Lineage extrinsic and intrinsic control of immunoregulatory cell numbers by SHIP
See accompanying Commentary by Corey et al.
Abstract
We previously showed that germline or induced SHIP deficiency expands immuno-regulatory cell numbers in T lymphoid and myeloid lineages. We postulated these increases could be interrelated. Here, we show that myeloid-specific ablation of SHIP leads to the expansion of both myeloid-derived suppressor cell (MDSC) and regulatory T (Treg) cell numbers, indicating SHIP-dependent control of Treg-cell numbers by a myeloid cell type. Conversely, T-lineage specific ablation of SHIP leads to expansion of Treg-cell numbers, but not expansion of the MDSC compartment, indicating SHIP also has a lineage intrinsic role in limiting Treg-cell numbers. However, the SHIP-deficient myeloid cell that promotes MDSC and Treg-cell expansion is not an MDSC as they lack SHIP protein expression. Thus, regulation of MDSC numbers in vivo must be controlled in a cell-extrinsic fashion by another myeloid cell type. We had previously shown that G-CSF levels are profoundly increased in SHIP−/− mice, suggesting this myelopoietic growth factor could promote MDSC expansion in a cell-extrinsic fashion. Consistent with this hypothesis, we find that G-CSF is required for expansion of the MDSC splenic compartment in mice rendered SHIP-deficient as adults. Thus, SHIP controls MDSC numbers, in part, by limiting production of the myelopoietic growth factor G-CSF.
Introduction
A role for SHIP in limiting immunoregulatory processes was initially revealed when it was found that a SHIP-competent, T-cell replete BM graft failed to mount a robust graft versus host (GVH) response in MHC-mismatched, SHIP-deficient hosts, resulting in improved transplant survival 1. We subsequently found that the number of myeloid-derived suppressor cells (MDSCs) 2, 3 and regulatory T (Treg) cells 4 are profoundly increased in secondary lymphoid tissues of SHIP−/− mice, and after ablation of SHIP expression in adult MxCreSHIPflox/flox mice. Graft versus host disease (GVHD) and organ graft rejection are primed by host APCs present in secondary lymphoid organs 5-8. MDSC and Treg cells can antagonize this process resulting in reduced GVHD 2, 3, 9, 10 or acceptance of allogenic organ grafts 11. Thus, a more complete understanding of how SHIP limits the numbers of these two key immunoregulatory cells in vivo might have important implications for clinical transplantation.
Several different hematolymphoid defects have been reported in SHIP-deficient mice 1, 12-18. In several cases, these genetic phenotypes have been shown to result from a cell autonomous role for SHIP in the affected cell type 17-20. However, others and we have documented instances where altered function on the part of a SHIP-deficient cell type impairs the function or development of a different cell type or lineage 21, 22. This appears to be due in large part to the altered production of key cytokines, growth factors, or chemokines that occurs in SHIP-deficient mice. SHIP−/− mice exhibit profoundly increased production of IL-6 17 and G-CSF 21 and greatly diminished SDF-1/CXCL12 production 21, all of which can contribute to altered function or localization of other cells, as seen specifically with hematopoietic stem cells (HSC) 21, 23, 24. The cellular and molecular basis of over- or underproduction of soluble factors in SHIP−/− mast cells, for example, indicate hyperactivation of NF-κB contributes to their increased production of IL-6 17.
Studies have revealed that, as anticipated, the primary role of SHIP in cell signaling is recruitment to receptor-signaling complexes where it can then oppose activation of PI3K effector kinases such as Akt by hydrolysis of the PI3K substrate, PI(3,4,5)P3 1, 13, 25. However, SHIP can also, in certain signaling contexts, “mask” cytoplasmic motifs on certain receptors and in so doing prevent the inappropriate recruitment of other phosphatases (for example SHP1) or PI3K 20, 26. This nonenzymatic role should be considered when attempting to decipher the role SHIP plays in signaling by a given receptor. Also, although largely considered to only exert a negative impact on cell function, survival, or proliferation; in some contexts, SHIP can also promote cellular functions. For example, analysis of NK cells in SHIP−/− mice indicated that SHIP is essential for target cytolysis and secretion of IFN-γ 1, 19, 20, 27, the two major NK-cell effector functions. Consistently, SHIP also promotes macrophage effector function 28 and cancer cell survival 29 by synthesis of its product, PI(3,4)P2, known to recruit GTPase Irgm1 28 and/or activate Akt 29-31.
