IL-33 attenuates EAE by suppressing IL-17 and IFN-γ production and inducing alternatively activated macrophages
Abstract
Interleukin (IL)-33, a member of the IL-1 cytokine family, is an important modulator of the immune system associated with several immune-mediated disorders. High levels of IL-33 are expressed by the central nervous system (CNS) suggesting a potential role of IL-33 in autoimmune CNS diseases. We have investigated the expression and function of IL-33 in the development of experimental autoimmune encephalomyelitis (EAE) in mice. We report here that IL-33 and its receptor ST2 (IL-33Rα) are highly expressed in spinal cord tissue, and ST2 expression is markedly increased in the spinal cords of mice with EAE. Furthermore, ST2-deficient (ST2−/−) mice developed exacerbated EAE compared with wild-type (WT) mice while WT, but not ST2−/− EAE mice treated with IL-33 developed significantly attenuated disease. IL-33-treated mice had reduced levels of IL-17 and IFN-γ but produced increased amounts of IL-5 and IL-13. Lymph node and splenic macrophages of IL-33-treated mice showed polarization toward an alternatively activated macrophage (M2) phenotype with significantly increased frequency of MR+PD-L2+ cells. Importantly, adoptive transfer of these IL-33-treated macrophages attenuated EAE development. Our data therefore demonstrate that IL-33 plays a therapeutic role in autoimmune CNS disease by switching a predominantly pathogenic Th17/Th1 response to Th2 activity, and by polarization of anti-inflammatory M2 macrophages.
Introduction
Experimental autoimmune encephalomyelitis (EAE) is an animal model of inflammatory demyelination in the central nervous system (CNS) 1. Although with apparent limitations, EAE is commonly used as a model of multiple sclerosis (MS) in humans for improving understanding and treatment of MS 2. The immune pathogenesis of MS and EAE is complex, but T cells are believed to play key roles in the disease development 3. Both Th1 and Th17 cells are thought to be responsible for the inflammatory demyelination in MS and EAE 4-8, while Th2 and Treg cells have been shown to be important in the resolution stages of the disease 9, 10. The dynamic balance between the effector Th1/Th17 and Treg cells controls the development of MS and EAE 10, 11. However, during the T cell-mediated neuroinflammation, macrophages also play indispensible roles in EAE development 12. Activated macrophages can be broadly classified into classically activated M1 macrophages and alternatively activated macrophages (AAMs or M2). While M1 macrophages are associated with pathologic CNS inflammation in EAE and MS development, M2 macrophages are protective against EAE 13. IL-33 has recently been shown to promote Th2-cell expansion and skew M1 to the M2 phenotype 14. We therefore investigated the potential role of IL-33 in EAE.
IL-33 was recently identified 15 as a new member of the IL-1 cytokine family, which also includes IL-1α, IL-1β, IL-18, and IL-1Ra. Human IL-33 was detected in epithelial cells, fibroblasts 15, and endothelial cells of the inflamed tissues from patients with rheumatoid arthritis and Crohn's disease 16. In rodents, IL-33 mRNA was detected in various tissues and organs including spleens and CNS 15. Different from IL-1 and IL-18, full-length IL-33 is bioactive and released most probably through cell necrosis and trigger inflammation in an autocrine or paracrine fashion 17. IL-33 signals via a heteromeric receptor that consists of ST2L (or ST2) and IL-1R accessory protein (IL-1RAcP) 18. ST2L 19, also known as T1 20, the membrane form of protein encoded by the ST2 gene, is a member of the Toll-like receptor (TLR)/IL-1R superfamily. ST2 is expressed on most cells especially on mast cells 21 and activated Th2 cells 22. The ST2 gene can also encode other isoforms by alternative splicing, including a secreted soluble ST2 (sST2) form that can serve as a decoy receptor.
IL-33 has pleiotropic effects on immune responses 14. IL-33 drives production of cytokines such as IL-5, IL-6, IL-13, and GM-CSF by mast cells and Th2 cells in naïve mice. Previous studies have shown that IL-33 plays an important inflammatory role in allergic diseases 23 but confers resistance to parasite infection 24 and atherosclerosis 25 by inducing type 2 and humoral immune responses. However, IL-33 is also able to induce inflammatory hypernociception 26 and is a proinflammatory cytokine in CIA 14, 27. Recent studies have also shown that the IL-33/ST2 pathway plays a significant role in the amplification of M2 polarization and chemokine production, which contribute to innate and antigen-induced airway inflammation 28 and protect against obesity-related metabolic events 29.
