Human γδ T-cell responses in infection and immunotherapy: Common mechanisms, common mediators?
Abstract
Upon receiving the Nobel Prize in Physiology or Medicine in 1987, Susumu Tonegawa referred to the then recent discovery of the γδ T-cell receptor and stated that “while the function of the T cells bearing this receptor is currently unknown (…) these T cells may be involved in an entirely new aspect of immunity”. [Tonegawa, S., Scand. J. Immunol. 1993. 38: 303–319]. Twenty-five years of intense research later this ambivalent view still holds true. Immunologists now appreciate that γδ T cells indeed represent a highly intriguing “new aspect of immunity” that is unique and distinct from conventional lymphocytes, yet even scientists in the field still struggle to understand the molecular basis of γδ T-cell responses, especially with respect to the enigmatic mode of antigen recognition. Here, we portray the peculiar responsiveness of human Vγ9/Vδ2 T cells to microorganisms, tumor cells and aminobisphosphonates, in an attempt to integrate the corresponding — and at times confusing — findings into a “theory of everything” that may help explain how such diverse stimuli result in similar γδ T-cell responses via the recognition of soluble low molecular weight phosphoantigens.
Human γδ T cells: Unconventional T cells responding to unconventional antigens
“My goal is simple. It is a complete understanding of the universe, why it is as it is and why it exists at all.”
(Stephen Hawking)
Immunology is an increasingly complex topic, and immunology teaching has benefited from grouping different aspects of the immune system into easily digestible portions such as “innate” or “adaptive” immunity. However, certain cells such as γδ T cells do not fit into either category and — due to a lack of better terminology — are described as “bridging” innate and adaptive immunity 1. Historically, adaptive immunity was believed to be mediated by two types of lymphocytes with rearranged antigen-specific receptors: B cells and T cells. The unexpected discovery of a third rearranged receptor, the γδ TCR, fundamentally changed this simplistic view (Fig. 1). It is now recognized that the immune system of all jawed vertebrates harbors three classes of lymphocytes (B cells, αβ T cells, and γδ T cells) which must have coevolved for over 400 million years, arguing for a crucial and nonredundant role of each. Unfortunately, progress in our understanding of γδ T cells has been hampered by the fact that these cells cannot be studied by applying models established for conventional B and T cells, not the least to their lack of MHC restriction and their frequent responsiveness to nonproteinaceous agents 1, 2. In addition, γδ T cells display striking species-specific differences with respect to their TCR repertoires, activities, and anatomical locations such as highly specialized dendritic epidermal Vγ5/Vδ1+ γδ T cells that are present in the murine but not the human skin 3. Most γδ T-cell antigens therefore remain elusive.
Vγ9/Vδ2+ γδ T cells comprise the major γδ T-cell subset in human peripheral blood. As bona fide “unconventional” lymphocytes, Vγ9/Vδ2 T cells integrate features reminiscent of Th1, Th2, Th17, TFH, and Treg cells as well as CTLs, NK cells, and APCs — depending on the priming conditions activated Vγ9/Vδ2 T cells can produce a range of cytokines and chemokines, kill infected and transformed target cells, regulate survival and differentiation of monocytes and neutrophils, induce maturation of dendritic cells, provide B-cell help and present antigens to CD4+ and CD8+ T cells 2, 4. Normally constituting only 1–5% of circulating T cells in healthy adults, Vγ9/Vδ2 T-cell numbers increase dramatically in response to many microbial infections, at times to more than 50% of the peripheral T-cell pool within days 5. This is one of the most impressive physiological expansions of any human immune cell and indicates a fundamental role of Vγ9/Vδ2 T cells in acute disease. In addition to microbial infection, Vγ9/Vδ2 T cells respond to many tumor cell lines, especially those of B-cell origin, and are stimulated by a class of anti-bone resorption drugs, the nitrogen-containing or aminobisphosphonates (NBPs) 5-7. For reasons that are not yet understood, Vγ9/Vδ2 T cells and their unique responsiveness are only found in higher primates, and hence no small animal model replicates the interaction between Vγ9/Vδ2 T cells and other immune and nonimmune cells in the human body. Nevertheless, with a plethora of different experimental stimuli available, Vγ9/Vδ2 T cells have become the most widely studied γδ T-cell population, as they are readily accessible in human blood, easy to culture and expand in vitro, and of direct relevance for infection and immunotherapy.
Human γδ T-cell responses in microbial infection: The nature of phosphoantigens
“It is a good morning exercise for a research scientist to discard a pet hypothesis every day before breakfast. It keeps him young.”
