Hematopoietic and nonhematopoietic cells promote Type I interferon- and TLR7-dependent monocytosis during low-dose LCMV infection
Abstract
Release of inflammatory monocytes from the bone marrow (BM) into the blood is an important physiological response to infection, but the mechanisms regulating this phenomenon during viral infection are not completely defined. Here, we show that low-dose infection with lymphocytic choriomeningitis virus (LCMV) caused rapid, transient inflammatory monocytosis that required type I interferon (IFN) and Toll-like receptor (TLR) 7 signaling. Type I IFN and TLR7 signals were critical for induction of IFN-stimulated gene expression and CCR2 ligand upregulation in the BM microenvironment in response to LCMV infection. Experiments utilizing BM chimeric mice demonstrated that type I IFN and TLR7 signaling on either hematopoietic or nonhematopoietic cells was sufficient to initiate monocytosis in response to LCMV infection. BM plasmacytoid dendritic cells (pDCs) generated type I IFN directly ex vivo, suggesting that pDCs are a hematopoietic contributor of type I IFN in the BM early during LCMV infection. Overall, we describe novel roles for type I IFN and TLR7 signaling in nonhematopoietic cells and BM pDCs in directing IFN-stimulated gene and CCR2 ligand expression in the BM to initiate an increase in blood inflammatory monocytes during viral infection.
Introduction
The bone marrow (BM) acts as a reservoir of inflammatory monocytes, which can be released into peripheral circulation in response to microbial challenge 1. Inflammatory monocytes express high levels of the chemokine receptor CCR2, which binds to the chemokines CCL2, 7, and 12. Inflammatory monocyte egress from the BM into the blood in the steady state and in response to inflammation requires interactions between CCR2 and CCL2 or 7, whereas the role of CCL12 in this process is still unclear 2-4. CCR2 ligands can be induced in response to either Toll-like receptor (TLR) stimulation or type I interferon (IFN) signaling or both during infection with bacterial, fungal, and viral pathogens, but how these signals are integrated in the BM niche and what effects these have in shaping the innate response to virus infection is still not completely defined 3-7. Recent work has suggested that viral infection can drive monocytosis that is critical for viral clearance and survival 8, 9. However, the mechanisms by which viral infection drives BM egress of inflammatory monocytes have not been fully elucidated.
Cells of hematopoietic and nonhematopoietic origin populate the BM microenvironment. The contribution of cells from both lineages in mounting an effective inflammatory response is an area of active study. Recent work has indicated that both nonhematopoietic mesenchymal stem cells (MSCs) and CXCL12-abundant reticular (CAR) cells and hematopoietic cells in the BM are capable of producing of Ccl2, and inducing monocytosis in response to low, systemic doses of LPS, and infection with the bacterium Listeria monocytogenes 4. Other work has shown that infection with murine CMV, a virus with a dsDNA genome, induces CCR2 ligands in the BM primarily from F4/80+ hematopoietic cells in a manner that depends largely on type I IFN 6. What inflammatory factors, innate receptors, and specific cell types determine the response of the BM microenvironment to infection with an ssRNA virus is unknown.
Plasmacytoid dendritic cells (pDCs) are sentinel cells of the innate immune system that express high levels of TLR7 and 9 and are capable of rapidly secreting IFN-α. pDCs are unique among other DC subsets in that they fully mature and reside in appreciable numbers in the BM 10. BM pDCs have been found in mice, primates, and humans, suggesting an evolutionary conserved role for these cells in the BM microenvironment 11-13. BM pDCs may represent a source of rapid, local type I IFN that uniquely influences early innate immune responses originating in the BM, such as the release of inflammatory monocytes into the circulation. Here, we investigated the mechanisms and cell types involved in modulating BM gene expression changes that promote monocytosis during infection with the Arenavirus lymphocytic choriomeningitis virus (LCMV) strain Armstrong, and revealed novel roles for type I IFN, TLR7, nonhematopoietic cells, and BM pDCs in this process.
