Retinoic acid alleviates Con A-induced hepatitis and differentially regulates effector production in NKT cells
See accompanying Commentary by Nagy
Abstract
Retinoic acid (RA) is a diverse regulator of immune responses. Although RA promotes natural killer T (NKT) cell activation in vitro by increasing CD1d expression on antigen-presenting cells (APCs), the direct effects of RA on NKT-cell responses in vivo are not known. In the present study, we demonstrated the effect of RA on the severity of Con A-induced hepatitis and molecular changes of NKT cells. First, we demonstrated that Con A-induced liver damage was ameliorated by RA. In correlation with cytokine levels in serum, RA regulated the production of IFN-γ and IL-4 but not TNF-α by NKT cells without influencing the NKT-cell activation status. However, RA did not alleviate α-GalCer-induced liver injury, even though it reduced IFN-γ and IL-4 but not TNF-α levels in serum. This regulation was also detected when liver mononuclear cells (MNCs) or NKT hybridoma cells were treated with RA in vitro. The regulatory effect of RA on NKT cells was mediated by RAR-α, and RA reduced the phosphorylation of MAPK. These results suggest that RA differentially modulates the production of effector cytokines by NKT cells in hepatitis, and the suppressive effect of RA on hepatitis varies with the pathogenic mechanism of liver injury.
Introduction
Liver damage induced by various agents, such as viral infection, results in serious problems accompanied by an excessive immune response 1. Uncontrolled severe responses in the liver by immune cells are observed in diverse animal models, including Con A-induced hepatitis. Following the administration of Con A, T cells, granulocytes, and Kupffer cells infiltrate into the liver, resulting in the death of hepatocytes 2-4. NKT cells are responsible for liver injury in this model 5-10. NKT cells are a distinct T-cell subset with an invariant T-cell receptor (TCR) that recognizes glycolipids loaded on the cell-surface protein CD1d, and they rapidly secrete cytokines upon stimulation 11-14. In Con A-induced liver injury, inflammatory cytokines, such as IFN-γ, TNF-α, and IL-4, from NKT cells are pathogenic 5, 7, 9, 10. In addition, a specific ligand of NKT cells, α-GalCer, can induce liver injury mediated by FasL and TNF-α rather than IFN-γ 15-17. Although NKT cells are critical to induce both Con A- and α-GalCer-induced liver injury, their pathologic mechanisms are different from each other.
Retinoic acid (RA), an active metabolite of vitamin A, regulates various diseases through anti-tumor and anti-inflammatory effects 18, 19. RA is associated with anti-inflammatory effects in diverse diseases 20. RA also enhances T-cell effector responses and is critical in vaccine responses 21-25. These contradictory findings imply that the exact physiological function of RA remains to be discovered. RA promotes the proliferation and activation of NKT cells indirectly in vitro by increasing CD1d expression in APCs 26-28. However, the direct effects of RA on NKT cells and NKT cell-dependent diseases in vivo have not been examined. In the current study, we observed that RA differentially regulates NKT cell-mediated hepatitis and cytokine production by NKT cells. Our results suggest the selective regulatory effects and the therapeutic potential of RA in NKT cell-dependent diseases.
