Rationale and design of the ESC Heart Failure III Registry – Implementation and discovery
ABSTRACT
Aims
Heart failure outcomes remain poor despite advances in therapy. The European Society of Cardiology Heart Failure III Registry (ESC HF III Registry) aims to characterize HF clinical features and outcomes and to assess implementation of guideline-recommended therapy in Europe and other ESC affiliated countries.
Methods
Between 1 November 2018 and 31 December 2020, 10 162 patients with chronic or acute/worsening HF with reduced, mildly reduced, or preserved ejection fraction were enrolled from 220 centres in 41 European or ESC affiliated countries. The ESC HF III Registry collected data on baseline characteristics (hospital or clinic presentation), hospital course, diagnostic and therapeutic decisions in hospital and at the clinic visit; and on outcomes at 12-month follow-up. These data include demographics, medical history, physical examination, biomarkers and imaging, quality of life, treatments, and interventions – including drug doses and reasons for non-use, and cause-specific outcomes.
Conclusion
The ESC HF III Registry will provide comprehensive and unique insight into contemporary HF characteristics, treatment implementation, and outcomes, and may impact implementation strategies, clinical discovery, trial design, and public policy.
Introduction
Heart failure (HF) is a global pandemic affecting more than 64 million people worldwide.1, 2 The incidence appears stable but the prevalence is increasing,3 especially of HF with preserved ejection fraction (HFpEF). Mortality and risk of HF hospitalization remain high and quality of life and functional capacity are poor.1, 2, 4 Compared to matched controls, patients with HF also appear to have lower socioeconomic status and have greater risk of not only HF and cardiovascular (CV) events but also of non-CV death and hospitalization, and longer duration of hospitalization if hospitalized for a non-CV reason.5
After the first heart transplantation in 1967, a large number of well-designed landmark randomized controlled trials (RCTs) in HF with reduced ejection fraction (HFrEF) demonstrated the efficacy of a number of classes of drugs that block maladaptive neurohormonal activation, as well as device, catheter-based and surgical interventions, and more recently neurohormonal modulation in the form of angiotensin receptor–neprilysin inhibitors (ARNi) and multi-mechanism treatment in the form of sodium–glucose cotransporter 2 inhibitors (SGLT2i),6 where the latter are effective also in HF with mildly reduced (HFmrEF) and preserved ejection fraction (EF).7
Despite these advances, outcomes in HF do not appear to be improving.8 There are two major challenges in improving outcomes and quality of life for patients with HF: (i) increasing appropriate use of existing evidence-based interventions; this is addressed in the field of implementation science9-13; and (ii) further characterizing clinical and biological characteristics of HF in order to develop novel interventions or new and more targeted use of existing interventions, and then testing these in properly designed RCTs; this is addressed in the field of discovery science.14-17 A well-designed HF registry can address and improve both implementation and discovery.13, 18 There are numerous completed and ongoing HF registries worldwide, including previous ones from the European Society of Cardiology (ESC),19-21 that indeed have considerably advanced quality of care, implementation, and our understanding of HF syndrome phenotypes.4, 22-31 However, there are few large HF registries with broad geographical coverage, covering HF that is chronic, acute (hospitalized), and worsening (hospitalized or managed in the emergency department or urgent outpatient setting), with all three relevant EF categories (HFrEF, HFmrEF, and HFpEF), and assessing detailed aspects of guideline adherence and treatment decisions, as well as both in-hospital and long-term outcomes.
Therefore, we designed the ESC HF III Registry to specifically study the implementation of contemporary evidence-based and guideline-recommended therapy, and to characterize HF phenotypes related to care setting, clinical status, EF category, and outcomes.