We have shown that in SHIP-deficient hosts, a significant number of T cells of the naïve phenotype CD4+CD25− express the transcription factor, forkhead box P3 (FoxP3) and are suppressive, suggesting T-lineage intrinsic control of FoxP3 expression and suppressive function by SHIP. Uncertainty remains as to how SHIP deficiency promotes the expansion of these CD4+CD25−FoxP3+ T cells, as well as of conventional CD4+CD25+FoxP3+ Treg cells, and MDSC numbers. In the present study, we examine this question further and find evidence for lineage intrinsic control of MDSC numbers and function and that SHIPs control of G-CSF production plays a role in this regulation. However, SHIP exerts control over CD4+CD25−FoxP3+ T cells and conventional CD4+CD25+FoxP3+ Treg-cell numbers through both lineage extrinsic and intrinsic mechanisms.
Results
SHIP1 is essential for innate immune control of adaptive immunoregulatory cell numbers
Several groups have used the LysCre transgenic mouse to create myeloid-restricted deletion of floxed gene loci 32. We generated LysCreSHIPflox/flox mice to determine if myeloid-restricted deletion of SHIP would lead to increased numbers of MDSCs in peripheral lymphoid tissues and, in turn, promote the expansion of Treg cells. LysCreSHIPflox/flox mice appear to have an essentially normal life span as they typically live well over a year with no apparent health complications as opposed to germline SHIP−/− mice that typically succumb within 6–10 weeks of life 2, 12. LysCreSHIPflox/flox mice also fail to develop the myeloproliferative disease (MPD) reported in germline SHIP−/− and MxCreSHIPflox/flox mice 3, 12, as we fail to observe MPD-associated splenomegaly in LysCreSHIPflox/flox mice.
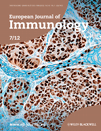
Analysis of spleen and lymph node (LN) tissue showed that the frequency of CD11b+Gr1+ MDSCs is significantly increased in both tissues of LysCreSHIPflox/flox mice relative to SHIPflox/flox controls (Fig. 1A). This increase is not pan-lineage, as developing myeloid cells that possess the same CD11b+Gr1+ phenotype as MDSCs in the periphery, are not significantly increased in the BM of LysCreSHIPflox/flox relative to SHIPflox/flox littermates (Fig. 1B). We also observe a significant decrease in the frequency of CD3+ T lymphocytes in spleen and LN tissue, although the absolute number of CD3+ T cells did not change (Fig. 1C). In contrast, the frequency and absolute number of Treg cells is significantly increased in both tissues (Fig. 1D). Importantly, Treg-cell frequency and absolute number was determined by measuring viable CD3+ T cells coexpressing CD4 and CD25. Alongside, an aliquot was stained similarly, fixed and then stained further for intracellular FoxP3 expression to confirm that 90–95% of CD3+CD4+CD25+ T cells also expressed FoxP3 and thus CD4+CD25+ T cells serve as an accurate representation of Treg cells (Fig. 1E). This was done for all instances in which Treg cells were analyzed. Furthermore, we confirmed that the T-cell compartment in LysCreSHIPflox/flox mice expressed normal levels of SHIP protein (Fig. 1F). Thus, the Treg-cell increase cannot be attributed to lineage-inappropriate ablation of SHIP expression in LysCreSHIPflox/flox mice. The increase in frequency in both myeloid and T-lymphoid immunoregulatory cell numbers we observe is physiologically relevant, as both LysCreSHIPflox/flox splenocytes and LN cells (Fig. 1G) exhibit significantly reduced capacity for priming of allogeneic T-cell responses by MHC-mismatched responder cells relative to SHIP-competent SHIPflox/flox controls. A similar increase in the immunoregulatory capacity of peripheral lymphoid organs is also observed in germline SHIP−/− mice 2, MxCreSHIPflox/flox mice after genetic ablation 3, and following treatment with a SHIP1 inhibitor 29. As anticipated, myeloid-restricted ablation of SHIP expression in LysCreSHIPflox/flox mice promotes an expansion of the MDSC compartment in secondary lymphoid tissues demonstrating that lineage intrinsic control of the production and/or survival of MDSCs are regulated by SHIP. The increased Treg-cell frequency that we also observe in these tissues indicates a myeloid cell that expresses SHIP, limits the frequency of Treg cells in peripheral lymphoid tissues in a lineage extrinsic fashion. Following cytokine administration or tumor formation, others have previously reported MDSCs can induce a significant increase in peripheral Treg-cell numbers 33, 34. Our findings demonstrate SHIP also limits this function of the myeloid compartment.
