-tailed Student's t-test. Data represent mean ± SEM. P ≤ 0.05 was considered statistically significant. All statistics were done using GraphPad Prism (GraphPad Software).
Study approval
All animal experiments were approved by the Swiss Cantonal Veterinary Office (33/2010 and 68/2013; Zurich, Switzerland).
Acknowledgments
The work was supported by grants from the Swiss National Science Foundation (316030_150768 (BB), 310030_146130 (BB) and CRSII3_136203 (BB), European Union FP7 project TargetBraIn, NeuroKine and ATECT (BB), and the University priority project translational cancer research (BB). The authors would like to thank Florian Mair and Kathrin Nussbaum for manuscript preparation and discussion as well as Sabrina Nemetz Hasler, Jennifer Jaberg, and Jan Candreia for technical assistance.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
Abbreviations
-
- DETC
-
- dentritic epidermal T cell
-
- IL-23R
-
- IL-23 receptor
-
- IMQ
-
- imiquimod
-
- RM
-
- resident memory
-
- SLT
-
- secondary lymphoid tissue