Methods
Design
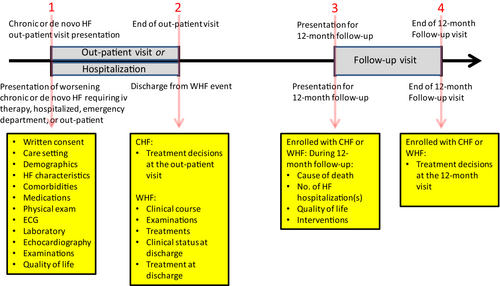
The ESC HF III Registry is a prospective, international, multicentre, observational registry study of patients with HF regardless of EF presenting with chronic or de novo HF for non-urgent outpatient visits (here termed chronic HF [CHF]) or with urgent worsening HF for care in the hospitalized, emergency department, or outpatient urgent care settings (here termed acute HF [AHF] if hospitalized with de novo HF, and worsening HF [WHF] if hospitalized or treated with intravenous medications for worsening of CHF). For CHF, data are collected on presentation and uniquely, at the end of the visit, reflecting therapeutic decisions. For AHF/WHF, data are collected at presentation and over the course of the AHF/WHF event (generally in-hospital), reflecting diagnostic and interventional decisions and in-hospital outcomes. For both CHF and survivors of the AHF/WHF event, data are collected at 12-month follow-up, reflecting changes in clinical status, medication changes, interventions, and outcomes (data collected shown in Figure 1). The study design is described in the protocol (online supplementary Appendix S1) and the detailed data captured are described in the case report form (CRF; online supplementary Appendix S2). The ESC HF III Registry complies with the 1975 Declaration of Helsinki; the locally appointed ethics committees have approved the research protocol, and informed consent has been obtained from the subjects (or their legally authorized representative).
Oversight
The ESC HF III Registry is sponsored by the ESC EURObservational Research Programme (EORP; https://www.escardio.org/Research/Registries-&-surveys/Observational-research-programme). The protocol was written by the HF III Chairperson with input from the EORP Oversight Committee (Appendix A1) and from the HF III Executive Committee, consisting of members of the Board of the Heart Failure Association (HFA) of the ESC (Appendix A2). National operations were overseen by the respective national coordinators (the Steering Committee, Appendix A3) who interacted with and liaised between the Chairman, the sponsor team at EORP, and local sites and principal investigators (Appendix A4).
Setting: Countries and sites
The ESC HF III Registry enrolled patients with HF in European, Mediterranean, and some non-European countries (Table 1). ESC country members, some who participated in previous ESC HF registries and some who were new, were invited through their National Cardiac Societies, who may have delegated responsibility to national HF working groups. In addition, and upon request, non-European countries with an affiliation with the ESC were invited to participate (information on ESC country members and affiliates is available on the ESC website: www.escardio.org).
| Country | Patients | Centres | Mean of patients per centre | Mean of patients per centre per month from first patient to enrolment close |
|---|---|---|---|---|
| Serbia | 976 | 7 | 139.4 | 5.68 |
| Poland | 892 | 13 | 68.6 | 3.26 |
| Spain | 700 | 8 | 87.5 | 6.07 |
| Italy | 695 | 17 | 40.9 | 2.44 |
| Russian Federation | 603 | 20 | 30.2 | 1.59 |
| Moldova, Republic of | 451 | 4 | 112.8 | 7.08 |
| Turkey | 427 | 19 | 22.5 | 1.02 |
| Morocco | 407 | 6 | 67.8 | 3.08 |
| France | 405 | 12 | 33.8 | 1.92 |
| Lithuania | 396 | 2 | 198.0 | 12.02 |
| Ukraine | 396 | 16 | 24.8 | 1.09 |
| Portugal | 376 | 6 | 62.7 | 4.27 |
| Egypt | 363 | 10 | 36.3 | 1.76 |
| Algeria | 351 | 9 | 39.0 | 1.53 |
| Romania | 251 | 8 | 31.4 | 1.57 |
| Israel | 209 | 4 | 52.3 | 2.50 |
| Bosnia and Herzegovina | 196 | 4 | 49.0 | 2.06 |
| Armenia | 190 | 4 | 47.5 | 2.15 |
| Croatia | 189 | 4 | 47.3 | 1.97 |
| Belgium | 168 | 6 | 28.0 | 2.39 |
| Slovenia | 164 | 2 | 82.0 | 7.84 |
| Kazakhstan | 150 | 6 | 25.0 | 1.08 |
| Malta | 132 | 1 | 132.0 | 6.99 |
| Kyrgyzstan | 124 | 1 | 124.0 | 5.90 |
| Cyprus | 120 | 2 | 60.0 | 3.00 |
| Hungary | 108 | 3 | 36.0 | 1.97 |
| Uzbekistan | 98 | 2 | 49.0 | 2.23 |
| FYR Macedonia | 85 | 2 | 42.5 | 1.67 |
| Finland | 82 | 1 | 82.0 | 4.00 |
| Belarus | 81 | 1 | 81.0 | 5.41 |
| Iraq | 59 | 1 | 59.0 | 3.65 |
| Mexico | 53 | 3 | 17.7 | 1.49 |
| Greece | 49 | 3 | 16.3 | 1.33 |
| Bulgaria | 45 | 3 | 15.0 | 0.90 |
| Slovakia | 44 | 3 | 14.7 | 1.18 |
| Norway | 42 | 2 | 21.0 | 1.15 |
| Estonia | 32 | 1 | 32.0 | 3.12 |
| Sweden | 22 | 1 | 22.0 | 5.49 |
| Brazil | 17 | 1 | 17.0 | 1.43 |
| Syrian Arab Republic | 8 | 1 | 8.0 | 0.62 |
| United Kingdom | 6 | 1 | 6.0 | 2.40 |