MDSCs isolated from naïve or tumor-bearing mice lack detectable expression of SHIP protein
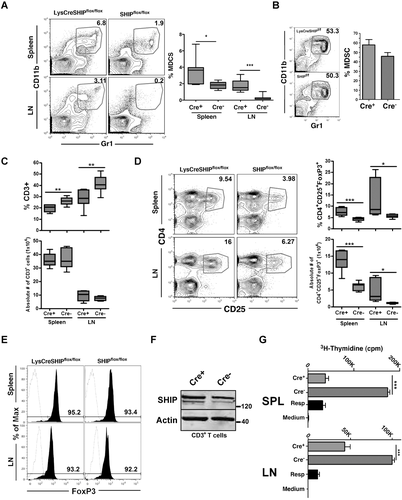
The expansion of MDSCs that we observe in LysCreSHIPflox/flox mice suggested that SHIP expression in these cells might oppose signals in these cells that limit their proliferation and/or survival in vivo. As an initial test of this hypothesis, we sorted MDSCs from SHIP+/+ spleen and analyzed SHIP expression by western blot (Fig. 2A). Surprisingly, SHIP protein expression was essentially undetectable in naïve MDSCs purified from spleen. In addition, we analyzed conventional MDSCs purified from the spleens and from the tumors of mice bearing MC38 tumors (Fig. 2A). MC38 tumors are known to harbor large number of potent MDSCs 35. As with naive MDSCs, these tumor-promoted MDSCs also lack detectable expression of SHIP. These findings indicate that MDSC expansion in LysCreSHIPflox/flox mice is unlikely to be the result of cell autonomous signaling by SHIP in MDSCs themselves, since they lack expression of SHIP. Thus, control of MDSC formation and/or survival by SHIP must be mediated by another cell type within the myeloid lineage that expresses SHIP. We further examined SHIP protein levels in immature CD11b+Gr1+ cells present in BM, as well as naïve T cells, Treg cells, and CD8+ T cells from normal mice (Fig. 2B). Although all T-cell lineages express SHIP, the immature myeloid cells in BM also lack detectable expression of SHIP.
T-lymphoid-restricted mutation of SHIP leads to expansion of Treg-cell numbers, but not MDSCs
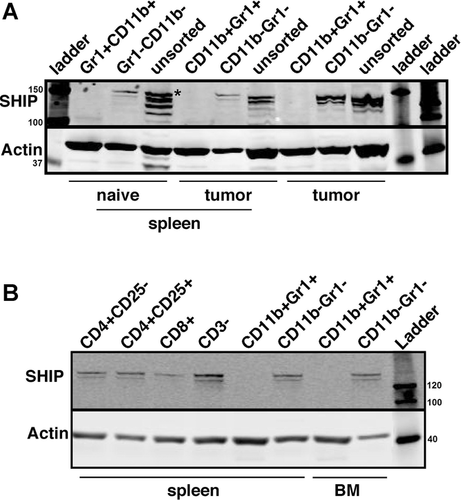
Efficient ablation of floxed loci in a T-lineage restricted fashion can be achieved with a Cre recombinase transgene driven by the Lck proximal promoter 36-39. We then developed LckCreSHIPflox/flox mice to assess whether T-lineage restricted ablation of SHIP expression also promotes expansion of Treg cells and whether these Treg cells might in turn facilitate an increase in the frequency of MDSCs in peripheral lymphoid tissues. As anticipated, the T-cell compartment in LckCreSHIPflox/flox mice exhibit significantly reduced expression of SHIP expression in T-lineage cells (Fig. 3A). We find there is a significant reduction in the frequency of splenic CD3+ T cells (Fig. 3B) and CD4+ and CD8+ T cells in the peripheral blood of LckCreSHIPflox/flox mice relative to SHIPflox/flox controls (Fig. 3C). The frequency of CD3+ T cells in LN tissue is unchanged relative to SHIPflox/flox littermates (Fig. 3B). Additionally, the absolute number of CD3+ T cells does not change in the spleen and LN tissue of LckCreSHIPflox/flox mice relative to SHIPflox/flox controls (Fig. 3B). In spite of this decreased frequency in total splenic and circulating T cells in both the CD4 and CD8 lineages, we observe a significant increase in the frequency and absolute number of Treg cells in both the spleen and LN tissue of LckCreSHIPflox/flox mice relative to SHIPflox/flox controls (Fig. 3D and E). In addition, there is a significant increase in the frequency and absolute number of T cells of the naïve CD4+CD25− phenotype that express FoxP3 in the spleen (Fig. 3F and G). These results indicate SHIP limits the expression of FoxP3 by CD4+CD25− T cells and homeostasis of CD4+CD25+ Treg cells in a T-lineage intrinsic fashion.