Results
Low-dose LCMV infection promotes monocytosis at 48 h postinfection
To interrogate whether RNA virus infection drives inflammatory monocytosis as seen in DNA virus and bacterial infections 3, 4, 8, 9, we first infected mice with 1 × 102, 1 × 104, and 2 × 105 plaque-forming units (PFU) of LCMV strain Armstrong and examined the number of leukocytes in the blood early after infection. Surprisingly, we found that doses higher than 1 × 102 PFU promoted dose-dependent panleukopenia beginning as early as 24 h after infection, with significant decreases in CD45.2+ leukocytes, neutrophils, resident monocytes, B cells, and T cells (Fig. 1A and Supporting Information Fig. 1A). To address whether high-dose infection caused monocytosis earlier than 24 h, we infected mice with 2 × 105 PFU of LCMV and assessed blood monocyte numbers at 3, 6, and 12 h after infection. High-dose infection did not differ from PBS injection in the first 24 h (data not shown), demonstrating we did not miss monocytosis that occurred with faster kinetics during high-dose LCMV infection.

As infection with 1 × 102 PFU did not cause leukopenia, we focused on this dose to investigate whether LCMV promotes monocytosis. Indeed, we observed a significant increase in the frequency and absolute number of inflammatory monocytes in the blood by 48 h postinfection with 1 × 102 PFU LCMV (Fig. 1A–C and Supporting Information Fig. 1B). The number of blood inflammatory monocytes peaked at 48 h postinfection and then began to wane by 72 h postinfection (Fig. 1D).
Low-dose LCMV monocytosis requires type I IFN receptor and TLR7 signaling
LCMV infection is characterized by a rapid burst of type I IFN followed by a later surge in IFN-γ 14. IFN-γ has been shown to cause de novo inflammatory monocyte production from BM myeloid precursor cells, which results in inflammatory monocytosis at day 8 postinfection with a high dose (1 × 105 PFU) of LCMV 15. However, in our system Ifng mRNA was not significantly upregulated in the BM at 36 or 48 h postinfection with 1 × 102 PFU LCMV (Supporting Information Fig. 2).
Therefore, we focused on whether type I IFN can modulate blood inflammatory monocyte homeostasis early in LCMV infection and with a low dose of virus. To this end, we inoculated WT and IFN receptor alpha deficient (IFNAR−/−) mice, which lacked cell surface expression of a functional type I IFN receptor, with 1 × 102 PFU LCMV and examined the number and frequency of inflammatory monocytes in the blood. At 48 h postinfection, there was a significant increase in the frequency and absolute number of inflammatory monocytes in the blood in WT mice, whereas there was no significant change in blood inflammatory monocyte frequency or absolute numbers in IFNAR−/− mice (Fig. 2A).
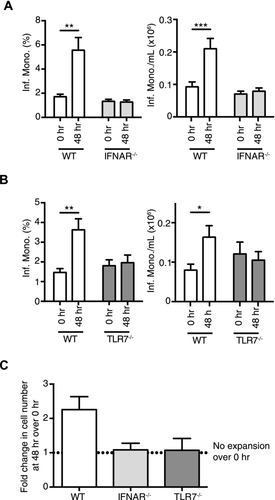
Detection of LCMV ssRNA genome by the innate immune system for type I IFN production can involve the endosomal sensor TLR7 or the cytoplasmic sensor RIG-I 16-18. We investigated whether TLR7 was important for the type I IFN-dependent monocytosis, we observed by infecting WT and TLR7-deficient (TLR7−/−) mice with 1 × 102 PFU LCMV. TLR7−/− mice did not display any signs of monocytosis at 48 h postinfection, whereas WT mice exhibited an increase in frequency and absolute number of blood inflammatory monocytes (Fig. 2B). Collectively, both IFNAR and TLR7 are required for an increase in the number of inflammatory monocytes in the blood at 48 h postinfection with 1 × 102 PFU LCMV (Fig. 2C).