Site selection in each participating country targeted a sample of cardiology centres of different levels of complexity (the epidemiology of HF and HF resources and care settings in Europe have been characterized in the HFA Atlas32). Centres were offered participation through national coordinators or through direct contact with the executive committee. Participation was voluntary and there was no financial compensation. National coordinators worked with the HF III Executive Committee to list potential sites focusing on a broad spectrum of cardiology and HF units that regularly follow patients with chronic and/or worsening or acute HF. The national coordinator outlined the profiles of the proposed medical centres and indicated whether they were tertiary/community, with/without cardiac surgery, and with/without interventional cardiology. As far as possible, the centres selected represent different health care setting types and geographical criteria within each country.
Patients
The enrolment period spanned from 1 November 2018 to 31 December 2020. The target enrolment was 10 000 and actual enrolment was a total of 10 162 patients. Inclusion criteria were HF diagnosis in the judgement of the investigator, and that patients with AHF/WHF also needed to be treated with intravenous medications for HF (diuretics, vasodilators, and/or inotropes) and/or mechanical circulatory support or had died shortly after acute presentation before such treatment was instituted. The only exclusion criterion was age <18 years. Patients were eligible regardless of EF (reduced, EF ≤40%, HFrEF; mildly reduced, EF 41–49%, HFmrEF; or preserved, ≥50%, HFpEF) and with pre-existing chronic or de novo HF, and with ‘stable’ symptoms and signs presenting for non-urgent outpatient visit (CHF), or with worsening symptoms and signs, presenting for urgent care and treated in the hospital, in the emergency department, or in an outpatient urgent care setting (AHF and WHF). The WHF concept is novel and of particular importance. WHF can be defined as ‘worsening symptoms and signs of HF in patients with pre-existing HF, requiring intensification of treatment, most often diuretic therapy’,33 or as ‘deterioration of HF signs and symptoms in patients with CHF, despite previous stable background therapy, that requires urgent escalation of therapy, including hospitalization, emergency department visit, or outpatient intravenous diuretic therapy ± outpatient oral therapy’.34 Traditionally, both trials and registries have captured WHF only if it resulted in hospitalization (AHF). However, it is increasingly common with WHF as defined above and this is now generally captured as a clinical trial endpoint. Increasing oral diuretic therapy also represents WHF, but may be more arbitrary than intravenous therapy and there is currently no consensus to include increasing oral therapy in the WHF definition. Accordingly, the HF III Registry includes as acute a HF event requiring intravenous therapy and/or mechanical circulatory support and hospitalized or treated in the emergency department or in an outpatient infusion clinic.
Baseline, clinical decision-making, worsening heart failure event course, and 12-month outcome data
Detailed data elements and time points are described in the CRF (online supplementary Appendix S2) and in overview format in Figure 1. For clinical characterization and phenotyping of patients with differences in EF and other characteristics, detailed data were collected on symptoms and signs, biomarkers, and imaging and ancillary data, as well as hospital course (in WHF) and 12-month outcomes. For assessment of guideline implementation, detailed data were collected on medical and device therapy, including doses and reasons for non-use or non-achievement of target doses. In many other registries, outcomes collected are often crude, such as all-cause mortality and possibly hospitalization events. In contrast, the HF III Registry captures not only death and hospitalization and their specific causes, but also changes over time in quality of life, clinical status, and importantly, changes in treatment and the reasons for treatment changes (Figure 1). The criteria for selecting data elements were (i) availability of sufficient detail to allow detailed clinical characterization and phenotyping, and to not only describe adherence to guideline recommendations, but also investigate reasons for non-use of recommended medications, doses and other interventions, but at the same time (ii) not require data that are not generally available and/or that would require extensive effort for investigators and staff to procure, as both of these would increase data missingness and reduce enrolment and generalizability (Figure 2).