Interestingly, the highest levels of IL-33 expression in naïve mice are found in the brain and spinal cord 15, indicating that IL-33 may have CNS-specific functions in addition to its role in immune modulation. Astrocytes express both ST2L and IL-1RAcP 30 and the expression of IL-33 in the CNS was increased in response to inflammatory stimuli 31. Recently, it was also reported that IL-33 levels were elevated in the periphery and CNS of MS patients, implicating IL-33 in the pathogenesis of MS 32. However, the precise role of IL-33 and its receptor in CNS under healthy and inflammatory conditions remain unclear.
In the present study, we show that ST2−/− mice developed significantly exacerbated EAE while WT EAE mice treated with IL-33 exhibited markedly attenuated disease. The reduced CNS inflammation of IL-33-treated mice was associated with a decreased level of IFN-γ and IL-17 but an increased production of IL-5 and IL-13. Furthermore, macrophages from IL-33-treated WT EAE mice showed an increased polarization toward the M2 phenotype, and adoptive transfer of M2 significantly reduced EAE. Together our results demonstrate that IL-33 may be a novel therapeutic agent against autoimmune CNS disease.
Results
Expression of IL-33 and ST2 in the spinal cord
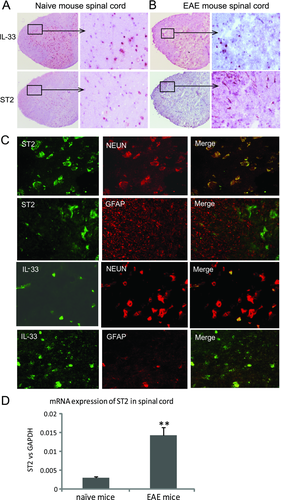
We first examined the expression of IL-33 and ST2 in the CNS tissue. Spinal cords were collected from naïve or EAE C57BL/6 mice 16 days after immunization with MOG35–55 in Complete Freund Adjuvant (CFA) together with pertusis toxin (PTX). The tissues were fixed, and 7 μm sections were stained immunohistochemically. Both IL-33 and ST2 were clearly expressed in the spinal cord tissues (Fig. 1A). Moreover, the expression of ST2 was markedly increased in mice with EAE (Fig. 1B), while the level of IL-33 change was not obvious. To identify the phenotype of CNS cells expressing IL-33 and ST2, we performed dual immunohistochemical staining. ST2 is expressed by NeuN+ neuron cells, but not glial fibrillary acidic protein (GFAP)+ astrocytes, while IL-33 is coexpressed by both GFAP+ astrocytes and NeuN+ neuron cells (Fig. 1C). Quantitative PCR analysis confirmed the upregulation of ST2 expression in Fig. 1B as it clearly shows the enhanced expression of ST2 mRNA in the CNS of mice with EAE compared with that of the naïve mice (Fig. 1D).
ST2−/− mice developed exacerbated EAE
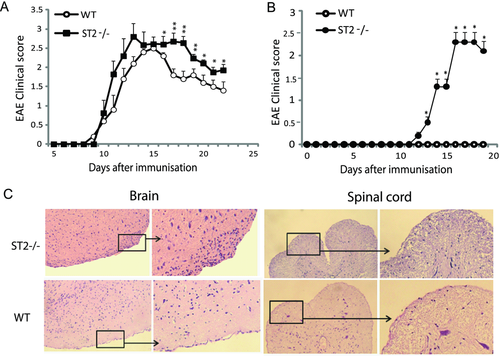
To identify an endogenous role of IL-33 in EAE, we next investigated the development of EAE in ST2−/− mice. EAE was induced in C57BL/6 WT and ST2−/− mice, Fig. 2A shows that the ST2−/− mice developed more severe EAE than that in the WT mice. In agreement with many previous reports, BALB/c mice are resistant to the induction of EAE. However ST2−/− BALB/c mice developed a mild but clearly detectable EAE while the WT BALB/c controls did not (Fig. 2B), the observation was further confirmed by histological analysis of the CNS tissues at day 19 after immunization (Fig. 2C). Minimal leukocyte infiltration was found in the CNS of WT BALB/c mice whereas significant leukocyte infiltration was found in the submeningeal infiltration in the CNS tissues of ST2−/− BALB/c mice (Fig. 2C).
