CD4+ T-cell immunity after pandemic influenza vaccination cross-reacts with seasonal antigens and functionally differs from active influenza infection
Abstract
Antigen-specific antibodies are well characterized after vaccination with pandemic H1N1 or seasonal influenza vaccines. However, knowledge on cellular immunity toward pandemic H1N1 after vaccination and infection and cross-reactivities toward seasonal antigens is limited. Nineteen individuals were vaccinated with the pandemic H1N1 vaccine. Among those, ten had been prevaccinated against seasonal influenza. CD4+ T cells specific for pandemic H1N1 and for seasonal vaccine, and antibodies were monitored using flow cytometry and ELISA/neutralization assays, respectively. In addition, seven patients with acute pandemic influenza infection were analyzed. Pandemic H1N1 vaccination induced a strong 4.63-fold (IQR 4.16) increase in antigen-specific CD4+ T cells that was more pronounced in individuals not prevaccinated with seasonal influenza (p = 0.01). T-cell levels toward seasonal vaccine concomitantly rose by 2.71-fold (IQR 2.26). Likewise, prevaccination with seasonal influenza induced a less pronounced increase in specific antibodies. Influenza-specific T cells in vaccinees had a Th1 phenotype mainly coexpressing IFN-γ and IL-2, whereas patients with active pandemic influenza showed a shift toward cells predominantly expressing IFN-γ. In conclusion, T cells toward seasonal influenza antigens cross-react with pandemic H1N1 antigens and affect induction of specific T cells after pandemic influenza vaccination. In addition, the cytokine patterns of specific T cells during acute H1N1 infection and after vaccination differ, and the predominantly dual-positive cytokine profile of vaccine-induced T cells suggests sufficient functionality to confer successful virus control.
Introduction
Worldwide, seasonal influenza epidemics cause millions of cases of severe illness and about 250,000 to 500,000 deaths every year 1. Due to recombination of gene segments and spontaneous mutations in the viral genome, new pandemic strains, particularly of influenza virus type A, emerge. The global transmission of these strains is largely based on escape from existing immunity in the human population. Well-documented pandemics were caused by different virus subtypes, such as the 1918 H1N1 Spanish influenza, the 1957 H2N2 Asian influenza, or the 1968 H3N2 Hong Kong influenza. The influenza outbreak of a novel H1N1 strain (pdmH1N1) in April 2009 was officially declared a global pandemic by the WHO, but in contrast to the severity of preceding pandemics, most of the infections documented had caused milder symptoms than seasonal influenza, especially in the elderly 2. As specific antibodies generally protect against influenza 3, 4, this was partially attributed to the presence of preexisting antibodies with cross-protective potential in individuals born before 1957 5. Yet, in the majority of individuals born thereafter, cross-reactive antibodies were not present or had not been induced by seasonal influenza vaccination 5. Nevertheless, although controversially discussed 6, recent evidence suggests that seasonal vaccination could provide partial protection against pandemic influenza 7, 8, which may be due to the induction of T-cell immunity with cross-protective potential. In silico studies comparing seasonal and pandemic strains have indeed provided evidence for the existence of cross-reactive T-cell epitopes, which may blunt pandemic infection in vivo 9. While several reports have recently been published on cellular immunity after seasonal influenza vaccination, few data are available on the induction of cellular immunity in response to the pandemic H1N1 vaccine. In addition, knowledge on their cross-reactivity with seasonal antigens and on the influence of previous encounters with seasonal strains on the induction of specific immune responses toward pandemic strains is limited. Disease severity in pandemic influenza infection may also be modulated by functional exhaustion of antigen-specific T-cells, as has been shown for other symptomatic infectious diseases such as HIV infection, CMV disease, or active tuberculosis 10, 11. Up to now, however, the role of functional properties of influenza-specific T cells in the control of symptomatic infections is poorly characterized.
This prompted us to analyze the induction and functional characteristics of specific CD4+ T cells as well as of specific antibodies in response to pandemic influenza vaccination. We also analyzed cross-reactivity with seasonal antigens and its impact on induction of specific immunity after immunization with the pandemic vaccine. Finally, to assess evidence of impaired immune functionality on viral control, cytokine expression profiles of influenza-reactive T cells induced after vaccination were compared with those of patients with acute pandemic influenza infection.