Low-dose LCMV infection induces IFNAR- and TLR7-dependent ISG and CCR2 ligand upregulation
We hypothesized that the type I IFN- and TLR7-dependent inflammatory monocytosis we observed in response to low-dose LCMV infection was due to inflammatory monocyte egress from the BM, and that BM gene expression changes regulated this process. Therefore, we examined gene expression changes at 36 h postinfection in the whole BM, which includes both hematopoietic and nonhematopoietic cells 4, 19, as this time point preceded the spike in blood inflammatory monocytes seen at 48 h postinfection. We observed that the IFN-stimulated genes (ISGs) Isg15 and Ifit1 were upregulated ∼6× and ∼18×, respectively, in total BM of WT mice by 36 h postinfection compared to uninfected control mice (Fig. 3A). The upregulation of ISG was completely dependent on IFNAR signaling, as infection of IFNAR−/− mice did not drive increased BM ISG expression over uninfected control mice (Fig. 3A). We found no significant induction of Ifit1 by LCMV infection in TLR7−/− mice, whereas Isg15 was upregulated only ∼2× over uninfected mice in TLR7−/− mice, much lower than the ∼6× induction in WT mice (Fig. 3A). Therefore, ISG expression during low-dose LCMV infection is reliant on IFNAR signaling and partially dependent upon TLR7 signaling. This suggests that whereas TLR7-induced responses predominate for type I IFN production at this time after infection, other viral sensing receptors, such as RIG-I, also contribute to this process.
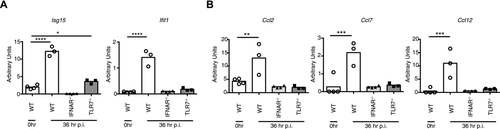
CCL2 is the principal ligand of CCR2 and primary driver of inflammatory monocyte egress from the BM 1. We posited that type I IFN and TLR7 signaling in the BM were driving Ccl2 upregulation during low-dose LCMV infection. To test this hypothesis, we infected WT mice with 1 × 102 PFU LCMV and measured induction of Ccl2 at 36 h postinfection. Ccl2 was upregulated ∼3× in the BM of infected WT mice compared to uninfected control mice (Fig. 3B). Strikingly, this increase in Ccl2 was abrogated in both IFNAR−/− and TLR7−/− mice, paralleling the lack of monocytosis we observed in these animals after infection (Fig. 2). Ccl7 and Ccl12, also ligands for CCR2, were induced in an IFNAR- and TLR7-dependent manner similar to Ccl2 at 36 h postinfection with 1 × 102 PFU LCMV (Fig. 3B). At 48 h postinfection, ISG and CCR2 ligand expression in the BM microenvironment still depended entirely on type I IFN signaling, and optimal induction of BM ISG also required TLR7 signaling (Supporting Information Fig. 3A and B). We were not able to detect an increase in CCL2 protein by ELISA in BM lysates at this time after infection over the amount seen in lysates from uninfected mice, whereas we were able to detect an ∼5× increase in serum CCL2 protein (Supporting Information Fig. 3C). Overall, these data indicate that both type I IFN and TLR7 are critical for driving peak ISG and CCR2 ligand mRNA induction in the BM in response to low-dose LCMV infection at 36 and 48 h postinfection.
Hematopoietic and nonhematopoietic cells contribute to monocytosis during low-dose LCMV infection
Our previous experiments showed that significant changes in BM gene expression after LCMV infection depended on TLR7 and type I IFN signaling but they did not discriminate whether hematopoietic or nonhematopoietic cells were responsible for these gene changes, as both of these cell types are flushed from the BM using standard procedures 4, 19. To dissect whether type I IFN and TLR7 signaling were important on hematopoietic or nonhematopoietic cells in our infection system, we generated BM chimeric mice (WT→WT, IFNAR−/−→WT, WT→IFNAR−/−, TLR7−/−→WT, and WT→TLR7−/−) in which radiation-sensitive hematopoietic or radiation-resistant nonhematopoietic cells could selectively respond to type I IFN or TLR7 signals (Supporting Information Fig. 4A). In all cases, we used congenic alleles of CD45 to assess reconstitution and the frequency of donor and recipient cells in the BM (Supporting Information Fig. 4B).