To determine if SHIP expression by a myeloid lineage cell might also limit expression of FoxP3 expression by CD4+CD25− T cells, we performed the same analysis in LysCreSHIPflox/flox mice and SHIPflox/flox littermate controls and find that myeloid-restricted SHIP deficiency also promotes inappropriate FoxP3 expression by CD4+CD25− T cells (Fig. 3F and G). This suggests that SHIP limits conversion of naïve T cells to FoxP3+ Treg cells in both a T-lineage intrinsic and extrinsic fashion, with the latter regulation pathway mediated by SHIP-expressing myeloid cell. However, this increased frequency of FoxP3+ CD4+25− T cells and CD4+CD25+ Treg cells in LckCreSHIPflox/flox mice does not promote a corresponding increase in MDSC frequency in peripheral lymphoid tissues (Fig. 3H and I). Thus, the ability of SHIP-deficient immunoregulatory myeloid and T lymphoid cells to influence each other's homeostasis and formation is unidirectional, with only SHIP-deficient myeloid lineage cells capable of translineage control of immunoregulatory cell numbers in another lineage.
G-CSFpromotes expansion of MDSCs in mice rendered SHIP-deficient
We previously showed there is a profound increase in production of the myelopoietic growth factor G-CSF in SHIP−/− mice 21. We postulated that this myelopoietic growth factor might promote the MDSC expansion and consequently expansion of Treg-cell numbers. To test this, we treated mice with neutralizing G-CSF-specific antibodies for 1 week after ablating SHIP expression using the MxCreSHIPflox/flox mouse model 3. Consistent with this hypothesis, anti-GCSF treatment prevented the expansion of the splenic MDSC compartment after induction of SHIP deficiency; however, the splenic Treg-cell expansion was still observed (Fig. 4A and B). Thus, expansion of MDSCs in the spleen, but not in the LNs of SHIP-deficient mice is dependent upon increased G-CSF production. However, the Treg-cell expansion that occurs in peripheral lymphoid tissues following induction of SHIP-deficiency in adults 4 is not prevented by G-CSF neutralization and thus not dependent upon increased G-CSF production or expanded numbers of MDSCs. In MxCreSHIPflox/flox mice where SHIP deficiency is systemic, the Treg-cell expansion likely results from the T-lineage intrinsic effects of SHIP deficiency, or increased production of another soluble or cell-bound ligand expressed by SHIP-deficient myeloid cells that is required for Treg-cell expansion, but which does not impact MDSC numbers.
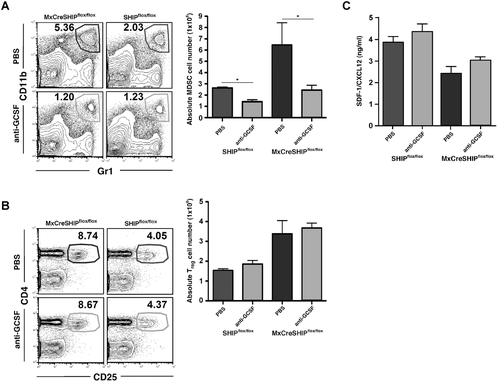
G-CSF can also suppress SDF-1/CXCL12 production by osteoblasts, which is critical for their support of quiescent HSC in the endosteal BM niche 40-42. We previously found that BM niche expression and plasma levels of SDF1/CXCL12 are substantially diminished in SHIP−/− mice and MxCreSHIPflox/flox mice following ablation of SHIP expression 21. We speculated that G-CSF neutralization might then prevent suppression of SDF-1/CXCL12 production following induction of SHIP deficiency. However, suppression of SDF-1/CXCL12 following induction was not prevented by G-CSF neutralization (Fig. 4C). These results indicate suppression of SDF-1/CXCL12 production following SHIP deficiency is not a result of increased G-CSF production and may reflect a direct role for SHIP in promoting expression of SDF-1/CXCL12.
