Caspase 3 is not essential for the induction of anergy or multiple pathways of CD8+ T-cell death
Abstract
T-cell death is a fundamental process that is intricately regulated at multiple phases during T-cell differentiation, tolerance induction and the decline of the immune response. Caspase 3 is a crucial molecule regulating both mitochondrial and death receptor apoptotic pathways and therefore we were interested in examining the role of caspase 3 in T cells. Using P14 and H-Y CD8+ TCR-transgenic models, our analysis has shown that caspase 3 is not required for thymic negative selection. In addition, caspase 3 does not play a prominent role in the contraction phase following acute viral infection, nor clonal deletion of CD8+ T cells under tolerizing conditions. Surprisingly, our studies demonstrate that caspase 3 was not required for the induction of CD8+ T-cell anergy in vivo, contrary to published reports using CD4+ T cells. Therefore, these results demonstrate that caspase 3 is not essential in CD8+ T cells for multiple forms of thymic or peripheral tolerance, nor the contraction phase after an acute anti-viral response.
Introduction
T-cell death is strictly controlled in the thymus and periphery to maintain self tolerance and lymphocyte homeostasis. Two distinct programs of cell death can occur in the thymus. Thymocytes expressing T-cell receptors (TCR) that which have high affinity for peptide/MHC ligands are eliminated via a process termed negative selection. Double positive (DP) thymocytes expressing TCR that are unable to engage self-peptide/MHC complexes are destined to die by a process often referred to as death by neglect 1.
Although many molecules have been shown to play a role in negative selection, the pathway that regulates this process remains controversial. Many members of the TNF receptor (TNFR) superfamily play a role in apoptosis and studies have suggested that molecules such as Fas/CD95 and TRAIL are involved in negative selection 2, 3. There are, however, conflicting reports and this remains an area of controversy 4, 5. Molecules downstream of the TNFR superfamily have also yielded controversial results. Some studies examining FADD, Bim and caspases supported the importance of these molecules in negative selection 6-8. Other reports analyzing FADD and caspase 8 concluded that these molecules are not involved in negative selection 9, 10. Few papers have examined the molecules that regulate the process called death by neglect.
In the mature T-cell repertoire, cell death is important for maintaining homeostasis and inducing tolerance. The presentation of self-antigens by tolerogenic dendritic cells (DC) induces T-cell tolerance by various mechanisms including clonal deletion. The molecular mechanism that induces the death of mature self-reactive T cells also remains unclear. It has been shown that members of the TNFR superfamily such as Fas or TNFRI play an important role in peripheral deletion of self-reactive T cells 11, 12. However, others suggested that TNFR superfamily members are not critical for peripheral deletion or the contraction of an anti-viral response 13-15. In addition, there is evidence for alternative caspase-independent death pathways in T cells 16.
Caspase 3 is a 32 kD cysteine protease that has a key role during apoptosis triggered both by the mitochondrial and the death receptor pathways. It has been reported that caspase 3 is cleaved during negative selection and that a pharmacological caspase inhibitor inhibits thymic deletion 6, 17. In contrast, there is a report demonstrating that expression of a viral caspase inhibitor in thymocytes did not affect negative selection in vivo 18. Caspase 3 activation has also been reported to play a role in early T-cell activation not only upon anti-CD3 stimulation in vitro, but also in lymphocytic choriomeningitis virus (LCMV)-infected mice 19. Studies have also shown that caspase 3 is induced during T-cell anergy and plays an important role in this process 20. Despite the multiple proposed roles for caspase 3 in T-cell biology, the physiological importance of caspase 3 has not been carefully investigated. In this study we evaluated the importance of caspase 3 in CD8+ T cells during central and peripheral tolerance, as well as the contraction phase of an immune response, using caspase 3-deficient mice.
Results and discussion
Negative selection is not impaired in the absence of caspase 3
To examine the role of caspase 3 in thymocyte selection in vivo, H-Y TCR-transgenic mice expressing the TCR specific for the H-Y male antigen and H-2Db were crossed onto a caspase 3−/− background. Western blot analysis confirmed the absence of caspase 3 in CD8+ T cells (Fig. 1A). In female H-Y transgenic mice, the CD4+CD8+ DP and CD8+ single positive populations were unaffected by the absence of caspase 3, suggesting that caspase 3 is not essential for positive selection (Fig. 1B). In H-Y male mice, H-Y-specific T cells recognize the male antigen and typically show a reduction in DP cells and thymic cellularity. In H-Y caspase 3+/+ male mice, less than 10% of thymocytes were DP and the total number of thymocytes (1.4×107±0.6 cells) was about 10 times less than in female mice (1.4×108±4.8 cells). In H-Y caspase 3−/− male mice, the number of thymocytes was also reduced approximately 10-fold, compared with H-Y caspase 3−/− female mice (female: 1.3×108±6.0 cells, male: 1.5×107±0.3 cells). In addition, the percentage of DP thymocytes in H-Y caspase 3−/− male mice was similar to male control mice. These results indicated that caspase 3 was not essential for negative selection.