After infecting these chimeric mice with 1 × 102 PFU LCMV, we assessed whether monocytosis was observed at 48 h postinfection. Surprisingly, we observed an increase in blood inflammatory monocytes after infection in all chimeric mice (Fig. 4A). This increase was specific to inflammatory monocytes, as we observed no change in overall leukocytes at 48 h postinfection (Fig. 4B). These data demonstrate that functional redundancy exists between hematopoietic and nonhematopoietic cells in driving monocytosis in response to low-dose LCMV infection—intact type I IFN and TLR7 signaling in either compartment is sufficient to promote this response.

Next, we asked whether BM gene expression changes reflected the monocytosis observed across all chimeric mice. Indeed, WT→IFNAR−/−, TLR7−/−→WT, and WT→TLR7−/− chimeric mice expressed nearly equivalent levels of BM Isg15 and Ifit1 compared to WT→WT chimeras, all of which were increased at least 10× over uninfected WT mice at 48 h postinfection (Fig. 4C). The data from WT→IFNAR−/− chimeric mice demonstrate that WT hematopoietic cells in the BM are sufficient to generate ISG when nonhematopoietic cells cannot respond to IFNAR signaling. Our observations in TLR7−/−→WT and WT→TLR7−/− chimeric mice show that TLR7 expression on either hematopoietic or nonhematopoietic cells is sufficient to generate robust ISG in response to LCMV. Interestingly, compared to uninfected control mice, infected IFNAR−/−→WT chimeras had no induction of BM Isg15 and reduced induction of BM Ifit1 compared to infected WT→WT chimeras or WT→IFNAR−/− chimeras (Fig. 4C).
We then examined the induction of CCR2 ligands in the chimeric mice at 48 h postinfection. WT→IFNAR−/−, TLR7−/−→WT, and WT→TLR7−/− chimeras, all produced similar amounts of Ccl2, Ccl7, and Ccl12 as control WT→WT chimeras after LCMV infection. These data show that TLR7 expression on either hematopoietic or nonhematopoietic cells is sufficient to generate robust CCR2 ligand expression in response to LCMV. Similar to what was found for Ifit1 induction, IFNAR−/−→WT chimeras had reduced induction of Ccl2, Ccl7, and Ccl12 compared to WT→WT chimeras (Fig. 4D). The reduced CCR2 ligand induction observed in IFNAR−/−→WT did not affect inflammatory monocytosis in these chimeras, corroborating evidence that very low amounts of CCR2 ligands are capable of promoting monocytosis 4. Overall, these results demonstrate that IFNAR expression on hematopoietic cells in the BM, contribute more to ISG and CCR2 ligand induction than nonhematopoietic cells in this tissue after low-dose LCMV infection, and that the low amount of CCR2 ligands produced in WT→IFNAR−/− chimeras is sufficient to induce monocyte egress.
pDCs generate type I IFN in the BM after infection with low-dose LCMV
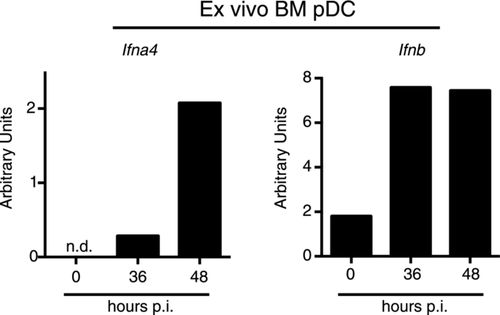
We posited that pDCs, cells that express high levels of TLR7, are capable of producing prodigious amounts of type I IFN and reside in the BM, might be contributing to the TLR7- and IFNAR-dependent inflammatory monocytosis after low-dose LCMV infection. To investigate this, we sorted BM pDCs from uninfected mice and mice infected with 1 × 102 PFU LCMV at 36 and 48 h postinfection. We found that BM pDCs produced increased amounts of Ifna4 and Ifnb mRNA after infection, compared to the undetectable or low amounts seen in pDCs from uninfected mice (Fig. 5). These data demonstrate that pDCs are hematopoietic contributors of type I IFN in the BM early in infection with low-dose LCMV.