Discussion
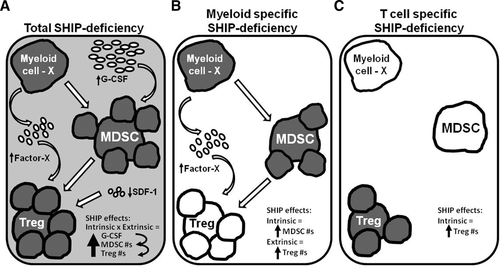
By selectively ablating SHIP expression in either myeloid or T-lymphoid lineage cells, we show here that SHIP exerts both lineage intrinsic and extrinsic control over the peripheral immunoregulatory cell compartment. Myeloid-restricted ablation of SHIP expression causes a significant expansion of MDSCs and SHIP-competent FoxP3+ T cells in both the spleen and LNs. Consistently, priming of allogeneic T-cell responses by these tissues is significantly compromised. However, we failed to find evidence for reciprocal regulation of MDSC numbers by SHIP-deficient T cells despite a significant increase in Treg cells and CD4+CD25−FoxP3+ T-cell frequency in LckCreSHIPflox/flox mice. Thus, SHIP possesses both lineage intrinsic and extrinsic control over peripheral Treg-cell accumulation; and lineage-intrinsic control over MDSC accumulation when specifically manipulating SHIP in the T-cell and myeloid compartments.
Surprisingly, we found that MDSCs from naïve mice or tumor-bearing mice lack detectable levels of SHIP protein expression. This suggests that regulation of MDSC formation occurs via a lineage-intrinsic mechanism mediated by another SHIP-expressing myeloid cell that perhaps produces a soluble factor that promotes peripheral MDSC expansion. Consistently, neutralization of G-CSF, which is significantly increased in SHIP-deficient mice, blocked splenic MDSC expansion in mice with induced SHIP deficiency. Furthermore, immature CD11b+Gr1+ myeloid progenitors in the BM lack SHIP expression, suggested that the myeloid cell that limits MDSC accumulation is a differentiated myeloid cell in the periphery. In addition, as seen with tissue macrophages 28, activation of SHIP expression may be required for myeloid cell differentiation and acquisition of effector function. Inability to activate SHIP expression may cause SHIP-deficient myeloid cells to default to cells that possess immunoregulatory rather than effector function. Conversely, inappropriate activity, such as increased production of G-CSF, by other SHIP-deficient myeloid cells may promote MDSC accumulation.
Previous studies have indicated a potential role for MDSCs in promoting expansion of Treg cells 2-4, 34. Although, our G-CSF neutralization studies suggest otherwise, MDSC-mediated Treg-cell expansion cannot be conclusively dismissed as a potential mechanism at play in our model of induced SHIP deficiency. Specifically, only splenic MDSC expansion, and not MDSC expansion in the LNs, was prevented after G-CSF neutralization. Possibly, MDSCs in the LNs mediated Treg-cell expansion in these mice. Otherwise, another myeloid cell population may be responsible for this effect on the Treg-cell compartment via a soluble factor or cell-bound ligand other than G-CSF, since G-CSF neutralization only prevented splenic MDSC expansion, and not Treg-cell compartment expansion (see model, Fig. 5). Also, the unaffected increase in peripheral Treg cells may have been due to their ineffective recruitment to their BM reservoir by SDF-1/CXCL12, which is suppressed in the BM of SHIP-deficient mice and was not reversed by G-CSF neutralization. Treg-cell migration to tissue sites, including the BM reservoir, is controlled by SDF-1/CXCL12 43, 44.