IL-33 treatment attenuates EAE development
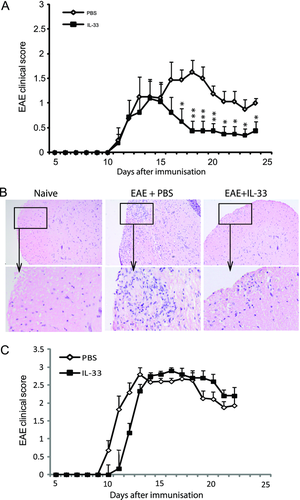
We next investigated the effect of exogenous IL-33 in the development of EAE. C57BL/6 mice were immunized as described in Materials and Methods. Recombinant IL-33 (1 μg/100 μL) or PBS was injected intraperitoneally to each mouse daily from day 12 to day 20 after immunization. Both groups of mice developed similar degree of EAE from day 10 to day 15. However, from day 15, mice treated with IL-33 recovered significantly faster than the control mice treated with PBS (Fig. 3A). In induced experimental autoimmune diseases, disease severity could vary between experiments even with the same protocol. Analysis using multiple comparisons suggests that the effect of IL-33 treatment on the outcome of EAE mice could be time course dependent. Histological examinations of the spinal cord tissue collected at day 25 postimmunization revealed marked reduction of infiltrating cells in the spinal cord tissues of IL-33-treated mice (Fig. 3B). Importantly, IL-33 had no effect on the disease development in ST2−/− C57BL/6 mice (Fig. 3C) demonstrating the specificity of the IL-33 effect.
IL-33 alters the cytokine production of EAE mice
To investigate the immunological mechanisms associated with the reduced severity of EAE in the IL-33-treated mice, C57BL/6 mice were immunized and treated with IL-33 or PBS from day 12 to day 20 after immunization as above. Spleen, LN, and serum samples were collected at 26 days after immunization. Cytokine levels in the serum samples were measured by Luminex. IL-33-treated mice produced significantly reduced amounts of serum IL-17 and IFN-γ but increased levels of IL-5 and IL-13 compared with PBS-treated control mice (Fig. 4A). We then examined the cytokine production by the draining LN (DLN) and spleen cells ex vivo. Single cell suspensions were cultured in the presence or absence of MOG35–55 for 72 h and the supernatant harvested and analyzed by ELISA for IL-5, IL-13, IL-17, IFN-γ, and other cytokines/chemokines by Luminex. Cells from IL-33-treated mice produced significantly less IFN-γ and IL-17, but more IL-5 and IL-13 in response to MOG35–55 compared with cells from control mice (Fig. 4B and C). Other cytokines (IL-10, TGFβ, TNF-α, IL-6, IL-4, FGF, GM-CSF, IL-1α, IL-1β, IL-2, IL-12p40/70) and chemokines (IP-10, KC, MCP-1, MIG, MIP-1α, VEGF) were detected in low levels and were not significantly different between the two groups. These results therefore show that the IL-33 treatment switched a predominately Th17/Th1 response to a Th2 phenotype.