Results
RA regulates Con A-induced hepatitis and differentially regulates cytokine levels in serum
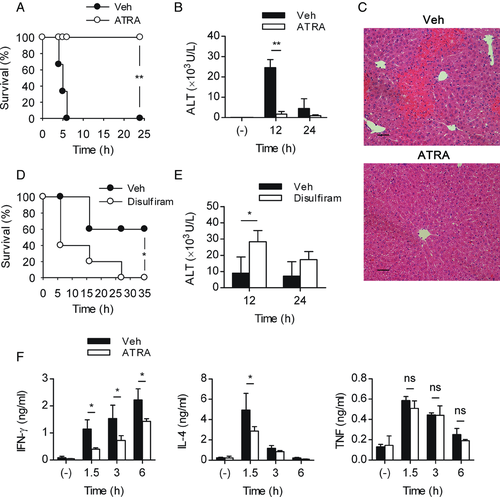
To determine the effect of RA itself, we injected RA directly into normal mice, and liver injury was induced by injecting Con A. The RA-treated group had a 100% survival rate, whereas the entire control group succumbed to the lethal dose (30 mg/kg) several hours after the Con A injection (Fig. 1A). In addition, when the ALT activity was measured in animals with nonlethal (20 mg/kg) Con A-induced hepatitis, significantly less ALT activity was observed in the RA-treated group (Fig. 1B). And also, liver histology showed massive necrosis in vehicle-treated mice, but in RA-treated mice, the liver tissue maintained the structure (Fig. 1C). Treatment with disulfiram, a blocking agent of RALDH that synthesizes RA, aggravated the survival rate and serum ALT activity, indicating the protective effect of endogenous RA against Con A-induced hepatitis (Fig. 1D and E). The pathogenesis and maintenance of Con A-induced liver injury is mediated by inflammatory cytokines, such as IFN-γ, IL-4, and TNF-α 5, 7, 9, 10. Interestingly, treatment with RA reduced the levels of IFN-γ and IL-4 in serum significantly but failed to affect the level of TNF-α (Fig. 1F). These data show that RA regulates Con A-induced hepatitis and that this effect is correlated with IFN-γ and IL-4 levels in serum.
Differential regulation of cytokine production from hepatic NKT cells by RA
Since NKT cells are responsible for early cytokine production in Con A-induced hepatitis 7, 8, the production of each effector cytokine in NKT cells was analyzed. As with the cytokine levels in serum, RA reduced the percentage of IFN-γ- or IL-4-producing NKT cells but not TNF-α-producing NKT cells (Fig. 2B and C). Conventional T cells did not seem to be critically involved in the reduced cytokine level (Fig. 2A and D, and Supporting Information Fig. 2). In the RA-treated group, NK cells included a considerably reduced percentage of IFN-γ-producing cells 6 hours postinjection compared to the control, but they were not required for the regulation or pathogenicity of liver injury (Fig. 2E and Supporting Information Fig. 3A). The percentage of IL-4- or TNF-α-producing T or NK cells was below 1% (data not shown). Furthermore, we found that Treg cells, which can be induced by RA, were not altered by treatment of RA and they were dispensable in the protective effect of RA on hepatitis (Supporting Information Fig. 3B–E). Our observations indicate that NKT cells can play a predominant role in the regulation of cytokine production and the modulation of liver injury by RA.

Treatment with RA does not modulate the activation of NKT cells or the expression of FasL
The suppression of cytokine-producing NKT cells by RA could be caused by an impaired activation of NKT cells. We therefore sought to determine if the observed effects of RA resulted from the inhibition of NKT-cell activation. The population of NKT cells in the liver rapidly decreases in Con A-induced hepatitis 8, which may be considered a parameter of NKT-cell activation. NKT-cell populations decreased shortly after Con A injection, with no significant difference between the RA and control groups (Fig. 3A and B). In addition, the expression of CD69 and CD25 showed no difference before or after Con A injection between the two groups (Fig. 3C and D).

Some studies have suggested that FasL, which is upregulated upon stimulation in NKT cells, may act as an effector molecule during liver injury, even though such a role is controversial in Con A-induced hepatitis 29, 30. We observed that the expression of FasL on the surface of NKT cells after injection of Con A was similar between the two groups (Fig. 3C and D). Collectively, these data indicate that RA does not modulate the activation of NKT cells.