Data capture and storage
Data were entered manually by investigators and/or coordinators into a registry specific eCRF (data elements listed in online supplementary Appendix S2). The eCRF was accessible via a unique username and password distributed by the EORP team. Personal data were and are managed in accordance with General Data Protection Regulation (GDPR). Collected patient data are pseudonymized, meaning they are personal data that cannot be attributed to any specific person without the use of additional information. This additional information is unique data codes that can identify a patient in the EORP database. Data are stored in the registry specific central database with limited access protected by individual passwords. The ESC HF III Registry database is stored at the EORP Department, European Heart House, 2035 route des Colles, 06903 Sophia Antipolis, France. (Pseudonymization is in contrast to anonymization, where personal identifiers or codes are irreversibly destroyed, and data can no longer be traced to any individual).
Data quality and queries
The ESC HF III Registry database is designed, managed, controlled, and validated according to the ESC EORP standards. The eCRF has numerous built-in automatic cross-checks for data completeness, internal consistency, and validity that automatically raises alerts in case of data incompleteness or inconsistency, enabling investigators to resolve issues directly in conjunction with data entry. In addition, data were regularly assessed and validated by the EORP HF III Data Management Team, according to the Data Validation Plan. No formal central source data verification or adjudication was performed.
Access to data and data availability
Direct access to the ESC HF III Registry dataset is limited to the EORP HF III Data Management and Statistical Analysis teams. Country-specific datasets may be provided to the national cardiology societies/national coordinators upon request, for subsequent analysis of the country-specific data.
Aims of and potential research questions to be addressed by the ESC Heart Failure III Registry
The aims of the ESC HF III Registry are to characterize HF clinical characteristics and outcomes and to assess implementation of guideline-recommended therapy in Europe and ESC affiliated countries. These aims are broad but can be grouped according to many different narrower dimensions or domains. The 2021 ESC HF Guidelines6 and the 2022 ACC/AHA/HFSA HF Guidelines35 both list specific ‘Gaps in evidence’. Many gaps are related to treatment effect of existing interventions in new populations (e.g. HFmrEF) or to new or emerging interventions (e.g. novel drugs or catheter-based valve interventions). Because the HF III Registry is observational, no conclusions can be made about treatment efficacy. However, extensive analyses can be performed related to implementation and use of novel interventions, as well as patient characteristics associated with indications for, and associated with the cause-specific outcomes addressed with, these interventions. Table 2 lists some potential research domains and examples of research questions, together with specific gaps in evidence from the ESC and ACC/AHA/HFSA Guidelines, and potential impact for clinical care, implementation and future discovery and trial design. Table 3 lists specific research directions and specific research questions to be addressed by the HF III Registry.
| Unmet needs, objectives, research questions, and/or gaps in evidence | Examples and potential impact for clinical care, implementation and future discovery and trial design |
|---|---|
| Implementation science | |
| Implementation of diagnostics and therapy | There are now extensive diagnostic tools and extensive general and targeted interventions, but implementation is poor and variable, and little is known about which patient factors are associated with sub-optimal implementation. |
| Optimal models for patient follow-up | What is the role of HF specialty care versus general cardiology versus internal medicine, primary care or geriatrics care? It is known that cardiology versus primary care is associated with greater use of guideline-recommended diagnostics and treatment and with better outcomes, but little is known about the specific role of HF specialists and how the HF sub(−sub)-specialty may affect treatment decisions, quality of care and outcomes. |
| Patient care setting and systems of care | WHF is now treated in different settings: hospital, emergency department, and outpatient infusion clinics. Increasingly, all of these are being counted as endpoints in HF clinical trials. However, little is known about patient characteristics, treatment decisions and outcomes in these settings. |
| Association with use of diagnostics and therapy and association with outcomes | Clinical trial participation in different care settings in Europe has not been systematically characterized and this information may help in trial design and operations and enhance cooperation between academia and industry. |
| Unwarranted disparities and inequity in patient care | What is the role of age, sex, ethnicity, education, family status and work status on quality of care, use of GDMT, and outcomes? |