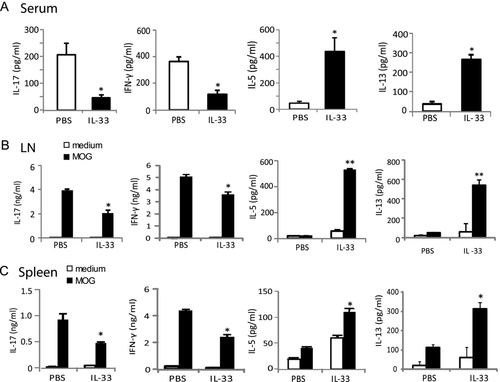
IL-33 alters the cellular phenotype in vivo
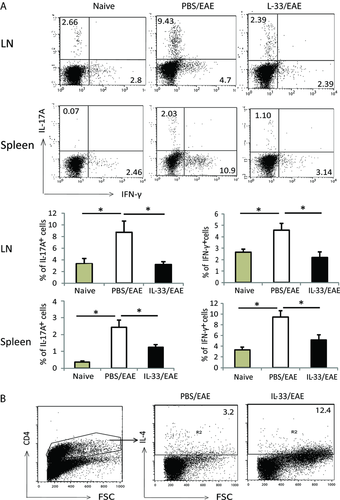
We further examined the cellular phenotypes of mice treated with IL-33. Single cell suspensions were prepared from the spleen and DLN of mice 20 day after immunization as above, and stained for cell surface and intracellular markers by FACS. Consistent with the cytokine profile observed above, there was a significant reduction in the frequency of IL-17+ and IFN-γ+ cells in both the DLN and spleen from IL-33-treated mice (Fig. 5A), while IL-4-secreting CD4+ T-cell frequency in the spleen was increased (Fig. 5B). Furthermore, we observed an increase of CD4+Foxp3+ cells in the DLN from 1.8 to 15%. However, there was no difference between the PBS- and IL-33-treated mice in term of total CD4+ and CD8+ T cells. We also examined the spinal cord tissue and found that the number of infiltrating cells was reduced in the IL-33-treated mice (0.8 × 105 cells per spinal cord) compared with PBS-treated group mice (1.65 × 105 cells per spinal cord). However, there was no significant difference in the percent of IL-17+ and IFN-γ+ cells (Fig. 6A). In contrast, the frequency of CD4+Foxp3+ Treg cells in the spinal cord was significantly increased in the IL-33-treated mice compared with that of the PBS group (Fig. 6B).

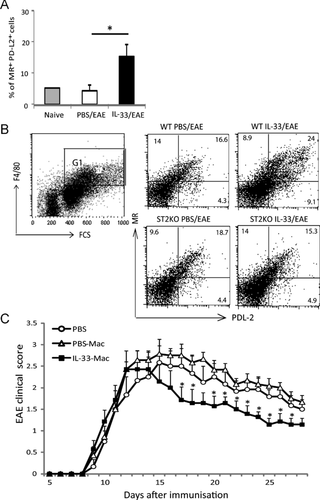
Given the well-defined role of IL-33 in macrophage polarization toward an M2 phenotype 33 and the protective role of M2 in EAE development 34, we also analyzed the macrophage phenotype in the IL-33-treated EAE mice. Spleen and DLN F4/80+ macrophages were stained for cell surface MR (CD206) and PD-L2 (CD273), two markers closely identified with M2 35. Macrophages (F4/80+ cells) from IL-33-treated mice had significantly increased frequency of MR+/PD-L2+ cells in the DLN and spleen (Fig. 7A and B). Such IL-33-induced increase in the frequency of M2, however, was not seen in ST2−/− mice (Fig. 7B). To demonstrate directly the effect of IL-33-treated M2 macrophage on the outcome of EAE, we performed adoptive transfer experiments. Naïve C57BL/6 mice were injected intraperitoneally with IL-33 or PBS on 5 consecutive days. Spleen and LN cells were harvested on day 7 and F4/80+, CD4−CD8− (T cells), and Siglec-F− (eosinophils) macrophages were purified by sorting with FACSAria. The cells (1 × 106) were then adoptively transferred i.v. to EAE mice on day 12 and day 16 after immunization. EAE mice that had received macrophages from IL-33-treated mice (IL-33-Mac) developed significantly reduced disease severity and accelerated recovery compared with mice that had received macrophages from PBS-treated mice (PBS-Mac) or control PBS-treated mice (Fig. 7C). These results therefore indicate that IL-33 treatment facilitates the polarization of AAM that at least partially account for the IL-33-mediated reduction of CNS inflammation in EAE.
Discussion
Data presented here demonstrate that IL-33 administered after EAE onset is protective against the disease. The effect of IL-33 is likely endogenous as ST2−/− mice developed exacerbated disease following EAE induction. Furthermore, we show that the beneficial effect of IL-33 is accompanied by a switch from the predominant pathogenic Th17 and Th1 response to the protective Th2-type immune activity. Moreover, IL-33 treatment also resulted in an increase in Treg-cell frequency and the polarization of M2, which has been previously demonstrated to be associated with a favorable outcome of EAE.