Results
Pandemic influenza vaccination induces a CD4+ T-cell response toward pandemic and seasonal antigens
The induction of influenza-specific cellular immunity was assessed in a total of 19 individuals before as well as 1, 2, and 10 weeks after immunization with the pandemic vaccine Pandemrix. Among those, ten individuals had been prevaccinated using the seasonal influenza vaccine. The frequencies of CD4+ T cells specific for both the pandemic (pdmH1N1) and cross-reactive seasonal influenza antigens (Flu09) were determined directly ex vivo from whole blood samples. Antigen-specific CD4+ T cells were identified using flow cytometry based on the induction of the act-ivation marker CD69 and the cytokine IFN-γ after specific stimulation in vitro. When basal T-cell frequencies in all 19 individuals were assessed, low levels of CD4+ T cells specific for pdmH1N1 (median (interquartile range, IQR) 0.03% (0.04%)) and seasonal influenza antigens were found (0.10% (0.07%), Table 1). As shown in a representative example (Fig. 1), low levels of specific T cells present prior to vaccination increased thereafter and highest values were found 1 week after vaccination (Fig. 1 and Table 1). In general, pandemic H1N1 vaccination resulted in the strongest induction of specific CD4+ T-cell frequencies toward the pdmH1N1 antigen present in the vaccine (4.63-fold median increase after 1 week, Table 1). Interestingly, although less pronounced, this was associated with a concomitant increase in the percentage of cells cross-reacting toward seasonal influenza antigens (2.71-fold median increase). In contrast, frequencies of T cells reactive against the positive control stimulus Staphylococcus aureus Enterotoxin B (SEB) were largely stable over time and only slightly increased after 10 weeks (Fig. 1 and Table 1). These results show that vaccination with the pandemic influenza vaccine induces a specific CD4+ T-cell immunity toward pandemic H1N1 antigens that cross-reacts with that of the seasonal influenza vaccine.
| pdmH1N1 | Flu09 | SEB | ||
|---|---|---|---|---|
| Median frequencies % | Basal (0 weeks) | 0.03% (0.04%) | 0.10% (0.07%) | 3.40% (2.53%) |
| (IQR) | 1 week | 0.18% (0.18%)*** | 0.24% (0.18%)*** | 3.21% (3.34%) |
| 2 weeks | 0.14% (0.19%)*** | 0.21% (0.19%)** | 3.13% (2.45%) | |
| 10 weeks | 0.12% (0.14%)** | 0.24% (0.18%)** | 4.01% (3.05%)* | |
| p-values (Friedman test) | p < 0.0001 | p < 0.0001 | p = 0.031 | |
| Median fold increase in specific T cells (IQR) | Week 1 versus basal | 4.63 (4.16) | 2.71 (2.26) | 1.03 (0.25) |
- a) Shown are median (IQR) frequencies of antigen-specific CD4+ T cells (CD69+ IFN-γ+) that were calculated from values subtracted for the respective negative controls; pdmH1N1, pandemic H1N1; Flu09, seasonal influenza vaccine; SEB, Staphylococcus aureus Enterotoxin B; T-cell frequencies over time were compared using the Friedman test, *, **, and *** denote p < 0.05, p < 0.01, and p < 0.001, respectively in Dunn´s posttest.

Vaccination mainly induces Th1-cells that change differentiation status directly after vaccination
In order to analyze changes in phenotypical properties of vaccine-induced pdmH1N1-specific CD4+ T cells in more detail, we monitored the expression of different functional markers (IFN-γ, IL-2, IL-17, CD69, CTLA-4) and of the early differentiation marker CD127 that is known to be downregulated by antigenic stimulation 12. As shown in representative dotplots of pdmH1N1-specific T cells 1 week after pandemic H1N1 vaccination, predominant coexpression of effector cytokines IL-2 and IFN-γ with CD69 indicated that specific T cells were mainly Th1 effector-type cells (Fig. 2A). IL-17-expressing cells were not detected after pdmH1N1-specific stimulation (Fig. 2A), whereas it was readily detected after stimulation with SEB (data not shown). In line with the kinetics in frequency, CTLA-4, the most important negative costimulatory surface receptor of T-cell activation, peaked 1 week after vaccination and then decreased to almost prevaccination levels (p < 0.0001, Fig. 2B). Median (IQR) percentages of CD127-expressing influenza-specific CD4+ T cells showed an inverse relationship in that they initially decreased from 97.6 (5.0%) to 80.0% (12.1%) during the first week postvaccination, but increased again in the follow-up to 98.0% (6.0%) after 10 weeks (p < 0.0001, Fig. 2C), consistent with transient downregulation after antigenic stimulation in vivo. The CD4+ T cells that were specific for the seasonal influenza vaccine showed similar expression profiles and properties (data not shown). In summary, phenotypical characterization of the influenza-specific CD4+ T cells showed that the vaccine leads to induction of mainly Th1 cells that change in differentiation status in the immediate postvaccination period.