Tarasenko et al. 45 also examined SHIP's intrinsic role on T cells and reported results that are inconsistent with those shown here. Specifically, using CD4CreSHIPflox/flox mice, they found that T-cell specific deletion of SHIP had no affect on T-cell development, activation state, or Treg-cell numbers. The discrepancy may be because deletion of SHIP in CD4CreSHIPflox/flox mice may occur at a different time point during T-cell development compared to SHIP deletion in LckCreSHIPflox/flox mice. Nonetheless, the only supporting data provided was a table depicting the percentage of splenic FoxP3+ cells without distinguishing CD4 and CD25 expression, thus not being an accurate representation of the Treg-cell compartment. In fact, it was reported that 19–21% of splenocytes in CD4CreSHIPflox/flox mice were FoxP3+ that is an abnormally high percentage for splenic Treg cells. The more precise Treg-cell phenotype analysis provided here, it can be said that SHIP expression in the T lineage also limits the size of the Treg-cell compartment in vivo. In addition, experiments performed by Locke et al. 46 further support SHIPs intrinsic role in Treg development. Specifically, they showed that a significantly higher proportion of SHIP-deficient CD4+FoxP3− T cells acquired FoxP3 expression compared to WT T cells in an in vivo adoptive transfer experiment.
Because of SHIP's potential to influence many PI3K-mediated signaling pathways during the lifespan of a T cell, SHIP may control the Treg-cell compartment size by limiting the acquisition of FoxP3 expression by peripheral T cells (Treg-cell induction) and/or limiting the survival or proliferation of existing Treg cells in the periphery (Treg-cell homeostasis). Consistent with this hypothesis, NF-κB and NFAT, transcription factors whose activation is limited by SHIP 18, 47, have recently been identified as critical for promoting FoxP3 expression during T-cell development 48. CD28 engagement activates the PI3K/Akt pathway, which SHIP opposes 49, and promotes FoxP3 expression and differentiation of Treg cells from naïve CD4+ T cells 50. In addition, others have found that Akt can promote iTreg-cell formation independent of CD28 signals 51. Furthermore, Treg-cell numbers and function are compromised in p110delta PI3K mutant mice 52 and increased in mice with enforced expression of Akt/PKB 51. Although, it must be noted that Treg cells show reduced Akt phosphorylation 46 and specifically require limited Akt activation to induce, but not maintain, FoxP3 expression 53, 54. Furthermore, SHIPs function in T cells may be more than just negative regulation of PI3K and instead also include activation of qualitatively different PI3K effector pathways. Specifically, it has been shown that the catalytic product of SHIP, PI(3,4)P2, recruits different signaling proteins from those recruited by PIP3 and the PTEN product, PI(4,5)P2 55.
The findings presented here and our previous studies of MDSCs, Treg cells, and NK cells in SHIP-deficient mouse models 1-4, 19, 20, 27, indicate that SHIP deficiency promotes an immunosuppressive environment that preferentially impairs cell-mediated immunity, particularly those involving MHC-mismatched responses. Recently, a SHIP-1 selective inhibitor that also increases MDSC numbers in vivo and fosters a potent immu-nosuppressive environment in peripheral lymphoid tissues was identified 29. Thus, pharmacological targeting of SHIP1 activity in vivo could be used to improve the efficacy and utility of allogeneic organ and bone marrow transplantation. We propose that downregulation of SHIP expression serves as a molecular switch to promote an immunosuppressive state in peripheral lymphoid organs 56. Such a “SHIP switch” is certainly plausible as SHIP expression is readily modulated posttranscriptionally by miR155 57 and posttranslationally by ubiquination 58. Indeed, SHIP expression varies significantly within both the myeloid (Fig. 2) and NK-cell lineages 59, suggesting that SHIP regulation occurs during normal fluctuations in immune status and differentiation. Rather than acting as a global immune suppressor, the increased MDSC and Treg-cell numbers promoted by a “SHIP switch” may act to dampen low-affinity T-cell responses or self-reactive T-cell responses during intense immune responses allowing more focused, and thus more effective, immune responses to major pathogen challenges.
Materials and methods
Mice
Mice with germline transmission of a SHIPflox allele were generated previously in our laboratory 1 and propagated by intercrossing with SHIPflox/flox mice (F10 to the C57BL/6J background). MxCre transgenic mice were obtained from Jackson Laboratory. MxCreSHIPflox/flox and SHIPflox/flox controls, on a C57BL/6 background, were created by intercrossing SHIPflox/flox and MxCreSHIPflox/flox mice. LysCreSHIPflox/flox, LckCreSHIPflox/flox mice, and SHIPflox/flox controls were created the same way. Studies were performed in accordance with the guidelines and approval of the Institutional Animal Certification and Use Committee at the University of South Florida.
Conditional deletion of SHIP
Conditional deletion of SHIP in MxCreSHIPflox/flox mice was achieved by injecting 625 μg polyinosinic–polycytidylic acid (polyI/C; Sigma-Aldrich, St Louis, MO, USA) into the peritoneum of mice on days 0, 3, and 6 as described by Desponts et al. 24.