(Konrad Lorenz)
Soon after their discovery 8, Vγ9/Vδ2 T cells were shown to display a unique reactivity to mycobacteria and other microbes 9. Surprisingly, this reactivity was not directed toward classical protein antigens because the active compound in microbial extracts turned out to be resistant to proteinase K treatment but susceptible to degradation by alkaline phosphatase. This puzzling phenomenon resulted in the identification of natural and synthetic low molecular weight “phosphoantigens” with potent in vitro activity on Vγ9/Vδ2 T cells 10, 11 (Fig. 2). Although the isoprenoid precursor isopentenyl pyrophosphate (IPP), which is found in all living cells, was isolated from mycobacteria as the first natural phosphoantigen 12, microbial IPP levels may not reach the minimum required for T-cell activation and thus do not explain Vγ9/Vδ2 T-cell responses in infections 13. However, as we discuss in Human γδ T-cell responses to aminobisphosphonates: The potential for immunotherapy, IPP or IPP by-products may nevertheless play a role in vivo under certain conditions, such as cellular stress or pharmacological manipulation, leading to intracellular accumulation of isoprenoid precursors in host cells 5.

The search for the true microbial activator of Vγ9/Vδ2 T cells eventually led to the purification of a novel metabolite, (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP), an intermediate of the alternative, nonmevalonate pathway of isoprenoid biosynthesis 13. The extraordinary bioactivity of HMB-PP toward Vγ9/Vδ2 T cells — 10,000 times more effective than IPP — explained why HMB-PP-producing bacteria but not HMB-PP-deficient bacteria induce activation and expansion of Vγ9/Vδ2 T cells in vitro and in vivo 13-15. Pathogens producing HMB-PP include the causative agents of diseases such as anthrax, cholera, diphtheria, dysentery, gonorrhoea, leprosy, pertussis, plague, syphilis, tetanus, tuberculosis, and tularaemia, as well as malaria and toxoplasmosis 5, 16. Of note, HMB-PP is also produced by many commensal and opportunistic bacteria in skin, mucosa, and faeces, even though these sites are not routinely patrolled by Vγ9/Vδ2 T cells under homeostatic conditions. The rapid and sensitive response of Vγ9/Vδ2 T cells to HMB-PP producing microbes thus evokes cardinal features of innate immunity, as HMB-PP fulfils Janeway's definition of a "pathogen-associated molecular pattern" 15, 16. HMB-PP-negative bacteria of clinical relevance include staphylococci, streptococci and the causative agents of Lyme and Legionnaires’ disease.
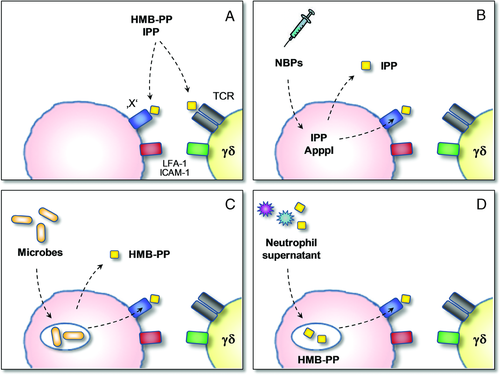
The mode of action of HMB-PP and other phosphoantigens remains enigmatic. With a molecular size similar to that of a single amino acid — and thus a very limited number of possible atomic contacts — it is difficult to envisage how HMB-PP may become ‘presented’ on a cell surface and be recognized by the γδ TCR in a manner analogous to the MHC/peptide/αβ TCR complex (Fig. 3A). Indeed, most Vγ9/Vδ2 T cells lack CD4 and CD8 on the cell surface and their TCR-mediated activation is MHC and CD1 independent 17, 18. Alternatively, HMB-PP may bind directly to the γδ TCR and trigger signaling events such as activation of MEK/Erk and PI-3K/Akt pathways 19. However, an interaction with the TCR has never been demonstrated biochemically, and attempts to cocrystallize the γδ TCR in the presence of HMB-PP or related molecules have failed so far 20. Of note, all chemical changes in the molecular structure of HMB-PP drastically reduce its potency. Yet, the resulting derivatives often still have bioactivities comparable or even superior to that of IPP, demonstrating that many chemical features of HMB-PP are not necessarily required to exert its specific effect on Vγ9/Vδ2 T cells 21-24. The most prominent of these analogues are 2-methyl-3-butenyl pyrophosphate (2M3BPP), 3-bromo-3-hydroxybutyl pyrophosphate (BrHPP, "Phosphostim"), and a nonhydrolysable diphosphonate analog of HMB-PP (HMB-CPP, "Picostim"), all three of which have entered preclinical and clinical stages of development for γδ T-cell targeting immuno-therapy 2, 5, 7 (Fig. 2).