The role of IL-33 in inflammatory EAE is consistent with the high levels of IL-33 and ST2 expression in the CNS 15. However, the association of EAE and the distribution of these molecules in the CNS cells are less clear. A previous report 30 suggested that ST2 is expressed by the astrocytes based on ST2 gene transcript in the cultured cells. Using immunohistochemical staining in CNS tissues, we show here that ST2 protein is exclusively expressed by the neuron cells in the spinal cord, but not the GFAP+ astrocytes in situ, whereas IL-33 protein is expressed by both neuron cells and astrocytes, in agreement with an earlier report 36. Thus, IL-33 may function in an autocrine and a paracrine manner. Intriguingly, ST2 expression was upregulated in the CNS of EAE mice, suggesting that in addition to its well-known role in modulating immune response, the IL-33/ST2 signaling pathway may have a CNS-specific function as IL-33 levels were also elevated in tissues of MS patients 32. Recently, IL-33 gene expression in the CNS was associated with a protective outcome for human Alzheimer's disease 37. This intriguing observation warrants further investigation.
The initiation, development, and resolution of EAE are determined by a dynamic balance between effector and regulatory immune cell populations. Activated macrophages are required for EAE induction by autoreactive proinflammatory Th17 and Th1 cells 38. The polarization of macrophages into M1 or M2 depends on the cytokine milieu in the tissue. Macrophages develop into the proinflammatory M1 phenotype in response to IFN-γ and microbial products such as LPS, while the anti-inflammatory M2 are induced by type 2 cytokines including IL-4, IL-13, and IL-21 33. The IL-33/ST2 signaling pathway leads to the selective induction of type 2 immune responses 22, 39. IL-33 can also drive the quiescent alveolar macrophages toward M2 and amplifies the polarization of M2 by the enhanced induction of the type 2 cytokines 28, 29. Thus, IL-33 may attenuate EAE by two related pathways: (i) IL-33 diverts the predominant pathogenic Th17/Th1 cells to Th2 cells; (ii) the type 2 cytokines thus produced by Th2 cells would then polarize the anti-inflammatory M2. An important role of M2 in attenuating EAE has been demonstrated previously 34 and our data using adoptive transfer experiments further confirm that M2 induced by IL-33 in vivo attenuates the severity of EAE (Fig. 7C). The molecular mechanism by which IL-33 selectively polarize Th2 has been addressed previously and was associated with the preferential expression of ST2 on activated Th2 cells 22. It should also be noted that M2 macrophages express a significant level of ST2 28 and may thus also be able to respond directly to IL-33-mediated activation.
We also showed here that IL-33 induced an increase in the frequency of CD4+Foxp3+ Treg cells in the LN and the CNS. The role of Treg cells in suppressing EAE has previously been reported 10, 40. Furthermore, a recent study demonstrates that M2 can also preferentially induce Treg cells 41. Thus, a further mechanism in IL-33-mediated attenuation of EAE could be the induction of Treg cells via the IL-33-indcued M2.
Our results are in apparent contrast to a recent report showing that a strain of IL-33-deficient mice exhibited EAE similar to the WT mice 42. It should however be noted that a lower immunizing dose of antigen was used in our model in order to demonstrate the effect of ST2 and IL-33 on EAE. It is clear that both ST2−/− and IL-33−/− mice are able to mount an EAE response, suggesting that IL-33 is but one of several factors that could induce the complex disease such as EAE. Our results show that high levels of IL-33 induced during EAE could function as a self-limiting mechanism to control the chronic nature of the disease. It may be that the absence of a low dose of IL-33 could be compensated by other cytokine (such as IL-1), while a high dose of IL-33 could limit EAE by preferentially inducing Th2 and M2. In our study, the endogenous role of ST2 in EAE is evident from the two strains of ST2−/− mice used, both of which developed exacerbated EAE. Furthermore, IL-33 was not able to change EAE severity in ST2−/− mice. However, the possibility that IL-33 may bind any signal via a receptor in addition to ST2 cannot be excluded; neither can the likelihood that ST2 may recognize ligands other than IL-33.
In summary, the present study demonstrates that IL-33 administered after disease onset has a protective role in EAE and thus, by extension, IL-33 may be a potential therapeutic reagent for MS. IL-33 attenuates the disease development possibly through the induction of IL-5 and IL-13 production together with M2 polarization. However, the exact function of IL-33 in the CNS, and whether IL-33 influences the balance of demyelination and remyelination in the development of EAE remains unclear and merits further investigation.