Flow cytometry
For flow cytometric quantitation of viable T cells or MDSCs, splenocytes and mesenteric LN cells, single cell suspensions were Fc-blocked and stained using fluorescent-conjugated antibodies specific for these surface markers: CD3, CD4, and CD25 for T cells; and CD11b and Gr1 for MDSCs. 4,6-diamidino-2-phenylindole (DAPI), a viability dye was also used. For intracellular FoxP3 expression, cells were stained as mentioned above, then fixed and permeabilized as per manufacturer's instructions using the eBioscience Fixation/Permeabilization kit (eBioscience, San Diego, CA, USA), and stained with anti-FoxP3 (FJK-16a). An LSRII (BD Biosciences, San Jose, CA, USA) was used to analyze all stained samples. All antibodies were obtained from BD Biosciences PharMingen (San Diego, CA, USA) or eBioscience.
Western blots
Fluorescence-activated cell sorter (FACS)-sorted CD3+ and CD3+CD4+CD25+CD8– T cells were lysed for 30 min on ice in a modified TNE buffer (50 mM Tris-HCl, 1% Nonidet P-40, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM sodium orthovanadate (Na3VO4), 1 mM NaF, and protease inhibitors). Using equal cell equivalents, samples were resolved on a 4–12% Bis-Tris gel (Invitrogen, Carlsbad, CA, USA) and transferred onto a Hybond-ECL nitrocellulose membrane (GE Healthcare, Little Chalfont, United Kingdom). After blocking with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE, USA), blots were probed with antibodies against SHIP1 (P1C1, 1:200) or FoxP3 (eBio7979, 1:500) and β-actin (C-11, 1:500) followed by a fluorochrome-tagged secondary antibody. Probed blots were developed using a LI-COR Odyssey imager to quantify band intensities and normalize SHIP levels or FoxP3 levels to β-actin, displayed as arbitrary fluorescence units (AFU).
G-CSF neutralization and SDF1ELISA
SHIP was conditionally deleted in MxCreSHIPflox/flox mice by administering an i.p. injection of 625 μg polyI/C on days 0, 3, and 6 as described in Paraiso et al. SHIPflox/flox mice were treated similarly to serve as control mice. On day 2, polyI/C-treated MxCreSHIPflox/flox mice and SHIPflox/flox control mice were also injected subcutaneously with monoclonal anti-mouse G-CSF antibodies (R&D systems, 10 μg per animal per day) or PBS (diluent) for 1 week. Then, MDSCs and Treg cells in the spleen and mesenteric LNs were quantitated by flow cytometry (as described above).
Purification of MDSCs from naïve and tumor-bearing mice
MC-38 colon carcinoma tumor cells (provided by D. Gabrilovich) were cultured in complete DMEM (10% FBS and antibiotics). MC-38 tumor cells (5 × 105) or PBS were injected subcutaneously in the right flank of C57BL/6J mice. Whole splenocytes from naïve (PBS treated) mice or from MC-38 tumor-bearing mice (3 weeks after injection) were processed into a single cell suspension. MC-38 tumors were extracted and digested with collagenase D (400 U/mL, Roche) to process into a single cell suspension. Splenic and intratumoral MDSCs were isolated by staining the single cell suspensions with antibody specific for CD11b and Gr1 and with DAPI. CD11b+Gr1+ cells were sorted using a BD FACSAria cell sorter. CD11b−Gr1− cells were sorted in parallel to provide an internal control for SHIP expression. Population purity was more than 95% as determined by post-sort analysis. Sorted cells were lysed and the lysates used for western blot analysis.
Acknowledgements
This work was supported in part by grants from the NIH (RO1 HL72523, R01 HL085580, RO1 HL107127) and the Paige Arnold Butterfly Run. W. G. K. is the Murphy Family Professor of Children's Oncology Research and an Empire Scholar of the State University of New York.
Conflict of interest
W.G.K. is the inventor, or co-inventor, on patents, both issued and pending, related to modulation of SHIP expression and activity for therapeutic purposes. The other authors have no financial or commercial conflicts of interest to declare.
References
Abbreviations
-
- FoxP3
-
- forkhead box P3
-
- MDSC
-
- myeloid-derived suppressor cell