Human γδ T-cell responses to aminobisphos-phonates: Potential for immunotherapy
“We are to admit no more causes of natural things, than such as are both true and sufficient to explain their appearances. Therefore to the same natural effects we must, as far as possible, assign the same causes.”
(Isaac Newton)
During the search for natural and synthetic Vγ9/Vδ2 T-cell-specific ligands, NBPs emerged as another interesting class of bioactive molecules. NBPs such as zoledronate, pamidronate, alendronate, and ibandronate (Fig. 2) were originally developed to treat osteoporosis and metastatic bone disease, due to their chemical similarity to the pyrophosphate crystals of the bone matrix. In vivo activation of Vγ9/Vδ2 T cells by NBPs was first observed in patients who experienced acute-phase reactions upon first-time treatment 25 and shown to depend on the TCR 26. The fact that NBPs are approved drugs with good safety profiles opened the way for clinical studies exploiting the cytotoxic activity of Vγ9/Vδ2 T cells for immunotherapy of tumor patients 2, 5, 7.
Subsequent studies, however, have revealed that the capacity of NBPs to activate Vγ9/Vδ2 T cells is not merely due to their structural similarity with the natural ligands (Table 1). Unlike soluble phosphoantigens, NBPs can be pulsed stably onto accessory cells serving as "presenting" cells 27, suggesting that these two classes of compounds operate via distinct molecular mechanisms. In fact, NBPs require endocytic uptake by cells like monocytes, DCs or tumor cells 28. Once inside the cell, NBPs act as inhibitors of farnesyl pyrophosphate (FPP) synthase, a key enzyme of the classical, mevalonate pathway of isoprenoid biosynthesis that is utilized by all higher eukaryotes including humans (and by HMB-PP-negative prokaryotes) 5. NBPs block the FPP synthase-mediated condensation of IPP and its isomer dimethylallyl pyrophosphate (DMAPP) to FPP, leading to intracellular accumulation of upstream metabolites, including IPP and DMAPP 28, 29.
| Experimental stimulus: | HMB-PP | IPP | ApppI | NBPs | S/N (DCs) | S/N (PMNs) |
|---|---|---|---|---|---|---|
| Dependent on TCR | Yes | Yes | Yes | Yes | Yes | Yes |
| Resistant to proteinase K | Yes | Yes | Yes | Yes | ? | ? |
| Sensitive to alkaline phosphatase | Yes | Yes | No | No | ? | Yes |
| Dependent on presence of APCs or bystander cells | No | No | Yesb | Yes | No | Yes |
| Sensitive to statins | No | No | No | Yes | No | No |
| Active phosphoantigen involved | HMB-PP | IPP | IPP? | IPP? | IPP?c | HMB-PP?d |
- a S/N (DCs), supernatant from NBP-treated DCs. S/N (PMNs), supernatant from neutrophils harboring HMB-PP producing bacteria.
- b In the absence of APCs, the bioactivity of ApppI depends on exogenously added nucleoside pyrophosphatase releasing free IPP 52.
- c Experimental data suggest a greater potency of supernatants from zoledronate-treated DCs than exogenous IPP used at equimolar concentrations 6.
- d Experimental data suggest a greater potency of supernatants from microbe-pulsed neutrophils than exogenous HMB-PP used at equimolar concentrations (HMB-PP was below the biochemical detection limit in those supernatants) 15.
The rate-limiting enzyme of the mevalonate pathway is 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase. Pharmacological inhibition of HMG-CoA reductase by statins lowers the synthesis of all downstream isoprenoids including cholesterol, which is exploited in the clinic to prevent cardiovascular diseases 30. Of relevance for this review, statins counteract the NBP-induced accumulation of IPP and DMAPP and thereby inhibit the NBP-driven activation of Vγ9/Vδ2 T cells in vitro 18, 28, 31. Taken together, these biochemical investigations imply that NBPs act indirectly on Vγ9/Vδ2 T cells, via accumulation of intracellular phosphoantigens as secondary mediators in target cells that are then recognized by Vγ9/Vδ2 T cells (Fig. 3B).