| Implementation of SGLT2 inhibitors | SGLT2 and SGLT2/1 inhibitors are now proven effective across the EF spectrum and in patients with and without diabetes and CKD, but so far little is known about the extent of uptake in clinical practice. |
| Use of more and less available diagnostics | ECG, echocardiography, chest X-ray, and biomarkers are now nearly universally available, but use is variable. Indications for, availability of, and use of lung ultrasound, right heart catheterization, coronary angiography, CT angiography, cardiac MRI, and endomyocardial biopsy are highly variable. |
| Use of targeted HF therapies | Numerous interventions are effective in HF sub-populations but may be complex, expensive, scarce, and/or otherwise difficult to implement, for example, potassium-binders, iv iron, percutaneous valve interventions, rhythm control in AF, short-term MCS, LVAD, heart transplantation, invasive and non-invasive remote monitoring, nurse-based HF clinics, exercise rehabilitation programmes, palliative care. |
| Screening for HF and outcomes | There is likely a considerable number of patients with undiagnosed HF in the community, and screening may improve diagnosis, treatment, and outcomes. Specifically, among patients with confirmed HF, there is under-use of evidence-based interventions. Screening patients in a HF registry for non-use of recommended therapy may nudge clinicians to implement these therapies and improve outcomes. |
| Cost-effectiveness of different strategies to screen for HF | |
| Clinical discovery | |
| Characteristics, pathophysiology and diagnosis of HFmrEF/HFpEF | Based on current definitions and literature, HFmrEF seems on average more similar to HFrEF than to HFpEF. However, HFmrEF remains poorly understood and there are only weak guideline recommendations and limited consensus on how to treat patients with HFmrEF. Furthermore, there is continued uncertainty in clinical trial design, whether to include patients with HFmrEF in HFrEF trials or in HFpEF trials. It is unclear if a simpler division into reduced and normal EF may be more practical. |
| Classification of HFrEF/HFmrEF/HFpEF | |
| Understanding advanced HF and AHF/WHF | Advanced HFrEF and AHF/WHF are common, associated with poor outcomes, and there is little evidence-based treatment. Additional profiles include hypotension, CKD, congestion, cardiorenal syndrome, diuretic resistance, pulmonary hypertension, right ventricular failure. Each of these profiles is associated with distinct risk markers/risk factors, with distinct outcomes, and have often been excluded from clinical trials. Large registries can address these and provide hypotheses for targeted treatments and ultimately improve outcomes. |
| Characterizing distinct patient profiles | |
| Characterization of advanced HFrEF | Characterization of advanced HFrEF to facilitate development of new interventions and new inotropes/myotropes/contractile agents for the many patients with advanced HFrEF who cannot undergo heart transplantation or placement of an LVAD. |
| Therapies targeting patients with advanced HF and patients excluded from clinical trials, for example, advanced kidney failure or hypotension | Understanding the tolerance of, use of, adherence to and persistence with GDMT in patients with advanced HFrEF, who often do not tolerate or adhere to GDMT. |
| Diagnostic modalities and outcomes | Lung ultrasound, right heart catheterization, coronary angiography, CT angiography, cardiac MRI, and endomyocardial biopsy have variable availability, or general availability but variable adoption, and how their results are associated with outcomes and/or improve care is uncertain. |
| AHF/WHF triggers | WHF is increasingly relevant as a clinical profile, risk marker for poor outcomes, and endpoint in clinical trials. But the risk markers/risk factors for, triggers of, and clinical characteristics of, patients treated in different settings, such as intensive care, hospital ward, emergency department, or outpatient infusion clinic, are poorly understood. |
| Hospital course | In-hospital complications and mortality remain a significant source of poor outcomes and high costs in HF. Detailed information on clinical presentation in WHF and AHF together with detailed information on hospital course can identify patients characteristics that may need special attention in the urgent setting, as well as patient characteristics more amenable to participation in clinical trials in AHF and WHF. |
| Asymptomatic left ventricular dysfunction and transition to symptomatic HF | Which detailed patient characteristics are associated with transition from NYHA class I to symptomatic HF? |
| Biomarkers for targeting and optimizing therapy | NT-proBNP (and BNP) is indicated for HF diagnosis and is useful for HF prognosis. But most literature on natriuretic peptides has studied ‘snap-shot’ concentrations rather than changes over time. There is limited data to support NT-proBNP guided therapy. NT-proBNP assessed over time and in relation to changes in other clinical characteristics and outcomes can improve the understanding of the longitudinal role of natriuretic peptides and biomarkers. |