Next, we examined the effects of RA on other cells, such as Kupffer cells and other APCs that might participate in the regulatory effects of RA on NKT cells. As illustrated in Fig. 3E, the percentages of Kupffer cells before and after Con A injection were comparable in each group (Supporting Information Fig. 4A). In addition, RA tended to reduce ALT activity in Kupffer cell-depleted mice (Supporting Information Fig. 4B). Moreover, the expression of costimulatory molecules or CD1d was not modulated by RA (Fig. 3F and Supporting Information Fig. 4C). Overall, these data indicate that treatment with RA reduces IFN-γ and IL-4 but not TNF-α production in NKT cells without affecting Kupffer cells or other APCs.
RA treatment reduces cytokine levels but not liver injury in α-GalCer-induced hepatitis
We next examined whether RA could also regulate α-GalCer-induced hepatitis. Consistent with Con A-induced hepatitis, RA reduced the levels of IFN-γ and IL-4 but not TNF-α in α-GalCer-induced hepatitis (Fig. 4A). Although α-GalCer-induced hepatitis is mediated by activated NKT cells, its pathogenic mechanism is not consistent with Con A-induced liver injury. For example, whereas TNF-α is important in both liver injury models, IFN-γ is critical in Con A-induced hepatitis but not in α-GalCer-induced hepatitis 17, 30. We found that treatment with RA failed to regulate α-GalCer-mediated liver injury, with comparable ALT levels to the control (Fig. 4B), correlating with an unaltered level of TNF-α (Fig. 4A). These results indicate that RA can alleviate Con A-induced hepatitis but not α-GalCer-induced hepatitis. The differential regulation of RA on cytokine production can explain the contrary effects of RA in two hepatitis models.

RA directly regulates the secretion of IFN-γ and IL-4 from NKT cells through RAR-α
The observations described above led us to hypothesize that RA acts on NKT cells directly. Therefore, we examined the effects of RA on liver MNC cultures in vitro to exclude the environmental factors present in the liver. Consistent with the in vivo results, in the presence of RA, the secretion of IFN-γ and IL-4 but not TNF-α was reduced compared to vehicle in the presence of Con A or α-GalCer stimulation (Fig. 5A and B). RA has been suggested to act upon various cell types via its specific receptors. In NKT cells, the mRNA expression of RAR-α, RAR-γ, and RXR-α was detected (data not shown), and the addition of Ro 41–5253, a specific RAR-α antagonist, recovered the suppression of IFN-γ and IL-4 secretion induced by RA (Fig. 5C). Taken together, these results indicate that RAR-α mediates the regulation of cytokine production by RA.
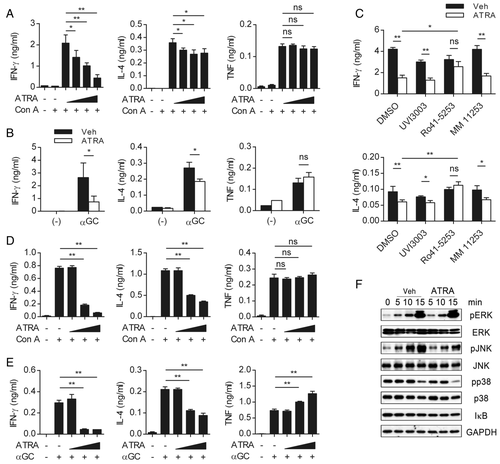
Next, to determine if RA directly affects NKT cells, the CD1d-expressing NKT-cell line DN32.D3 was stimulated with Con A or α-GalCer in the presence of RA (Supporting Information Fig. 5A). As shown in Fig. 5D and E, the secretion of IFN-γ and IL-4 but not TNF-α was reduced by RA. The mRNA expression was consistent with the quantitation data of the secreted cytokines (Supporting Information Fig. 5B). Because TNF-α production, which is regulated by NFAT, was not reduced by RA, we examined the changes in other signaling molecules that are activated upon TCR stimulation. As a result, the phosphorylation of MAPK, especially JNK, was reduced by RA (Fig. 5F). We measured the amount of IκB, as an indicator of NFκB signaling, by western blot, and it was not influenced by RA. Therefore, these data suggest that RA regulates cytokine production in NKT cells directly, the mechanism of which might include a modification of MAPK signaling pathway.