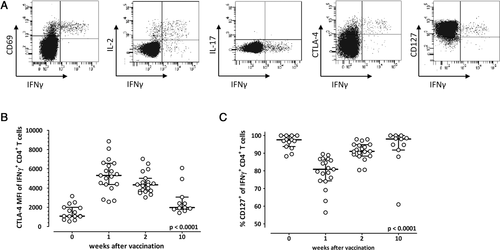
Existing T cells due to recent seasonal vaccination decrease influenza-specific T-cell induction
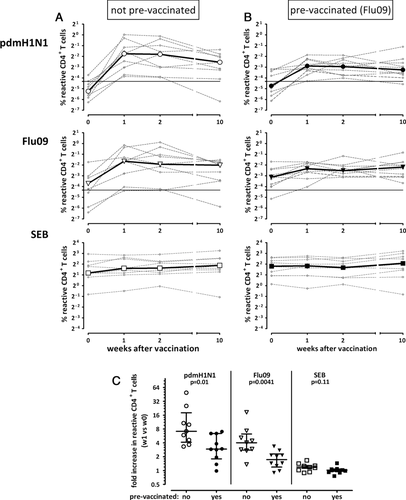
To assess the impact of preexisting cellular immunity against seasonal influenza antigens on basal levels and on the induction of influenza-specific immunity after pandemic H1N1 vaccination, individuals were separated into those not prevaccinated and prevaccinated with the seasonal influenza vaccine. The kinetics in influenza- and SEB-reactive CD4+ T cells in both groups are shown in Fig. 3A and B. Interestingly, although basal frequencies of pdmH1N1 specific T cells were not different (p = 0.32), their median increase was significantly stronger in individuals that had not been prevaccinated against seasonal influenza (7.15-fold), as compared with individuals that had been prevaccinated (2.94-fold, p = 0.01, Fig. 3C). Of note, this difference between the two groups was also found for CD4+ T cells reacting toward seasonal influenza antigens (4.03-fold versus 1.74-fold, p = 0.0041). As expected, the frequencies of SEB-reactive T cells did not increase in any of the groups (p = 0.11, Fig. 3C). Taken together, the results indicate that a preexisting immunity induced by recent seasonal vaccination is associated with a less pronounced induction of influenza-specific CD4+ T cells after pandemic H1N1 vaccination.
Vaccine-induced antibodies show a certain extent of cross-reactivity with seasonal antigens
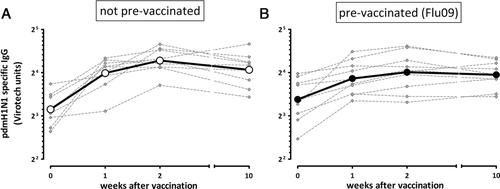
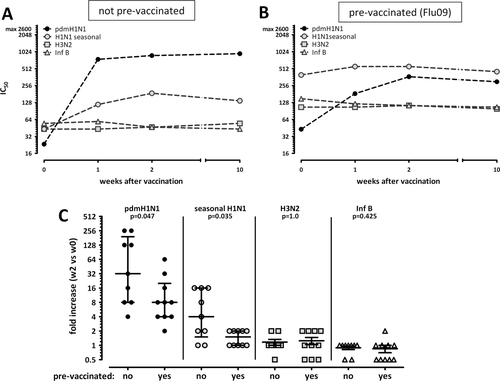
To compare the effects of the pandemic vaccine on cellular and humoral responses, induction of influenza-specific antibodies was determined. Importantly, vaccination induced a significant continuous increase in pdmH1N1-specific IgG titers that peaked about 2 weeks postvaccination and remained almost constant over the following 8 weeks (Fig. 4A and B). In line with T-cell results, baseline titers were unaffected by history of seasonal prevaccination (p = 0.34), whereas the increase after pdmH1N1 vaccination was slightly lower in individuals prevaccinated with seasonal influenza (1.64 ± 0.46 fold versus 2.30 ± 0.77 fold increase after 2 weeks, p = 0.04).