| Serial and changes in NTproBNP | |
| Clinical trial design | Clinical trials, especially in HFpEF, may be diluted by a low risk of CV and HF events (and high risk of competing non-CV events). With detailed information on clinical risk markers, biomarkers, and imaging data, HF III can provide enrichment markers that can be used in isolation or as a ‘menu’ of choices among many inclusion criteria in clinical trials. |
| Machine learning and artificial intelligence | Both HFrEF, HFmrEF and HFpEF are heterogeneous. This makes targeted therapy and clinical trial design difficult. Novel machine learning and artificial intelligence methods could identify clusters with for example, especially high or low risk or ventricular arrhythmia, non-CV and non-modifiable events, or risk of incident hypotension, worsening kidney function, or hyperkalaemia. This information can inform both clinical decision-making and clinical trial design. |
| Phenotyping HFpEF | |
| Precision medicine and systems biology models | |
| Rapid implementation of GDMT | Clinical trials and guideline recommendations increasingly emphasize rapid and concurrent initiation and up-titration of multiple GDMTs, both in the chronic HF and hospital setting, and for some medications, also in the acute phase of WHF. However, the extent of, factors associated with, and safety of, these approaches are still poorly understood. |
| Order of adding disease-modifying drugs for HFrEF | |
| Safety of initiating multiple medical treatments concurrently and in de novo HF, AHF, stabilized in hospital, and in relation to blood pressure and eGFR | |
- The table lists and consolidates objectives and research questions from the ESC HF III Registry (in black text). If these gaps were specified in guidelines, they are color coded as follows: “Gaps in evidence” from the ESC 2021 HF Guidelines (in blue text) and “Evidence gaps and future research directions” from the 2022 ACC/AHA/HFSA HF Guidelines (in red text).
- AF, atrial fibrillation; AHF, acute heart failure; BNP, brain natriuretic peptide; CKD, chronic kidney disease; CT, computed tomography; CV, cardiovascular; ECG, electrocardiogram; EF, ejection fraction; eGFR, estimated glomerular filtration rate; GDMT, guideline-directed medical therapy; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; iv, intravenous; LVAD, left ventricular assist device; MCS, mechanical circulatory support; MRI, magnetic resonance imaging; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; SGLT, sodium–glucose cotransporter; WHF, worsening heart failure.
| Domain | Research question |
|---|---|
| Implementation science | |
| Physician specialty training | Which patient care settings and physician specialty training are common and how is physician specialty associated with patient characteristics, use of diagnostics and therapy, and outcomes? |
| Diagnostics | In patients with de novo and with pre-existing HF in the outpatient or acute HF setting, which diagnostic tools are used, such as echocardiography, lung ultrasound, right heart catheterization, endomyocardial biopsy, coronary angiography, and cardiac CT and MRI? How is use of diagnostics consistent with guidelines? What factors are associated with use of diagnostics and and how is use of diagnostics associated with outcomes? |
| Implementation of GDMT | What is the implementation of GDMT and adherence to guidelines regarding GDMT? Which factors are associated with use of GDMT? What is the extent of use and timing of use in different clinical settings? How common is discontinuation and which factors are associated with more or less initiation and discontinuation in the outpatient and hospitalized setting? |
| Targeted HF therapies | What is the implementation of interventions that are or may be indicated in some but not all patients with HF, for example, CRT, ICD, potassium binders, iv iron, rhythm control in AF, short-term MCS, LVAD, heart transplantation, invasive and non-invasive remote monitoring, nurse-based HF clinics, exercise rehabilitation programmes, palliative care? |
| Percutaneous valve interventions | Transcatheter edge-to-edge repair and valve replacement are rapidly evolving treatment options for patients with HF and secondary mitral and/or tricuspid regurgitation. How do patients in the HF III Registry resemble patients in trials of percutaneous valve interventions? What is the extent of secondary valvular regurgitation in relation to other parameters on echocardiography such as EF and LV and RV dimensions? |
| Indications for SGLT2/1 inhibitors and GLP-1 receptor agonists | SGLT2/1 inhibitors are now indicated in HF and GLP-1 receptor agonists are being extensively studied in various syndromes related to HF. Have these agents and are these agents being studied in ‘real-world’ patients with HF? What proportions of HF patients would meet indication for example, GLP-1 receptor agonists based on a BMI criterion? |
| Clinical discovery | |