Discussion
In the current study, we demonstrated, for the first time, how RA regulates NKT cell-mediated diseases and NKT-cell responses in vivo. We showed that RA ameliorated Con A-induced hepatitis but not α-GalCer-induced hepatitis. This distinct role of RA can be explained by the finding that RA differentially regulated the secretion of various pathogenic cytokines from NKT cells, with unaltered NKT-cell activation. Mechanistically, our observations indicate that RA affects NKT cells directly by modulating signaling molecules such as RAR-α and MAPK.
We first attempted to examine the influence of endogenous RA using vitamin A-deficient mice; however, the results did not correlate with the data obtained from RA-pretreated animals (unpublished observation). We found that RA deficiency affected the activation status of cells in naïve mice by an unknown mechanism. RA signaling is biphasic and has the potential to display opposite effects in various models 20-25. These controversial findings have not been explained completely, and our observations and future studies may explain this discordance. In this study, to minimize the effect of vitamin A-deficiency on NKT cells, disulfiram was used to pretreat the animals for 3 days to reduce the amount of endogenous RA. Aggravated liver injury was observed in disulfiram-treated mice, demonstrating the regulatory role of endogenous RA in Con A-induced hepatitis (Fig. 1D and E). Disulfiram can induce liver injury by hitherto unknown mechanisms when it is administered to treat alcohol abuse 31, 32. Our observations suggest that a defect in RA synthesis via disulfiram treatment might cause the liver to become susceptible to inflammation and increase liver injury in patients. In addition, we speculate that RA supplementation might be useful to protect against disulfiram-induced liver injury.
The present study shows that the regulatory effect of RA is restricted to liver injury induced by Con A but not α-GalCer. We also demonstrated that RA regulates IFN-γ and IL-4 but has no effects on TNF-α in Con A-induced hepatitis or α-GalCer-induced hepatitis. NKT cells mediate the liver injury caused by Con A and by α-GalCer, but by different mechanisms. Several papers have demonstrated differences in the levels of effector cytokines between Con A-induced hepatitis and α-GalCer-induced hepatitis 17, 30. Although the papers could not demonstrate the cellular and molecular mechanism of how the same cytokine can function differently in two hepatitis models, they showed that IFN-γ was dispensable in α-GalCer-induced hepatitis but critical in Con A-induced hepatitis. Several possibilities might explain this difference between Con A-induced hepatitis and α-GalCer-induced hepatitis. For example, CD1d-expressing antigen presenting cells could counteract tissue-destructive effect of IFN-γ in α-GalCer-induced hepatitis via an unknown mechanism. In fact, the decrease of IFN-γ production does not ameliorate liver injury in α-GalCer-induced hepatitis. Moreover, the previous studies have established that α-GalCer-induced hepatitis is dependent on TNF-α 17, 30. We observed that the treatment of RA did not alter liver injury induced by α-GalCer (Fig. 4B). This observation supports that RA does not reduce TNF-α production of NKT cells and that RA does not inhibit activation of NKT cells. RA regulated effector cytokines in the same manner in both hepatitis models. That is, the production of IFN-α and IL-4 was inhibited by RA but not TNF-α upon stimulation with Con A or α-GalCer. We speculate that the differential effect of RA treatment on the two hepatitis models is because of the difference of the pathologic effect of each cytokine in each model via an unknown mechanism. It is unclear how the pathogenic aspects of the same molecule in the liver have different effects. However, our observations expand the understandings on α-GalCer- and Con A-induced hepatitis. More important, the differential regulatory effects of RA could be important for the possible clinical application of RA to prevent potential liver damage.