In order to assess potential cross-reactivity of antibodies induced by the pandemic vaccine, plasma samples from individuals that were not prevaccinated against seasonal influenza were analyzed for the presence of neutralizing antibodies against pdmH1N1 and the individual compounds of the seasonal influenza vaccine (H1N1 seasonal, H3N2, Influenza B). Consistent with results from ELISA (Fig. 4A), maximum titers of pdmH1N1-specific neutralizing antibodies were induced already during the first 2 weeks following vaccination and remained stable throughout week 10 (Fig. 5A and C, 32-fold increase). In contrast, only a minor increase in seasonal H1N1 titers was observed indicating a certain extent of neutralizing cross-reactivity against the seasonal H1N1 influenza strain (Fig. 5C, 4-fold increase). There was no effect on neutralizing antibody titers toward H3N2 and Influenza B. As expected, individuals that had been prevaccinated against seasonal influenza had higher levels of neutralizing antibodies toward the components of the seasonal vaccine that largely remained stable after pandemic vaccination (Fig. 5B and C). Titers of pdmH1N1 neutralizing antibodies before immunization with the pandemic vaccine did not differ from those in not prevaccinated individuals (p = 0.22). In line with cellular immunity, those titers showed an increase after pandemic vaccination, but this was less pronounced (p = 0.047, Fig. 5C) and did not reach the level of those in individuals that had not been prevaccinated against seasonal influenza. Nevertheless, all individuals turned seropositive as defined by a titre above 1:40. Interestingly, there is a correlation between the increase of pdmH1N1-specific antibody and T-cell responses in individuals not prevaccinated (Spearman r = 0.78, p = 0.02), whereas this did not reach statistical significance in individuals with prior seasonal vaccination. Taken together, pandemic vaccination induces a pdmH1N1-specific antibody response with a certain extent of cross-reactivity toward seasonal H1N1 antigens, although this effect was less pronounced than that in T cells.
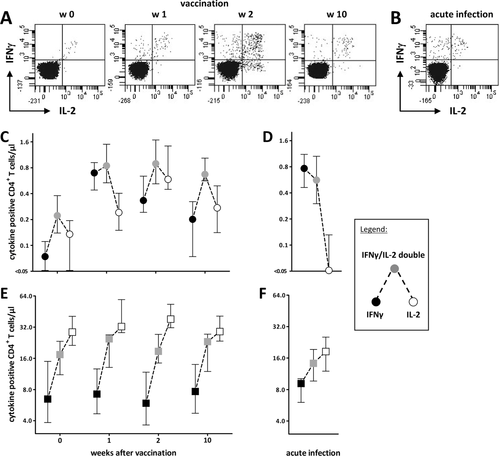
Dual-cytokine-expressing cells decrease in patients with acute infection
Previous studies have indicated that multifunctionality of antigen-specific T cells correlates with pathogen control, whereas acute viral or bacterial infections are associated with changes in cytokine expression profiles of antigen-specific T cells 11, 13. To examine whether this is also the case in control of pandemic H1N1 influenza virus, we comparatively analyzed the IFN-γ/IL-2 expression profiles of specific T cells induced after vaccination with those of seven patients who were suffering from a symptomatic infection with pandemic H1N1 influenza. Whole blood was stimulated with the pandemic antigen as described before, and CD4+ T cells were analyzed flow cytometrically for single or double expression of IFN-γ and IL-2. Stimulation with SEB served as positive control for cells with specificities unrelated to influenza. Representative dotplots of influenza-specific IFN-γ/IL-2 cytokine profiles before and after vaccination and in an acutely infected patient tested within 1 week of symptoms are shown in Fig. 6A and B. Interestingly, the vaccine-induced T-cell response was generally dominated by CD4+ T cells coexpressing both IFN-γ and IL-2, while single-cytokine-producing cells were less abundant (Fig. 6C). Among the latter, those exclusively expressing IL-2 generally dominated over those producing IFN-γ only. This ratio changed only 1 week after vaccination. Thereafter, the profile largely normalized to that found prior to vaccination (Fig. 6C). As shown in Fig. 6D, this cytokine profile was markedly different from that found in patients with acute infections, where IFN-γ only expressing cells were most abundant and IL-2 single producing cells were completely absent. These profiles were indeed influenza specific, as the cytokine profiles of SEB-reactive CD4+ T cells were unaffected by vaccination (Fig. 6E) or acute infection (Fig. 6F). This indicates that the pandemic vaccine induces polyfunctional T cells that differ from the more restricted functionality of T cells in patients with acute infection.