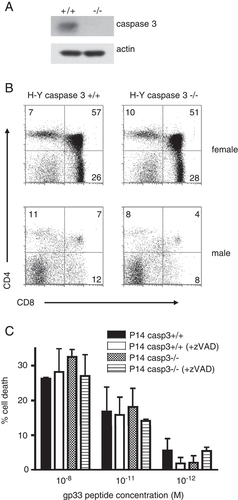
Normal thymocyte negative selection in the absence of caspase 3. (A) Expression of caspase 3 in CD8+ T cells isolated from caspase 3+/+ (n=3) or caspase 3−/− (n=3) spleens was evaluated by western blotting. (B) CD4 and CD8 expression on thymocytes was evaluated from H-Y male and female transgenic mice in the presence or absence of caspase 3. (C) Thymocytes from P14 caspase 3+/+ and P14 caspase 3−/− mice were incubated with macrophages pulsed with the gp33 peptide in the presence (n=6 per group) or absence (n=3 per group) of 20 μM zVAD. CD4+CD8+ cell populations were examined using flow cytometry in order to determine % cell death. Data are presented as the mean±SEM. Representative data from more than three independent experiments are shown (A–C).
In order to further examine the effect of caspase 3 on negative selection, we also generated P14 TCR-transgenic caspase 3−/− mice, where the transgenic T cells express a Vα2/Vβ8.1 TCR heterodimer specific for the LCMV glycoprotein (gp) peptide gp33 and H-2Db. With this system, thymocyte death can be evaluated using different concentrations of gp33 peptide pulsed on peritoneal macrophages. A similar degree of cell death was observed in P14 caspase 3+/+ and P14 caspase 3−/− DP thymocytes (Fig. 1C).
Previous reports using caspase 3-deficient mice and caspase 3/caspase 7 double-knockout mice demonstrated that thymocyte cell death was not affected, although models of negative selection were not examined 21, 22. To evaluate the possibility that other caspases would compensate for the lack of caspase 3, we used the pan-caspase inhibitor zVAD. Although zVAD inhibited etoposide-induced thymocyte cell death by more than 50% (data not shown), gp33 peptide-induced cell death was generally unaffected by zVAD (Fig. 1C). Therefore, caspase 3, together with other caspases, may not have a critical role in negative selection.
Caspase 3 does not have a crucial role during LCMV infection
Studies have shown that the active form of caspase 3 is detected in antigen-specific CD8+ T cells after LCMV infection 19 and that caspase 3 may be involved in T-cell proliferation 23, 24. To evaluate the role of caspase 3 during virus infection, caspase 3+/+ and −/− mice were challenged with LCMV and the CTL responses on day 8 and day 14 were examined. In caspase 3−/− mice, CD8+ T-cell expansion after LCMV challenge was comparable to that in control mice, as similar numbers of CD8+ T cells were observed in the spleen on day 8 (Fig. 2A). In addition, there were no differences in the number of LCMV-specific CD8+ T cells, CTL activity, or CD44 upregulation between caspase 3−/− and control mice (Fig. 2B, C and data not shown). By day 14 post-infection, control mice infected with LCMV had about 8-fold fewer total CD8+ T cells and LCMV-specific CD8+ T cellsin the spleen, compared with the numbers at 8 days after infection (Fig. 2A and B). Although most LCMV-specific CD8+ T cells were also deleted in caspase 3−/− mice 14 days after LCMV challenge, the number of CD8+ T cells and CTL activity were slightly higher than WT control mice (Fig. 2A, B and C). It does remain possible that caspase 3 plays a role in a small subset of memory T cells that are not readily detectable by this analysis.
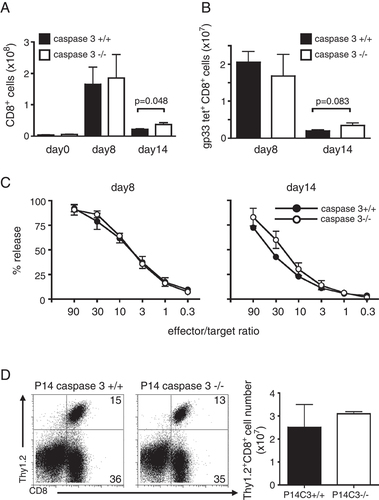
Caspase 3 deficiency does not affect viral responses following LCMV challenge. (A, B) Caspase 3+/+ and caspase 3−/− mice were injected i.v. with 2000 pfu LCMV and their spleens were harvested on day 0 (n=3 per group), day 8 (n=6 per group) and day 14 (n=6 per group). Cells were stained with anti-CD8 antibody and gp33/H2-Db tetramers and the number of cells was calculated. (C) Cytotoxic T-cell activity from splenocytes 8 days and 14 days after LCMV infection against gp33-pulsed targets. (D) 104 CD8+ T cells from P14 caspase 3+/+ or P14 caspase 3−/− mice (Thy1.2+) were adoptively transferred into Thy1.1+ congenic mice. Eight days after LCMV infection CD8+Thy1.2+ cells were evaluated by flow cytometry (two recipients per group in each experiment). Data are the mean±SEM. Statistical analysis of the data was performed with the unpaired Student's t test. Data from three (A–C) and two (D) independent experiments are shown.