| The role of EF | The EF measurement has been criticized as variable and arbitrary. How well does EF distinguish different underlying risk factors and aetiologies, clinical characteristics, and cause-specific outcomes? Are there other parameters from the echocardiogram (e.g. strain rate or LV volumes) or from other diagnostic examinations that better characterize and phenotype HF? Are such parameters feasible or even more suitable than EF for clinical use and clinical trial design? |
| Characterization of HFmrEF | From a perspective of aetiology, clinical characteristics and outcomes, does HFmrEF resemble more HFrEF or more HFpEF? How should future clinical trials consider HFmrEF – to be included in HFrEF trials or in HFpEF trials? |
| Understanding HFpEF | Is HFpEF a heterogeneous collection of multiple phenotypes or is there a universal common driver of HFpEF related to age, comorbidities, overweight, inactivity, and systemic inflammation? |
| Congestion in AHF and WHF | How common, how severe, and how reversible is congestion in de novo HF, chronic HF, and AHF/WHF, and how is congestion associated with EF, HF severity, and cardiovascular, HF and kidney outcomes? Can congestion scores be derived or validated as prognostic scores, treatment targets, and/or clinical phenotypes for future AHF trials? |
| Understanding iron deficiency | How common is iron deficiency and which parameters, such as ferritin, serum iron, and TSAT, are optimal for characterizing iron deficiency and its relation to anaemia, functional capacity, HF severity, and cause-specific outcomes? |
| Advanced HF | What is the hospital course in AHF/WHF and the 1-year cause-specific outcomes in CHF and post AHF/WHF in patients with advanced or severe HF? |
- AF, atrial fibrillation; AHF, acute heart failure; BMI, body mass index; CHF, chronic heart failure; CRT, cardiac resynchronization therapy; CT, computed tomography; EF, ejection fraction; GDMT, guideline-directed medical therapy; GLP-1, glucagon-like peptide-1; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter-defibrillator; LV, left ventricular; LVAD, left ventricular assist device; MCS, mechanical circulatory support; MRI, magnetic resonance imaging; RV, right ventricular; SGLT, sodium–glucose cotransporter; TSAT, transferrin saturation; WHF, worsening heart failure.
Discussion
In 2023, the two major challenges in heart failure are (i) implementation of existing evidence-based therapy, and (ii) discovery of new disease characteristics and targeted interventions and testing of these interventions in future optimally designed RCTs. The ESC HF III Registry may address both these needs. It is a comprehensive European and ESC affiliated HF registry with a target enrolment of 10 000 patients and actual enrolment of 10 162 patients. It includes data on chronic and acute/worsening HF, mild and advanced HF, all EF categories (HFrEF, HFmrEF, and HFpEF), demographic, clinical, biomarker and imaging data, treatment decisions and rationales, and in-hospital course and 12-month outcomes.
Poor implementation of evidence-based, guideline-recommended, regulatory approved, and payer reimbursed therapy for HF has been described in many registries and settings worldwide.4, 22-31 However, most reports have been merely descriptive, for example, providing percentage use for basic HF medications such as angiotensin-converting enzyme inhibitors, beta-blockers, and mineralocorticoid receptor antagonists. Details on diagnostic approaches and additional relevant medications, devices and other interventions are less often captured. Well-designed registries can, in addition to extensive descriptive data collection, also analyse and quantify underlying reasons for poor implementation, identifying specific patient groups with the greatest unmet needs or areas in most need of improvement, and thus allowing targeted initiatives to improve implementation. A majority of ‘real-world’ patients are eligible for HF drug therapy,36-38 so generalizability of trials should not be a major barrier to implementation. Instead, it appears that many barriers are addressable. For example, Swedish studies described poor implementation of evidence-based therapy for HFrEF.39, 40 Multivariable regression models suggested that one major reason was poor access to cardiology specialist physicians and HF nurses.12, 41, 42 As a result, in Sweden, the Stockholm region invested in additional HF specialist physicians and nurses at each large hospital, which resulted in greater use of HF medications and lower rates of HF hospitalization.43 Although this was not a randomized intervention or a strategy trial, it suggests that implementation science can identify targets for structural and strategic interventions that result in improved implementation of therapy and improved outcomes on a group level.
The ESC HF III Registry collects important information on factors that may determine use of therapy, such as age, blood pressure, estimated glomerular filtration rate, electrolytes, and also extensive additional information on for example, comorbidities and frailty-related aspects, and as such will be able to not only describe implementation but also suggest underlying independent reasons for poor implementation, and thus provide target for intervention on a policy level.