Materials and methods
Mice
C57BL/6 mice were purchased from Harlan (UK) and maintained at the Joint Animal Facilities, University of Glasgow under UK Home Office guidelines. ST2−/− mice were generated as described previously and kindly provided by Dr. Andrew McKenzie 43. The gene-targeted mice were backcrossed to the respective background for ten generations. ST2−/− and the WT littermates of the same background were kept in the same animal facilities for extended periods. ST2−/− and the WT littermates of BALB/c background were maintained in the animal facilities of the Faculty of Medicine University of Kragujevac (FMUK). ST2−/− and the WT littermates in C57BL/6 background were maintained at the University of Orléans (France) and at the University of Glasgow (UK). All experiments were performed under the guidelines of the University of Glasgow, UK Home Office, FMUK, and the University of Orleans Research Ethics Committee. Sex-matched mice at the age of 7–12 weeks were used in all experiments.
Recombinant IL-33
Mouse IL-33 was expressed in Escherichia coli and IL-33 proteins were purified by Ni-nitriloacetic acid affinity chromatography as described previously 27. Endotoxin was removed by purification with polymyxin B chromatography. The purity of IL-33 was >97% by silver staining. Endotoxin levels were <0.1 unit/μg of protein by the Limulus Amebocyte Lysate QCL-1000 pyrogen test (Cambrex). The specificity of IL-33 was also tested on WT versus ST2−/− cells. IL-33 was also obtained from Biolegend with similar results.
EAE induction and clinical evaluation
C57BL/6 mice were immunized subcutaneously on the back with 100 μg of MOG35–55 peptide (Sigma Genosys) in 50 μL of PBS emulsified with an equal volume of CFA (total 125 μg of Mycobacterium tuberculosis, strain H37RA, Difco, Detroit, MI, USA). Each mouse also received intraperitoneally 100 ng/200 μL of PTX (Sigma, UK) in PBS on days 0 and 2 post immunization. WT and ST2−/− male mice of the BALB/c background were immunized subcutaneously on the back with 200 μg of MOG35–55 peptide in 100 μL of PBS emulsified with an equal volume of CFA (800 μg of M. tuberculosis) together with 300 ng/100 μL of PTX on days 0 and 2. EAE was scored according to a 0–5 scale as follows: 0, no clinical sign; 1, complete loss of tail tone; 2, hind limb weakness; 3, hind limb paralysis; 4, forelimb involvement; 5, moribund.
Histological evaluation of disease
Mice were euthanized in CO2 chamber and their spinal cords flushed out with PBS by hydrostatic pressure using a syringe attached to a G18 size needle; the brains were also harvested. The tissues were then placed in 10% buffered formalin fixative overnight before changed to 75% ethanol. Tissues were then embedded in paraffin wax. Spinal cord and brain sections were stained with standard haematoxylin and eosin (H&E) staining.
Immunohistochemical staining
Tissues of naive and EAE mice (day 16 after immunization) were frozen in OCT embedding media and sections were stained with anti-IL-33 antibodies (Clone 396118 and YJE04, R&D System) or anti-ST2 antibody (Clone DJ8, MD Biosciences), followed by incubation with biotinylated secondary antibody (Vector Laboratories) and detected with substrate. To determine the localization of IL-33 and ST2 in the CNS resident cells, double immunohistochemistry staining was performed on the CNS tissues. After staining with antibodies against IL-33 or ST2 and followed by biotinylated antibody and streptavidin-FITC (e-Bioscience), the sections were restained with antibodies for murine astrocytes (Anti-GFAP, clone z0334, Dako) or neuron cells (Anti-NeuN, clone A60, Millipore). Isotypes with matching IgG were used as negative controls, ST2−/− mouse tissue was also used as negative control for ST2 staining. The sections were examined by an Olympus CX51 microscope.
Spinal cord cell isolation
Mice were killed by asphyxiation in CO2 and then perfused through the left cardiac ventricle with 20 mL of cold PBS buffer. Mouse spinal cords (thoracic and lumber region) were collected and then cut into small pieces and digested with collagenase A (2 mg/mL, Roche Diagnostics) and DNase (1 mg/mL, Sigma-Aldrich) at 37°C for 40 min. Mononuclear cells were isolated by passing the tissue through a cell strainer (100 mm), followed by a Percoll gradient (70/30%) centrifugation. Mononuclear cells were removed from the interphase, washed, and resuspended in culture medium for further analysis.