To further evaluate the expansion of antigen-specific T cells in vivo, 1×104 WT or caspase 3−/− P14 T cells were transferred to Thy1.1+ congenic mice, which were then infected with LCMV. Eight days later similar numbers of P14 T cells were observed in the spleen regardless of whether or not the T cells expressed caspase 3 (Fig. 2D). Previous studies have shown enhanced proliferation and/or reduced apoptosis of caspase 3-deficient T cells, when stimulated with anti-CD3 and anti-CD28 antibodies in vitro 25. Although we have also confirmed those findings using anti-CD3 and anti-CD28 antibodies, as well as peptide-specific stimulation of P14 cells in vitro (data not shown), we do not observe increased numbers of virus-specific T cells in vivo. Perhaps this phenotype is related to different requirements that arise during the in vitro versus in vivo expansion of T cells. Together these data demonstrate that caspase 3 is not absolutely required for the expansion or contraction phases of CD8+ T cells during an acute anti-viral response.
Caspase 3 is not essential for peripheral T-cell tolerance
Next we assessed whether caspase 3 played a role in clonal deletion of CD8+ peripheral T cells. CD8+ T cells from female H-Y caspase 3+/+ or −/− mice were adoptively transferred into CD45.1+ congenic male mice and transferred T cells in the spleen were evaluated. On day 3, the number of H-Y TCR-transgenic cells in male mice given H-Y caspase 3+/+ T cells was clearly increased (Fig. 3A). However, the majority of these cells were eliminated by day 6, indicating that H-Y caspase 3+/+ T cells were deleted in the presence of the physiological antigen in vivo. Unexpectedly, the expansion and contraction of H-Y caspase 3−/− T cells were very similar to control T cells.
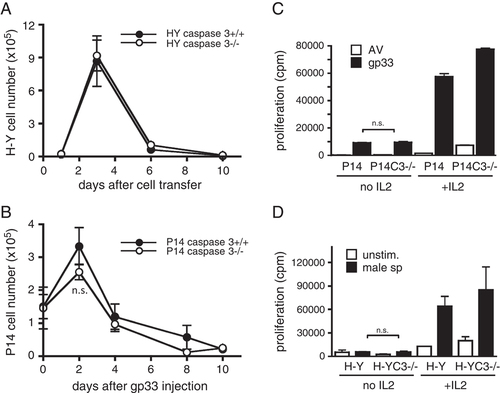
Normal peripheral T-cell deletion and induction of anergy in the absence of caspase 3. (A) CD8+ T cells isolated from female H-Y caspase 3+/+ or H-Y caspase 3−/− mice were transferred into CD45.1+ male mice. At the indicated time points, the splenic H-Y+CD8+CD45.2+ population was quantified by flow cytometry. (B) CD8+ T cells from P14 caspase 3+/+ and P14 caspase 3−/− mice were transferred into CD45.1+ mice, followed by the injection of gp33 peptide. At the indicated time points, the splenic CD45.2+CD8+ population was quantified by flow cytometry. Data are from three independent experiments using two recipients per time point in each experiment (A, B). (C) P14 caspase 3+/+ (n=4) and P14 caspase 3−/− (n=4) mice were treated with gp33 peptide to induce anergy. Mice were sacrificed on day 12 and 105 Vα2+CD8+ T cells were stimulated with gp33 or AV peptide in the presence or absence of 10 U/mL IL2, and proliferative responses were evaluated on day 2. (D) 105 H-Y+ T cells from male H-Y caspase 3+/+ and caspase 3−/− mice were stimulated with splenocytes from C57BL/6 male mice in the presence or absence of IL2 and proliferative responses were evaluated. Representative data from two independent experiments are shown (C, D). Data are expressed as the mean±SEM (A–D). n.s.: p>0.05 (t test).
To confirm that caspase 3 does not have an important role in deletion of peripheral CD8+ T cells, CD8+ T cells purified from P14 caspase 3+/+ and P14 caspase 3−/− mice were adoptively transferred into congenic CD45.1+ mice and 5 μg of gp33 peptide was administered i.v. on day 0, 3 and 6. The expansion and contraction of P14 caspase 3+/+ and P14 caspase 3−/− T cells were comparable in both genotypes (Fig. 3B). These results demonstrate that the clonal deletion of mature CD8+ T cells occurred normally in the absence of caspase 3.