RA skews conventional T cells toward a Th2 response in vitro 33-36. In our study, RA reduces the production of IFN-γ and IL-4 both in NKT cells (Fig. 5). Moreover, MAPK was affected by RA, but other TCR signaling molecules were not. The addition of RA during the initial stimulation suppresses Th1 and Th2 development, suggesting the involvement of AP-1 inhibition 33. Although we did not show any inhibition of AP-1 by RA directly, AP-1 activity might be affected by RA via reduced MAPK activity in NKT cells. In addition, the genes regulated by NFAT differ depending on the cooperative recruitment of AP-1 37-39. These findings might explain the differential regulation of cytokine production by RA, as TNF-α expression can be activated by NFAT without AP-1 activation, in contrast to IFN-γ and IL-4. Thus, RA might be able to change the balance of AP-1 and NFAT activity during T-cell activation, resulting in expression changes of specific genes.
In summary, RA ameliorated Con A- but not α-GalCer-induced liver injury. This protective effect of RA specific to Con A-induced hepatitis may be due to the different molecular mechanism of the liver injuries. According to our results, RA has therapeutic potential in protecting against liver damage by various agents, especially in the case of fulminant hepatitis. However, before administering therapy with RA, the pathogenic mechanism of specific hepatitis needs to be considered.
Materials and methods
Mice
Six- to 8-week-old female C57BL/6 mice were purchased from Orient Bio. All mice were bred and maintained in specific pathogen-free conditions. All studies conformed to the principles for laboratory animal research outlined by Seoul National University (Seoul, Korea).
Reagents
α-GalCer, kindly provided by Dr. Sanghee Kim (Seoul National University, Seoul, Korea), was dissolved in 0.5% Tween 20 in saline 40. ATRA (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in DMSO, further diluted in olive oil for injection, and 35 mg/kg of RA was intraperitoneally (i.p.) injected into the mice 16 h before injecting Con A or α-GalCer. Disulfiram was dissolved in DMSO, further diluted in olive oil, and injected i.p. at a concentration of 10 mg/kg. The antagonist of RAR-α (Ro 41–5253) was purchased from Enzo Life Science (NY, USA), and the antagonists against RAR-γ (MM11253) and RXR (UVI3003) were purchased from Tocris Bioscience (Bristol, UK). They were dissolved in DMSO.
Flow cytometry and intracellular cytokine staining
Intracellular staining was performed with BD Cytofix/Cytoperm Plus (BD Biosciences, San Jose, CA, USA) according to the manufacturer's instructions without additional stimulation ex vivo. The antibodies were purchased from BioLegend (San Diego, CA, USA). The stained cells were analyzed with a FACSCalibur flow cytometer (BD Biosciences) and CellQuest Pro software (BD Biosciences).
Con A- or α-GalCer-induced hepatitis
Con A (Sigma-Aldrich) was dissolved in PBS and intravenously (i.v.) injected into the mice at a concentration of 20 mg/kg. For the survival study, the Con A dosage was increased to 30 mg/kg. The mice were euthanized after becoming moribund. For the disulfiram treatment study, the Con A dosage used for alanine aminotransferase (ALT) detection was 15 mg/kg and for survival monitoring was 17 mg/kg. The level of ALT was measured using Fuji-Dri Chem (Fuji Film, Tokyo, Japan) in accordance with the manufacturer's instructions. Five micrograms of α-GalCer was further diluted in PBS and i.v. injected into the mice. For histology analysis, livers were fixed in 10% formalin and embedded in paraffin. Sections were stained with H&E at Reference Biolabs (Seoul, South Korea). Anti-asialoGM1 (200 μg) was administered i.p. to mice, followed by ATRA treatment (35 mg/kg) 16 h before Con A i.v. injection. To deplete Kupffer cells, 200 μg of gadolinium chloride (GdCl3) was injected i.v. 24 and 36 h before administration of Con A. To deplete Treg cells, 300 μg of anti-CD25 (PC61) was injected i.p. 16 and 40 h before Con A injection.