Discussion
The vaccine Pandemrix was developed in response to the pandemic H1N1 influenza outbreak in 2009 and has proven to be highly immunogenic in its potential to induce protective antibodies 14. Up to now, knowledge on the concomitant induction of pdmH1N1-specific cellular immune responses and potential T-cell cross-reactivity toward seasonal antigens is limited. In this study, a detailed quantitative and qualitative characterization of T-cell immunity toward pandemic H1N1 antigens was performed both in healthy individuals before and after pandemic H1N1 vaccination as well as in patients with acute pandemic H1N1 influenza infection. We showed that the vaccine led to a strong induction of both antibodies and T cells that mainly comprised Th1 cells. Besides induction of pdmH1N1-specific immunity, the pandemic vaccine led to a concomitant increase in T cells specific for components of the seasonal influenza vaccine, indicating cross-reactivity toward seasonal antigens. Moreover, prior contact with related antigens causes a preexisting immune condition that compromises a strong induction of vaccination responses, as individuals that had been immunized with the seasonal vaccine 2–3 weeks before pandemic vaccination showed a less pronounced increase of influenza-specific CD4+ T cells compared with those that had not been prevaccinated. Finally, we observed differential cytokine expression patterns of specific T cells during active H1N1 infection and after vaccination. In that respect, the predominantly dual-positive cytokine profile of vaccine-induced T cells may suggest sufficient functionality to confer successful virus control.
It may seem counterintuitive that individuals with a preexisting immunity toward seasonal influenza strains show a less pronounced induction of specific T cell and — to a minor extent — antibody responses after pandemic vaccination. However, similar circumstances may apply to other antigenic specificities. A BCG vaccination study that has compared vaccination outcomes in a Mycobacterium tuberculosis high-prevalence (Malawi) versus low-prevalence country (UK) has shown that vaccinees from Malawi not only had higher prevaccination levels of M. tuberculosis-reactive T cells, but also a less pronounced increase after vaccination 15. The same observation was made for induction of antibody responses in large trials of influenza vaccine effectiveness, where individuals with a history of influenza vaccination within the last 3 years showed a decreased induction of specific antibodies after the actual vaccination 16, 17. Thus, in general terms, continuous environmental exposure with pathogens and/or vaccines induces a specific immune response that may negatively affect subsequent induction of specific immunity toward related antigens. In addition, our data are in line with recent observations from antigen-specific CD8+ T cells after seasonal influenza vaccination, where repeated prevaccination with the same seasonal influenza strain resulted in limited boosting of humoral and cellular CD8+ T-cell responses, whereas respective responses toward new seasonal strains were more pronounced 18. Likewise, antigen-specific CD4+ T cell levels after live viral vaccination with seasonal influenza were lower in individuals that had higher levels of influenza virus-specific T cells prior to vaccination as compared to individuals with lower baseline levels 19.