Discussion
This study puts forth several novel findings in addition to building upon previous studies that have investigated how monocytes egress from the BM during infection. Other work has shown that monocytosis and CCR2 ligand production during infection is important for BM monocyte egress, but a specific sensor that initiates this response has not been identified. Here, we show that a single TLR, TLR7, is critical in early ISG and CCR2 ligand induction during LCMV infection. Our work is also the first to show that during LCMV infection, BM monocyte egress and CCL2/7/12 production require IFNAR signaling. Last, we found that both hematopoietic and nonhematopoietic cells contributed to TLR7- and IFNAR-dependent CCR2 ligand production and monocyte egress during LCMV infection.
It is clear that bacterial infection, such as with L. monocytogenes, can elicit monocytosis, but the literature is scant and conflicting on whether viral infection promotes this response early in infection. A study using a high dose of 1 × 105 PFU LCMV strain Armstrong delivered i.p. observed no monocytosis early in infection, but rather an increase in blood inflammatory monocytes by day 8 postinfection due to de novo production of inflammatory monocytes from myeloid progenitors 15. In contrast, work in which 1 × 102 focus-forming units of West Nile virus was delivered subcutaneously, described monocytosis, likely due to BM egress 9. We found that high doses of LCMV strain Armstrong that are typically used in the literature, from 1 × 104 to 2 × 105 PFU delivered i.p. promoted minor increases in the number of blood inflammatory monocytes with the concomitant development of panleukopenia. These observations highlight the sensitivity of the innate immune system in response to pathogens, and underscore the importance of the appropriate viral inoculum to study immune system function and behavior.
CCR2–CCL2 and CCR2–CCL7 interactions are important in mediating egress of inflammatory monocytes from the BM to the blood 1. The mechanistic underpinning of how this egress event occurs is unclear. It has been suggested that CCR2 ligands can be produced by cells that appose BM blood vessels, and that this event initiates the migration of inflammatory monocytes from BM cavities to vascular sinuses and into circulation 4. MSCs and CAR cells of nonhematopoietic origin and hematopoietic cells have been shown to mediate the reorganization and egress of BM monocytes during LPS administration and infection with L. monocytogenes, but only nonhematopoietic cells were shown to directly appose BM blood vessels and generate Ccl2, suggesting that they are crucial mediators of inflammatory monocyte egress in these models 4. Other work has suggested that BM-resident F4/80+ macrophages upregulate CCR2 ligands during murine CMV infection in a manner that requires type I IFN 6. These findings indicate that the BM is a dynamic microenvironment that has redundant mechanisms to ensure that inflammatory monocytes can be efficiently released into circulation in the event of bacterial, viral, or other microbial challenge. Our study contributes to this literature by definitively showing that type I IFN and TLR7 signaling can be important in initiating BM egress of inflammatory monocytes during viral infection, and that these signaling events can occur on either hematopoietic cells or nonhematopoietic cells.
We were not able to detect an increase in CCL2 protein in BM lysates of mice infected with a low dose of LCMV compared to uninfected mice, though we found strong induction in the serum. This is similar to data during i.v. L. monocytogenes infection where CCL2 is abundant in serum, but not in BM lysates [20], though this group was able to detect CCL2 induction in MSCs and CAR cells using a CCL2 reporter [4]. Together, our findings with LCMV and others’ findings with L. monocytogenes infection suggest a model in which the amount of total BM CCL2 is not increased during infection to promote monocyte egress, but rather new CCL2 expression in particular niches in the BM, such as in cells apposed to vascular sinuses, is critical in this process.