Isolation of liver mononuclear cells
The liver MNCs were isolated as described previously 41. Briefly, cells in supernatants were resuspended in 40% Percoll (GE healthcare), overlaid on 70% Percoll and centrifuged for 30 min at 750 × g. Cells in interphase were collected and washed. Adhesive cells in liver were isolated with collagenase solution as described previously 30.
Cell culture
The liver MNCs (3.5 × 105 cells) and the DN32.D3 hybridoma cells (5 × 104 cells, provided by Dr. Albert Bendelac, the University of Chicago, USA) were incubated with Con A (5 μg/mL) or α-GalCer (200 ng/mL) for 24 h in the presence of 100 nM ATRA. The supernatants were collected for ELISA. For the antagonist assay, chemicals were used at a concentration of 4 μM, and ATRA was used at a concentration of 10 nM.
Cytokine ELISA
The levels of IFN-γ, IL-4, and TNF-α in serum or supernatants were evaluated with ELISA kits in accordance with the manufacturer's instructions (BD Biosciences).
Western blot analysis
Con A-stimulated DN32.D3 hybridoma cells in the presence of vehicle (DMSO) or ATRA were lysed with Triton lysis buffer. SDS-PAGE was performed on 8% polyacrylamide gels, and then proteins were transferred to PVDF membranes. Following blocking using 5% BSA buffer, the blots were incubated in the presence of primary Abs specific for pERK, ERK, pJNK, JNK, phospho-p38 MAPK, p38 MAPK, IκB (Cell Signaling Technology, MA, USA), and GAPDH (Abcam, Cambridge, UK), followed by HRP-conjugated goat anti-rabbit IgG. The membrane was developed using WEST-one reagent (iNtRON Biotechnology, Gyeonggi-do, Korea) and detected on an X-ray film. The membrane was stripped and reblotted.
Quantitative real-time PCR
Total RNA was extracted from cells using RNeasy kit (Qiagen) and reverse transcribed into cDNA using oligo-dT primers and MMLV reverse transcriptase (Roche). Quantitative real-time PCR was performed using an ABI 7500 (Applied Biosystems) and SYBR green PCR MasterMix (Fermentas). Primer sequences were as follows: for Hprt, 5′-AAGACTTGCTCGAGATGTCATGAA-3′ (forward) and 5′-ATCCAGCAGGTCAGCAAAGAA-3′ (reverse); for IFN-γ, 5′-AACCCACAGGTCCAGCGCCA-3′ (forward) and 5′-CACCCCGAATCAGCAGCGACT-3′ (reverse); for IL-4, 5′-GGGCTTCACAGGTGCTTCGC-3′ (forward) and 5′-TCCAGGACATCGAAAAGCCCGA-3′ (reverse); for TNF-α, 5′-GCCAGCCGATGGGTTGTACC-3′ (forward) and 5′-CTTGGGGCAGGGGCTCTTGA-3′ (reverse). The reaction conditions were 10 min at 95°C, followed by 15 s at 95°C, 30 s at 57°C and 30 s at 72°C for 45 cycles, and 30 min at 72°C. The comparative Ct method for relative quantification was used, and all of the expression levels of the target genes were normalized to the expression of Hprt.
Statistics
The results are expressed as the mean values ± SD. To compare the differences between two groups, Student's t-test was used. The Kaplan–Meier method was used to analyze the statistical significance of differences in survival time. The log-rank test (Mantel–Cox) was applied using SPSS 16.0 for Windows.
Acknowledgments
This study was supported by the Public Welfare & Safety Research program (20110020963) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology.
Conflict of interest
The authors declare no financial or commercial conflicts of interest.
References
Abbreviations
-
- α-GalCer
-
- α-galactosylceramide
-
- FasL
-
- Fas ligand
-
- ALT
-
- alanine aminotransferase
-
- ATRA
-
- all-trans retinoic acid
-
- RA
-
- retinoic acid
-
- RALDH
-
- retinaldehyde dehydrogenase
-
- RAR
-
- retinoic acid receptor
-
- RXR
-
- retinoid X receptor