Preexisting immunity toward H1N1 antigens may be due to some exposure to the pandemic strain, although none of our vaccinees had any typical symptoms. Given that our study was conducted before the actual pandemic influenza wave in the Saarland region in Germany, preexisting immunity toward H1N1 antigens likely is based on cross-reactivity of CD4+ T cells specific for either seasonal vaccines or prior infections with seasonal strains. The existence of cross-reactivity is further supported by the fact that induction of specific immunity after pandemic H1N1 vaccination was paralleled by a concomitant induction of specific immunity toward seasonal antigens; notably, this was even observed in individuals that had not been prevaccinated with the seasonal vaccine. Alternatively, the T-cell response induced after pandemic vaccination could be directed at internal antigens derived from a common PR8-derived viral backbone that was used to produce both the pandemic and the seasonal vaccine. As hemagglutinins of the respective field strains were the predominant components of the two vaccines (90%), a significant contribution of PR8-derived antigens is considered rather unlikely. However, based on sequence similarity, neuraminidase (approximately 8% of vaccine proteins) may also contribute to the effect of cross-reactivity. Our observations are consistent with recent cross-sectional studies that have investigated CD4+ T-cell responses toward autologous cells infected with the pandemic H1N1 strain 20 or a selected panel of HLA-DR4 binding peptides derived from pandemic H1N1 proteins with substantial homology to immunodominant epitopes of a seasonal H1N1 influenza strain 21. Fifteen of the 17 analyzed peptides were able to stimulate a CD4+ T-cell response in individuals that had received seasonal influenza vaccine within the past 5 years but had no clinical history of pdmH1N1 infection. This was not exclusively due to de novo priming, as the main proportion of those T cells was expressing memory markers 21. Interestingly, cross-reactivity seems to be a typical feature of cellular immunity, as neutralizing antibody titers specific for components of the seasonal vaccine were only marginally induced after pandemic vaccination. This difference in the extent of cross-reactivity between cellular and humoral immunity may be directly related to the requirements for antigen recognition. Whereas T cells only require small peptides of a few amino acids presented by MHC molecules, antibodies frequently recognize larger motifs or whole proteins and are therefore more sensitive for strain-specific differences in individual epitopes. In line with this evidence, only 31% of the B-cell epitopes of recently circulating seasonal strains are conserved in pdmH1N1 whereas as high as 41% of CD4+ and 69% of CD8+ T-cell epitopes were identical 22, thereby providing a direct molecular basis for a higher degree of cross-reactivity among T cells.
Animal models have provided evidence for a protective role of cross-reactive T-cell immunity in pandemic H1N1 infection. The first US isolates of the pandemic H1N1 strain caused pathological lesions in the lungs of infected immunologically naïve mice, ferrets, and nonhuman primates that were more severe than those caused by seasonal H1N1 viruses 23. However, disease severity was much more reduced if animals were primed with seasonal influenza strains before challenge with the pandemic strain 24-26. Likewise, transmission of the pandemic H1N1 strain among guinea pigs was partially disrupted if animals had preexisting immunity to seasonal H1N1 strains 27. This protective effect was largely due to cellular immunity, as sera from primed mice did not confer protection in vivo and T-cell depleted mice failed to control infection 24, 26. Unlike neutralizing antibodies, T cells do not directly prevent infection; nevertheless, they contribute to blunting its severity by clearance of infected cells or mediating induction of humoral immunity. This may have direct implications for humans. Preexisting humoral immunity with cross-protective potential toward pandemic influenza is largely restricted to individuals born before 1957, whereas antibodies induced after seasonal vaccinations were not cross-reactive 5. Nevertheless, based on surveillance data, immunization with seasonal vaccines may have contributed to protection toward pandemic influenza even in individuals born after 1957 7, 8. This further emphasises the particular role of cross-protective cellular immunity and may provide a direct explanation for the considerably low disease severity of the 2009 H1N1 pandemic. In this respect, it is interesting to note that a large percentage of fatal cases was found among children and young adults 2, 28, 29 who may likely have been naïve for both specific humoral and cellular immunity. As a consequence, the less pronounced induction of pdmH1N1-specific T cells after pdmH1N1 vaccination in seasonally vaccinated individuals may indicate a beneficial effect of T cell cross-reactivity to control pandemic H1N1 strains. A protective effect of cross-reactivity is supported by a recent study in humans where preexisting CD4+ T cells induced by previous seasonal influenza encounters reduced virus shedding and severity of infection after challenge with the seasonal H3N2 or H1N1 subtypes 30. Of note, protection was not necessarily associated with a strong induction of specific immunity, as the magnitude of T-cell induction rather correlated with increased viremia and illness score 30. As viral control occurred even in the absence of specific antibodies 30, this emphasizes the particular role of CD4+ T cells in mediating cross protection.