The relative contributions of TLR- and RIG-I-like receptor (RLR) signaling during LCMV infection are unclear. For example, TLR7−/− mice have been shown to have diminished levels of type I IFN 1 day after infection compared to WT mice 17. On the contrary, it has been shown that mice lacking components of RLR signaling have significantly less type I IFN early in infection, whereas endosomal- or pan-TLR-deficient mice are not deficient in this respect 16, 18. Here, though we do not investigate a role for RLR-signaling, we reveal a clear function for TLR7 signaling in response to low-dose LCMV strain Armstrong infection as mediating egress of inflammatory monocytes from the BM to the blood early during infection. Interestingly, in the absence of TLR7 we found some induction of the ISG Isg15 at 36 h and both Isg15 and Ifit1 at 48 h after low-dose LCMV infection, suggesting a role for RLR such as RIG-I or other pattern-recognition receptors in type I IFN production in the BM at these times. Importantly, Ccl2, Ccl7, and Ccl12 were not induced in the BM at these times in TLR7−/− mice, nor was monocytosis, showing that TLR7 has a nonredundant role in this process. It will be interesting to investigate in future the role of other PRRs, such as RIG-I, in this process.
The precise role of pDCs in determining the host's ability to clear the virus and shape the innate immune response later in infection is unclear 21, 23. However, it has been demonstrated during LCMV infections of between 2 × 106 and 3 × 106 PFU that pDCs are activated in a TLR-dependent manner 16, 24, and that these cells are ephemeral secretors of type I IFN early after infection 18. In line with these reports, our data show that in response to 1 × 102 PFU LCMV strain Armstrong, BM-resident pDCs produce type I IFN mRNA by 36 h postinfection. pDCs are predominantly found in association with nonhematopoietic CAR cells in the BM 13. Therefore, we propose that BM pDCs act to secrete type I IFN that can then generate CCR2 ligands from other cells, such as CAR cells, but possibly also from MSCs or hematopoietic cells such as F4/80+ macrophages 4, 6.
CCR2-expressing inflammatory monocytes have been shown to be an important component of an effective response to infection with certain viruses 8, 9. Inflammatory monocytes, once released from the BM and into the blood may go on to mature into APCs, which then later influence T-cell responses. Corroborating this idea, monocyte-derived APCs have been shown to be important for restimulating tissue-resident effector T cell IFN-γ in situ in the vaginal mucosa after Herpes simplex virus-2 infection 8. We show here that cooperative signaling of type I IFN and TLR7, in both hematopoietic and nonhematopoietic cells, is crucial for modulating BM gene expression to influence inflammatory monocyte accumulation in the periphery during low-dose LCMV infection. These data suggest that the BM, as a reservoir of inflammatory monocytes, may play an important role in early antiviral defense in response to LCMV and possibly other RNA virus infection.
Materials and methods
Mice, BM chimeric mice, and LCMV infection
C57BL/6 and B6.SJL mice were purchased from Charles River or bred in our vivarium. IFNAR−/− mice were provided by D. B. Stetson (University of Washington) and TLR7−/− mice were purchased from Jackson Laboratories and bred in our vivarium. Mice were housed at Benaroya Research Institute and all experiments were performed under an IACUC-approved protocol (06HA01). BM chimeric mice were generated by lethally irradiating (1000 rad) recipient mice and reconstituting them with 4 × 106 donor BM cells. In all cases, recipients and donors differed in CD45 allele expression for assessment of chimerism. For infections, 1 × 102, 1 × 104, or 2 × 105 PFU of LCMV strain Armstrong were administered to mice i.p.
Cell isolation, cell surface staining, flow cytometry, and cell sorting
To obtain samples from the blood, mice were bled via saphenous vein into 10 μL of heparin (25 mg/mL; Sigma–Aldrich, cat. no. H3149). Thirty microliters of heparinized blood was depleted of red blood cells with ACK (Lonza, cat. no. 10–548E). Blood cells were blocked with unlabeled anti-CD16/32 mAb, followed by the addition of mAbs as indicated in a final volume of 50 μL. Cells were typically labeled with the following panel of monoclonal antibodies: CD19 AF647 (clone: eBio1D3; 1:200; eBioscience), TCRβ APC (clone: H57-597; 1:200; eBioscience), Ly6G Pacific Blue (clone: 1A8; 1:400; Biolegend), CD115 PE (clone: AFS98; 1:100; eBioscience), CD11b PerCp-Cy5.5 (clone: M1/70; 1:600; eBioscience), Ly6C FITC (clone: HK1.4; 1:400; Biolegend), and CD45.2 Alexa Flour 700 (clone: 104; 1:200; eBioscience). Samples were incubated at 4°C for 30 min for surface stains, then washed and fixed in 4% paraformaldehyde. Blood cell numbers were quantified using polystyrene microspheres (Polysciences, cat. no. 18328). The same animal was tracked longitudinally across all time points for determination of cell numbers in the blood. Data for all time points were acquired on the same day using the same bead lot on an LSRII (BD Biosciences) and analyzed using FlowJo (TreeStar). Live cells were analyzed by using Forward Scatter and Side Scatter. BM pDCs were isolated and sorted as described previously 25.