Flow cytometry analysis
Antibodies were purchased from BD Pharmingen unless mentioned otherwise. To examine the leukocyte phenotype in the DLN and spleen, the cells were stained with the following anti-mouse antibodies: anti-F4/80 (clone BM8, eBioscience), Anti-CD206 (or Mannose Receptor, MR, clone MR5D3, Serotec); Anti-CD273 (or PD-L2, clone TY25); Anti-IFN-γ (clone XMG1.2); Anti-IL-17A (clone eBio17B7). For Treg study, the cells were first stained for CD4 antibody, then permeabilized with Perm/Fix solution (eBioscience) before staining with FoxP3 antibody (clone FJK-16s, eBioscience). For measurement of intracellular cytokines, cells were restimulated with 50 ng/mL phorbol-12-myristate-13-acetate and 500 ng/mL ionomycin (both from Sigma) in the presence of GolgiStop (BD Bioscience) for 4 h, then permeabilized with Perm/Fix solution (eBioscience) and finally stained with anti-IL-17A and anti-IFN-γ. Isotypes-matched IgG were used as negative control. Cells were analyzed by FACSCalibur using CellQuest software (BD Biosciences).
Macrophage adoptive transfer experiment
Mice were injected i.p. with 0.5 μg of IL-33 or PBS for five consecutive days. On day 7 after the first injection, spleen and mesenteric LN of the mice were collected. Cells were stained with antibodies for F4/80, T cells (anti-mouse CD4 (clone RM4-5), and CD8 (clone 53-6.7, both from eBioscience) and eosinophils (anti-mouse Siglec-F, BD Biosciences). F4/80+CD4−CD8−Singlec-F− cells were purified using FACSAria cell sorter. Sorted macrophages from IL-33 (IL-33 Mac) or PBS (PBS-Mac)-treated mice were injected i.v. to EAE mice (1 × 106 cells per mouse) on day 12 and 16 after immunization. EAE mice received same volume of PBS were also used as control. EAE development was monitored using the clinical scoring system as described above.
DLN and spleen cell culture
DLN and spleen were collected and single cell suspension was cultured in 24-well plates at 4 × 106 cells/2 mL per well and stimulated with medium alone or with 50 μg/mL of MOG35–55 peptide. After 72 h, supernatants were collected and concentrations of selected cytokines were measured by ELISA and Luminex.
Measurement of cytokines in the serum and supernatant
Individual mouse serum was collected and assayed for cytokine concentrations using a 20-plex mouse cytokine assay (Luminex, Biosource, Invitrogen) for FGF, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40/70, IL-13, IL-17, IP-10, KC, MCP-1, MIG, MIP-1α, TNF-α, and VEGF according to manufacturer's instructions. Concentrations of selected cytokines in the spleen and LN cell supernatant were also measured by ELISA (IL-17, IFN-γ, IL-5 and IL-13) using paired antibodies (eBioscience).
Statistical analysis
Statistical evaluations of cell frequency, cytokine production, and histological analysis were performed with the two-tail unpaired Student's t-test. ANOVA were used to analyze EAE clinical grading. p < 0.05 was considered statistically significant.
Acknowledgment
We thank Diane Vaughan at the University of Glasgow for assistance in cell sorting. This study received financial support from Tenovus Scotland, the Royal Society and the Medical Research Scotland (Principle Investigator H. R. J ); Medical Research Council UK, the Wellcome Trust UK and the European commission (Principle Investigator F. Y. L.). Research by M. M. and M. L. L. was supported by grants 175069 and 175071 from the Ministry for Science, Serbia.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
Abbreviations
-
- AAM or M2
-
- alternatively-activated macrophage
-
- CIA
-
- collagen-induced arthritis
-
- GFAP
-
- glial fibrillary acidic protein
-
- IL-1RAcP
-
- IL-1R accessory protein
-
- M1
-
- classically-activated macrophage
-
- MOG
-
- myelin oligodendrocyte glycoprotein
-
- NeuN
-
- neuron nuclear
-
- sST2
-
- soluble ST2
-
- ST2−/−
-
- ST2-deficient