Besides IPP and DMAPP, the NBP-induced accumulation of another metabolite, triphosphoric acid 1-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester (ApppI) (Fig. 2), was recently reported 32. ApppI is synthesized from IPP and ATP in a reaction likely to be catalyzed by aminoacyl-tRNA synthetases, and exerts a proapoptotic function by inhibiting the mitochondrial ADP/ATP translocase in osteoclasts, macrophages, and tumor cells 32, 33. The similarity of ApppI to the nucleoside conjugates of HMB-PP, IPP, and DMAPP that had been described earlier 10, 12, 34, 35 immediately raised the question whether ApppI constituted a natural activator of Vγ9/Vδ2 T cells. Exogenous ApppI is indeed sufficient to expand Vγ9/Vδ2 T cells from PBMCs but requires the presence of accessory cells to do so, most likely due to cell surface-associated nucleoside pyrophosphatases cleaving ApppI into AMP and free IPP (Table 1) 36. Of note, ApppI is readily detectable in tumor cells such as Daudi cells in the absence of NBP treatment and may thus represent a natural “storage” form of IPP and/or an intermediate in a putative phosphoantigen processing/presentation pathway 36. It remains to be investigated whether other phosphoantigen-nucleoside conjugates with structures similar to ApppI (following a common “NpppX” motif) exist in vivo, especially in stressed and transformed cells, as well as in microbial pathogens and pathogen-infected target cells.
A theory of everything: Release of soluble phosphoantigens into the microenvironment?
“Everything should be as simple as it can be, but not simpler.”
(attributed to Albert Einstein)
The ease of manipulating Vγ9/Vδ2 T cells in vitro using soluble phosphoantigens left unanswered the question of how the immune system would actually “see” those compounds in vivo (as discussed in 16). Microbial uptake and processing was previously shown to be required for Vγ9/Vδ2 T-cell responses to mycobacteria, with the relevant Vγ9/Vδ2 T-cell epitopes remaining stably associated with the surface of the “presenting” cell 37, 38. However, those studies did not address whether HMB-PP itself was “presented”, or whether a new epitope was created or unmasked downstream of a putative HMB-PP “processing” pathway (Fig. 3C). While such studies were usually performed using APCs, the role of neutrophils was overlooked.
Neutrophils are the first immune cells infiltrating the site of infection and the main phagocytes responsible for early pathogen clearance. In a recent study, we demonstrated that neutrophils harboring phagocytosed bacteria release traces of HMB-PP into the microenvironment, in sufficient quantities to become accessible for Vγ9/Vδ2 T cells 15 (Fig. 3C). HMB-PP-deficient bacteria and bacteria in which HMB-PP production is inhibited by pretreatment with the antibiotic fosmidomycin induce no such Vγ9/Vδ2 T-cell responses above background. Of note, the Vγ9/Vδ2 T-cell response to HMB-PP-producing bacteria upon phagocytosis by neutrophils is crucially dependent on the presence of accessory monocytes 15, demonstrating that neutrophil-released HMB-PP requires uptake and processing (Fig. 3D). This endocytosis-dependent bioactivity of neutrophil-released HMB-PP is susceptible to inhibitors of macropinocytosis, endosomal acidification and vesicle trafficking (M. S. Davey and M. Eberl, unpublished observations), which is in striking contrast to that of exogenously added soluble HMB-PP but remarkably similar to the mode of action of NBPs (Table 1) 39. It remains to be investigated whether this is due to neutrophil-released factors such as cytokines, chaperones, or enzymes that enhance uptake, processing and/or presentation of microbial compounds. Furthermore, whether macrophages or other cells similarly release soluble HMB-PP upon phagocytosis of extracellular bacteria or infection by intracellular bacteria (Fig. 3C) is not known. It is also unclear whether the mevalonate pathway of these cells is somehow perturbed upon phagocytosis and whether this at all contributes to the activation of Vγ9/Vδ2 T cells 40.
Support for a scenario of phosphoantigens being either actively released or passively leaking into the microenvironment comes from observations with NBP-treated cells. We recently demonstrated that supernatants of zoledronate-treated DCs are sufficient to expand Vγ9/Vδ2 T cells, both in the absence or presence of monocytes 41. These supernatants contained up to 10–100 nM soluble IPP, concentrations at which purified IPP alone exhibits only very modest bioactivity (Table 1). Similar observations were made in a mouse xenograft model where tumors arising from implanted breast cancer cells showed a rich infiltrate of Vγ9/Vδ2 T cells upon treatment of mice with zoledronate, although the local intratumoral levels of IPP/ApppI were only 1–80 nM 42. These data imply that additional soluble components present in cellular supernatants enhance the bioactivity of the released IPP 41, 42 and evoke analogous findings with neutrophils harboring HMB-PP-producing bacteria 15.