It has been shown that caspase 3 is required for the induction of anergy in Th1 CD4+ T cells 20. Therefore, we next asked whether CD8+ T cells could be anergized in the absence of caspase 3. P14 caspase3+/+ mice and P14 caspase 3−/− mice were treated with gp33 peptide on day 0, 3 and 6 and sacrificed 12 days after the first injection. Splenocytes were normalized based on Vα2+ CD8+ cells and stimulated with gp33 peptide or negative control adenovirus peptide (AV) in the presence or absence of IL-2 and pulsed with [3H]-thymidine on day 2. The proliferative activity of WT T cells was minimal in the absence of IL-2, but was restored with the addition of IL-2. This is consistent with the induction of anergy. Notably the response of P14 caspase 3−/− T cells was similar as WT P14 T cells (Fig. 3C). We also confirmed this result using the H-Y TCR mouse model. T cells from H-Y caspase 3+/+ or H-Y caspase 3−/− male mice were incubated with splenocytes from C57BL/6 male mice with or without IL-2. As expected, H-Y caspase 3+/+ T cells from male mice were anergic. Furthermore, caspase 3 deficiency did not affect the anergy induction in the H-Y TCR model (Fig. 3D). Therefore, caspase 3-deficient CD8+ T cells can be rendered anergic in vivo.
Recent studies by Puga et al. have used a variety of models to induce anergy including ionomycin treatment and oral tolerance induction and have shown that caspase 3 is important for the induction of anergy in CD4+ Th1 cells 20. However, our models clearly demonstrate that CD8+ T-cell anergy induction in vivo does not require caspase 3. It is possible that ionomycin-induced anergy uses different mechanisms. Alternatively, there may be a difference between the molecular pathway that maintains anergy in CD4+ and CD8+ T cells. Further studies will be required to address these issues.
Concluding remarks
Caspase 3 has been shown to play a central role in both mitochondrial and death receptor apoptotic pathways. However, in this study we demonstrate that CD8+ T-cell death during thymic negative selection, the contraction phase upon LCMV infection and peripheral tolerance were normal in the absence of caspase 3. Compensation by other molecules and/or an important role for caspase-independent death pathways could be possible explanations. Since cell death is a fundamental process in multicellular organisms, alternative pathways are likely to exist that may play a more striking role in regulating T-cell death.
Materials and methods
Mice
The generation of P14 TCR-transgenic mice and caspase 3 knock out mice have been previously described 25, 26. C57BL/6 mice, CD45.1 mice, Thy1.1 mice and H-Y TCR-transgenic mice were obtained from The Jackson Laboratory. Mice were bred and maintained under specific pathogen-free conditions according to institutional guidelines. Mice used were between 6 and 12 wk of age. Caspase 3-deficient mice were born at a frequency lower than expected by Mendelian ratios. Runted or neurologically abnormal mice were not used for experiments.
Reagents
Anti-caspase 3 antibody from Cell Signaling was used for western blotting. For flow cytomeric analysis antibodies against CD4, CD8, CD45.2, Thy1.2 and H-Y TCR and Vα2 were obtained from eBiosciences. Db/gp33–41 MHC class I tetramers were used for tetramer analyses.
Cell suspension cultures
Thymocytes from P14 caspase 3+/+ and P14 caspase 3−/− mice were incubated with thioglycolate-activated macrophages pulsed with either gp33 peptide (KAVYNFATM) or non-stimulatory control AV peptide (SGPSNTPPEI) in the presence or absence of the pan-caspase inhibitor, zVAD (BD Pharmingen). After 20 h, cells were analyzed by flow cytometry and the percentage of cell death was calculated as (% DP cells after treatment with gp33 peptide)/(% DP cells after treatment with AV peptide)×100. Cell death in the presence of AV peptide was between 13 and 20%.
LCMV infection and CTL assay
Mice were immunized intravenously with 2×103 PFU LCMV Armstrong strain. For CTL assay, splenocytes were evaluated for killing of EL-4 target cells that were pulsed with 51Cr and LCMV-derived gp33 peptide or AV peptide. After a 5-h incubation the supernatants were counted using a γ counter. Percent-specific lysis were calculated as (cpm sample release − cpm spontaneous release)/(cpm maximal release − cpm spontaneous release)×100.
Acknowledgements
This work was supported by the National Cancer Institute of Canada with funds from the Canadian Cancer Society. P. S. O. holds a Canada Research Chair in Autoimmunity and Tumor Immunity.
Conflict of interest: The authors declare no financial or commercial conflict of interest.