When comparatively assessing functional properties of cellular immunity in vaccinated healthy individuals and in patients with active influenza disease, we provide first evidence that the cytokine profile of influenza-specific CD4+ T cells differs in both groups, although the overall number of individuals that was studied is still small. Differential cytokine expression profiles may in part be due to differences in the route of antigen exposure. T-cell responses induced by live replicating viral infection can be modulated by pathogen-associated molecular patterns that may be distinct from effects induced by adjuvants used in subunit protein immunization 31. In general terms, the dominance of IFN-γ/IL-2 dual-positive T cells found in vaccinated individuals is a typical functional property of specific T cells toward other pathogens that are well controlled 11, 13. In contrast, the relative loss of IL-2 production and the shift in the cytokine profile toward cells expressing IFN-γ only in patients with influenza disease is reminiscent of that found in patients with active cytomegalovirus infection 13 or active tuberculosis 11 and may therefore represent a general feature of specific immunity in patients with impaired pathogen control. This may directly relate to the functional role of IL-2 as a T-cell autocrine factor and indicates a decreased ability for expansion of influenza-reactive T cells in response to viral antigens. Of note, the changes in cytokine profile were influenza specific, as that of SEB-reactive CD4+ T cells did not differ in vaccinated individuals and patients. Thus, as with immunity toward other well-controlled pathogens, the predominance in dual-cytokine-expressing T cells induced after pandemic vaccination may indicate sufficient functionality to confer protection. At present, we do not have any longitudinal analyses in acutely infected patients or any comparative analysis with mildly symptomatic individuals. Therefore, the impact of time postinfection or of various stages of disease severity on cytokine profiles cannot be assessed and merits further study with larger sample sizes. Nevertheless, although diagnosis of acute influenza is largely guided by nucleic acid testing and clinical symptoms, influenza-specific cytokine profiling may have potential to be used to specifically identify patients with active influenza infection and to distinguish those from nonsymptomatic infections or vaccination responses.
Materials and methods
Study subjects
The study was conducted among 19 immunocompetent individuals in October/November 2009. Among them, ten persons (mean age 43.64 ± 10.05 years) had been prevaccinated against seasonal influenza (Begrivac 2009/2010, Novartis, Marburg, Germany) about 3 weeks before (mean 18.9±7.9 days), whereas nine (mean age 37.8 ± 15.9 years) had not. All individuals did not have any signs or symptoms of prior contact with pandemic influenza A/H1N1. They received a routine vaccination using one standard dose of the pdmH1N1 vaccine (Pandemrix, GlaxoSmithKline Biologicals s.a., Rixensart, Belgium). According to the manufacturers, both vaccines were produced using a common PR8-derived backbone, but hemagglutinin and neuraminidase were derived from the respective field strains and were the predominant components of both vaccine preparations (90% and approximately 8%, respectively, personal communication). The study was approved by the ethics committee and all participants gave written informed consent. Whole blood samples were drawn prior to vaccination with Pandemrix as well as 1, 2, and 10 weeks thereafter. To characterize cellular immunity in acute infection, additional whole blood samples of seven immunocompetent individuals (mean age 49.28 ± 11.07 years) who were newly diagnosed with influenza A/H1N1 infection on a clinical/epidemiological or virological basis were analyzed. All except one individual were hospitalized due to influenza-related symptoms and samples were collected within a median of 5 days (range 1–18 days) of symptom onset.
Quantitation and phenotypic analysis of influenza-specific CD4+ T cells
T cells from heparinized whole blood sample were stimulated in vitro for 6 h and a total of 450 μL blood was used per stimulatory reaction. Pandemic H1N1 antigen (pdmH1N1, 30 μg/mL HA content without adjuvant, derived from A/California (H1N1)v as was used in the pandemic vaccine Pandemrix, kindly provided by GlaxoSmithKline), and the seasonal influenza 2009/2010 vaccine Begrivac (Flu09, 30 μg/mL HA content; Novartis, Nürnberg, Germany) were used as stimuli to induce antigen-specific activation and cytokine expression. As negative and positive controls, cells were stimulated with phosphate buffered saline (PBS), and 2.5 μg/mL SEB (Sigma, Deisenhofen, Germany) respectively. All stimulations were carried out in the presence of 1 μg/mL anti-CD28 and anti-CD49d (clones L293 and 9F10 respectively; BD, Heidelberg, Germany), that increase the frequency of specifically stimulated T cells without concomitant increase in antigen nonspecific reactivity 32. Stimulation was carried out at 37°C at 6% CO2 for a total of 6 h. During the last 4 h, 10 μg/mL of Brefeldin A (Sigma) was added to block extracellular secretion of cytokines. Thereafter, the blood was treated with 2mM EDTA for 15 min. Subsequently, erythrocytes were lysed and leukocytes were fixed for 10 min using BD lysing solution (BD). Cells were washed once with FACS buffer (PBS, 5% filtered FCS, 0.5% BSA, 0.07% NaN3) and immunostained in FACS buffer containing 0.07% saponin (Sigma) using the following antibodies: anti-CD4 (clone SK3), anti-CD69 (clone L78), anti-IFN-γ (clone 4S.B3), anti−IL-2 (clone MQ1-17H12), anti-IL-17A (clone SCPL1362), anti-CD152 (CTLA-4, clone BNI3; all from BD), and anti-CD127 (clone eBioRDR5, eBioscience, Frankfurt, Germany). CD4+ T cells were analyzed on a FACS Canto II using FACS Diva software V6.1.3 (BD). For analyses of antigen-specific CD4+ T-cells, frequencies of activated CD4+ T cells after stimulation with the antigen were subtracted for the negative control. The detection limit was 0.05% IFN-γ-producing CD69+ CD4+ T cells.