If phosphoantigens are indeed released from DCs, neutrophils, or tumor cells into the microenvironment, a number of factors may considerably influence their extracellular levels. First, in the case of NBPs, fluid phase endocytosis is the main mechanism of drug entry into the cell; vesicular acidification is then necessary for drug delivery into the cytosol 39. Differences in these processes may thus explain the differences in NBP uptake and subsequent production of IPP/ApppI in a variety of cell types 42. Similarly, presentation of neutrophil-released microbial compounds varies greatly among distinct cell types, most likely due to different rates of uptake and intracellular processing, making primary monocytes excellent HMB-PP-presenting cells but rendering other monocytic cell types such as THP-1 and U937 cells refractory (M. S. Davey and M. Eberl, unpublished observations).
Second, the metabolism of phosphoantigens plays a role. Although the mevalonate pathway is ubiquitously found in mammalian cells, it is not equally active in all tissues. In this respect, sterol-synthesizing tissues such as liver, breast epithelia, adrenal glands, and gonads, as well as environmentally stressed cells, cells undergoing malignant transformation and invasive cancer cells have the highest activity of the mevalonate pathway 43-45. Consequently, these cell types are expected to release more IPP/ApppI upon NBP treatment than other tissues. Whether this correlates with an increased induction of Vγ9/Vδ2 T-cell activity by these tissues and/or predisposes them to Vγ9/Vδ2 T-cell mediated cytotoxicity remains to be investigated. Of note, IPP/ApppI may already be secreted by tumor cells in the absence of NBPs, although such secretion is greatly enhanced by prior treatment with NBPs 42.
The export rate of IPP/ApppI and HMB-PP is a third critical factor. Despite the low molecular weight of these molecules, their net charges make them unsuitable to cross the lipid bilayer by passive diffusion. To explain the presence of phosphoantigens in the extracellular environment, a carrier-mediated efflux or a less specific export via exocytic vesicles could be envisaged. Of note, APCs and tumor cells are rich sources of exosomes that export proteins, miRNAs, plasmids and oncogenic receptors 46; present antigens and have both activating and inhibiting effects on T cells 47; and exhibit ecto-nucleotidase/ecto-nucleosidase activity 48. Moreover, exosomes shed from infected macrophages carry pathogen-associated molecular patterns and stimulate bystander cells in a TLR- and MyD88-dependent manner 49, making exosomes intriguing candidates as potential carriers of both microbial and endogenous phosphoantigens.
Unconventional antigen presentation: In search of the “X” factor
“I don't believe that the ultimate theory will come by steady work along existing lines. We need something new. We can't predict what that will be or when we will find it because if we knew that, we would have found it already!”
(Stephen Hawking)
The complex system of phosphoantigen uptake, processing, and release is likely to have a role beyond immunity. Multicellular organisms have huge needs for phosphate, which is stored in bone but is also indispensable for cell growth, metabolic signaling, pH balance, and membrane transport. IPP and DMAPP are the building blocks for all higher isoprenoids including cholesterol, steroids, vitamin D, dolichol, carotenoids, and ubiquinones, which are essential in membrane biochemistry, electron transfer, and hormone synthesis 30. In addition, isoprenoids are substrates for posttranslational prenylation of small GTPases and lamins, and used as side chains in heme and certain tRNAs. The pivotal role of isoprenoids for the cellular metabolism is also evident by the notion that the first statins discovered, mevastatin and lovastatin, are actually fungal toxins 30. With such ancient and ubiquitous biochemical pathways involved it is surprising that the immune responsiveness to phosphoantigens is restricted to higher primates.
Given the observation that HMB-PP from internalized pathogens and IPP/ApppI accumulating in NBP-treated cells may exert their effects extracellularly, it remains to be resolved whether these compounds represent direct ligands for the TCR (and thus true antigens), or rather cofactors for surface receptors or even substrates for enzymes. It is tempting to speculate that all phosphoantigens and NBPs eventually yield the same end product, such as the modification or generation of a surface epitope that is then recognized by the γδ TCR. Recent experiments using TCR tetramers demonstrated that HMB-PP and other phosphoantigens can be displayed on the cell surface by a trypsin-sensitive structure, supporting the concept of a widely distributed and non-polymorphic ‘presentation’ molecule that is found on most human and primate cells but is absent from non-primate cells 27, 38, 50, 51.