Quantitative real-time polymerase chain reaction
BM was eluted directly into an RLT Plus buffer and RNA was generated using RNeasy Plus Mini Kit (Qiagen, cat. no. 74136). cDNA was synthesized using Applied Biosystems (cat. no. 4368814) or Qiagen reagents with random hexamers and Oligo(dT) primers. Quantitative real-time PCR was performed using SYBR green reagents (Takara) on a 7500 Fast Real-Time PCR System (Applied Biosystems). Relative expression was calculated as previously described 25. Primer sequences are as follows: Hprt: F, TGA AGA GCT ACT GTA ATG ATC AGT CAA C; R, AGC AAG CTT GCA ACC TTA ACC A, Isg15: F, AAG CAG CCA GAA GCA GAC TC; R, CAC CAA TCT TCT GGG CAA TC, Ifit1: F, GCC ATT CAA CTG TCT CCT G; R, GCT CTG TCT GTG TCA TAT ACC, Ccl2: F, AAC TGC ATC TGC CCT AAG GTC; R, AGT GCT TGA GGT GGT TGT GGA, Ccl7: F, GAT CTC TGC CAC GCT TCT GT; R, ATA GCC TCC TCG ACC CAC TT, Ccl12: F, ATT TCC ACA CTT CTA TGC CTC CT; R, ATC CAG TAT GGT CCT GAA GAT CA, and Ifng: F, GGC TGT TTC TGG CTG TTA CTG C; R, ACT CCT TTT CCG CTT CCT GAG G.
ELISA
BM lysates were generated by lysing flushed BM from two femurs in 500 μL T-PER Tissue Protein Extraction Reagent (Thermo Scientific) with protease inhibitors (cOmplete Mini Protease Inhibitor Cocktail, Roche). Cells were lysed at 4°C for 10 min, and insoluble material was removed by centrifugation. CCL2 protein in undiluted BM lysates or in diluted serum was measured by ELISA (BioLegend).
Data presentation and statistical analysis
Graphs were generated and statistical analyses were performed as noted in the figure legends using Prism (GraphPad).
Acknowledgments
This work was supported in part by NSF GRFP grant DGE-0718124 (MBB), NIH grants R01 AI081948 (J.A.H.), AI113325 (J.A.H.), AR055695 (J.A.H. and D.J.C.), AI067750 (D.J.C.), AI085130 (D.J.C.), and HL098067 (D.J.C.). The authors thank members of the Hamerman laboratory for helpful discussions and critical reading of the manuscript.
J.A.H. and M.B.B. designed experiments and wrote the manuscript; M.B.B., G.M.G., S.S., and J.A.H. performed experiments; and D.J.C. provided critical reagents and wrote the manuscript.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
Abbreviations
-
- CAR
-
- CXCL12-abundant reticular
-
- IFNAR−/−
-
- interferon receptor alpha deficient
-
- ISG
-
- IFN-stimulated gene
-
- LCMV
-
- lymphocytic choriomeningitis virus
-
- MSCs
-
- mesenchymal stem cells
-
- pDC
-
- plasmacytoid dendritic cell
-
- qPCR
-
- quantitative real-time PCR
-
- RLR
-
- RIG-I-like receptor
-
- TLR7−/−
-
- TLR7 deficient