Quantitation of influenza-specific antibody responses
Kinetics of pdmH1N1-specific IgG induction was analyzed using a standard ELISA (Virotech, Rüsselsheim, Germany), according to the manufacturer's instructions. Moreover, the presence of virus-neutralizing antibodies against pdmH1N1 and against the individual components of the seasonal influenza vaccine (H1N1seasonal, H3N2, Influenza B) was determined by respective microneutralization assays based on previously described procedures 33, 34. In detail, heat-inactivated sera were used to prepare duplicate serial dilutions from 1:10 to 1:2560 in virus diluent (EMEM/0.1% antibiotics/2% FBS, Biochrom, Germany) and mixed with an equal volume of virus diluent containing influenza A or B virus, the same strains that have been used for vaccine preparation (NYMC X-179A, H1N1 derived from A/California/7/2009; IVR-148, H1N1 derived from A/Brisbane/59/2007; NYMC X-175C, H3N2 derived from A/Uruguay/716/2007; or influenza B virus, B/Brisbane/60/2008) at 2 × 103 tissue culture infection dose 50/mL, incubated for 90 min (37°C, 5% CO2) in a moist chamber and 100 μL were transferred to Madin-Darby canine kidney cell monolayers (ECCC, Salisbury, UK) in a 96-well plate with appropriate controls. After 1 h incubation, 100 μL of overlay medium (1:1 virus diluent/3.2% carboxymethylcellulose, Sigma) was added to each well for 24–28 h. Monolayers were fixed with ice cold 40% acetone/60% methanol for 10 min. After blocking with 1% bovine serum albumin/0.1% thimerosal (Sigma) in wash buffer (137 mM NaCl, 20 mM Na2HPO4, 2.7 mM KCl and 1 mM K2HPO4, pH 7.3) for 30 min at 37°C, 5% CO2, fixed cells were incubated with anti-influenza A or anti-influenza B monoclonal antibody to nucleoprotein (MAB8251 and MAB8661, Millipore, Schwalbach, Germany). Foci were revealed by horseradish peroxidase-labeled rabbit anti-mouse IgG and counted using an automated ELISPOT instrumentation (AID Diagnostika GmbH, Straßberg, Germany). The neutralizing antibody titer was expressed as the reciprocal of the highest dilution that reduced the number of immunostained foci to 50% or less of the control value.
Statistical analysis
Statistical data analysis was carried out using GraphPad Prism V5.04 (Graphpad, San Diego, CA, USA). The unpaired t-test or the nonparametric Mann–Whitney test was used to compare results from two groups. The nonparametric Kruskal–Wallis test was used to compare results from nonpaired analyses over time. Paired analyses were performed using the Friedman test.
Acknowledgments
This study was supported by a grant from the “Förderverein zur Bekämpfung der Viruskrankheiten e.V.” The authors thank Candida Guckelmus for excellent technical assistance and Prof. Christoph Lange (Research center Borstel, Germany) for critical review of the manuscript.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
Abbreviations
-
- Flu09
-
- influenza vaccine
-
- IQR
-
- interquartile range
-
- pdmH1N1
-
- pandemic H1N1
-
- SEB
-
- Staphylococcus aureus enterotoxin B