A number of key players involved in the recognition of phosphoantigens has been identified over the last few years. Mitochondrial ecto-F1-ATPase may have a role in the shuttling of intracellular ApppI to the cell surface and presentation to Vγ9/Vδ2 T cells 36. Notably, cells that do not express surface F1-ATPase and hence do not activate Vγ9/Vδ2 T cells may become competent to do so when treated with pro-apoptotic concentrations of NBPs, thereby serving as sensor of the pro-immunogenic death mediated by intracellular accumulation of ApppI 52. Whether the same is true for free and/or nucleoside-conjugated HMB-PP in the context of microbial infection has not been addressed yet. The conspicuous difference in bioactivities between soluble HMB-PP and IPP remains baffling. However, supernatants derived from neutrophils harboring bacteria and from DCs pulsed with NBPs are significantly more potent than HMB-PP and IPP used alone, respectively, thus raising questions as to the true concentrations required to activate Vγ9/Vδ2 T cells in vivo. In fact, ApppI already activates Vγ9/Vδ2 T cells at nanomolar concentrations when presented by F1-ATPase, that is, at significantly lower levels than those required when applied as a soluble compound 52, indicating the importance of specific and directed “presentation” to Vγ9/Vδ2 T cells.
Another molecule recently implicated in Vγ9/Vδ2 T-cell responses is CD277, a member of the B7/butyrophilin family that is uniquely found in higher primates 53, 54. Agonist antibodies to human CD277 sensitize target cells to Vγ9/Vδ2 T-cell recognition, while blocking antibodies against CD277 inhibit Vγ9/Vδ2 T-cell activation by both endogenous and exogenous phosphoantigens 55. These findings may elegantly explain the species restriction of Vγ9/Vδ2 T-cell responses, as well as identify a common downstream player in the presentation pathway. However, the molecular mechanism of whether and how CD277 senses phosphoantigens and interacts with the TCR remains to be elucidated. The concerted action of molecules such as F1-ATPase and CD277 together with tight cell–cell contacts via LFA-1/ICAM-1 14, 15, 27 and costimulatory interactions involving CD27, CD28, CD6, NKG2D, and other factors (56, 57 and reviewed in 58, 59) may ensure the assembly of an effective “presentation” complex. It is conceivable that the biochemical and trafficking pathways involved in γδ T-cell activation may not be any less sophisticated than the multilayered interplay of proteins and cellular subcompartments in the processing, loading, and presentation of CD4+ and CD8+ T-cell antigens.
We are aware that, for the sake of simplicity in this review, we have regarded the recognition of phosphoantigens as an innate-like process. However, the true situation is likely to be far more complex in that the Vγ9/Vδ2 TCR is not just another pattern recognition receptor but undergoes considerable genomic rearrangement during thymic development and oligoclonal expansion in the periphery. Why the recognition of such simple bacterial and intracellular metabolites appeared so late during mammalian evolution and why it requires a rearranged TCR is entirely unclear. This conundrum will hopefully become solved once the nature of the cofactors, “presenting” molecules and intracellular trafficking pathways involved in Vγ9/Vδ2 T-cell activation is unveiled.
Acknowledgments
We thank the members of our research teams and our collaborators for their contribution. We gratefully acknowledge Marc Bonneville, Eric Champagne, Bernhard Moser, and Eric Oldfield for the critical discussion. Over the past 5 years, the Eberl lab has received support from Baxter Healthcare, Breast Cancer Campaign, Cancer Research UK, Medical Research Council, National Institute for Social Care and Health Research (NISCHR) and Tenovus. The Riganti lab has received support from the Italian Association for Cancer Research (AIRC) and Compagnia di San Paolo, Italy (Progetto Oncologia). The Massaia lab has received support from Regione Piemonte (Ricerca Sanitaria, Ricerca Scientifica e Progetto Strategico ImmOnc), Compagnia San Paolo di Torino (Progetto Oncologia) and Fondazione Neoplasie Sangue (Fo.Ne.Sa) (Torino, Italy). M.E. was recipient of a RCUK Fellowship in Translational Research and a Wellcome Trust VIP Award.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
Abbreviations
-
- ApppI
-
- triphosphoric acid 1-adenosin-5'-yl ester 3-(3-methylbut-3-enyl) ester
-
- DMAPP
-
- dimethylallyl pyrophosphate
-
- FPP
-
- farnesyl pyrophosphate
-
- HMB-PP
-
- (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HDMAPP)