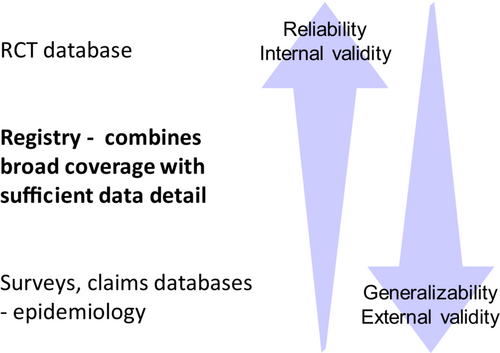
Although most patients with HF are eligible for HF treatment based on trial criteria, particularly on labels, there are substantial differences in generalizability of the study populations in different study settings. RCTs are complex and time consuming for both patients, investigators, and staff. Epidemiological surveys, often based on claims data and/or International Classification of Diseases (ICD) codes are large and generalizable but lack critical non-categorical variables such as EF, N-terminal pro-brain natriuretic peptide and other laboratory variables, and continuous vital parameters, such as blood pressure and heart rate. A well-designed registry strikes an optimal balance between sufficient data detail to reliably characterize HF, while avoiding excessively demanding data collection, leading to difficulties incorporating data collection into routine care and resulting in selective registration of few and less representative patients44 (Figure 2).
Despite effective therapy, and even in a setting of optimal implementation, HF remains a debilitating syndrome with a need for novel treatment strategies. This applies in particular to HFpEF, where only SGLT2i have been shown to improve outcomes,45-47 and to advanced HFrEF, where patients deteriorate despite, or may not tolerate, foundational HFrEF therapy.48-50 Well-designed registries can combine the collected data in emerging big data, machine learning, clustering or phenotyping approaches, to better define distinct clinical phenotypes, with clinical characteristics and/or outcomes that may be particularly amenable to intervention.51, 52 As such, registries do not inform treatment effects but do inform trial design for novel interventions or new use of existing interventions. The ESC HF III Registry combines clinical, biomarker, and detailed imaging data and may be able to identify novel HF phenotypes with particular characteristics or outcomes, and this information may inform subsequent HF trial design.
Finally, an emerging potential for HF registries is the actual conduct of randomized trials. The Registry-based RCT (RRCT) has received attention in particular in the setting of acute coronary syndrome, but may be extendable to chronic syndromes such as HF.44 Given that barriers to implementation are often specific and addressable, HF registries can also serve as foundations for strategy trials and implementation trials, using for example, cluster randomization or using screening approaches to identify therapeutic inertia and underuse.53, 54 An emerging initiative, the European Unified Registries for Heart Care Evaluation and Randomized Trials (EuroHeart), is an ESC and European initiative to further improve and standardize CV registries and may in the future be able to not only assess quality of care and implementation as well as clinical characteristics and phenotypes, but also serve as platforms for randomized trials.55 There is considerable interest in establishing local HF registries based on the success of large existing registries, but joint collaboration and participation in geographically diverse registries such as the present HF III and the future EuroHeart avoids ‘re-inventing the wheel’. National, regional, and local participants in large registries such as HF III and EuroHeart can participate in the overall registry and at the same time receive national, regional, or local data back for analysis, and as such can avoid the effort and expense of developing an independent registry.
Despite the combination of detailed and granular data collection and broad and generalizable coverage (Figure 2), the HF III has limitations. Participating sites were all cardiology centres, which means that patients and providers are selective. In many countries, patients with HF are cared for in primary care. Access to cardiology care differs according to age, sex, EF, income, education, and other factors, and access to cardiology care in turn affects quality of care and outcomes.18, 40, 56-58 Thus, patients in HF III will be younger, more commonly male, and receive higher quality of care and have better outcomes than the overall HF population in Europe, which compromises generalizability.
In conclusion, the ESC HF III Registry will provide comprehensive and unique insight into contemporary HF characteristics, treatment implementation and outcomes, and may impact implementation strategies, clinical discovery, trial design and public policy throughout Europe and ESC affiliated countries.
Acknowledgements
The EORP Oversight Committee, Registry Executive Committee acknowledges the contribution of patients, national leaders, local investigators, and staff, and the EORP project management team: Cecile Laroche as Statistical Project Lead, Quentin Escartin as Clinical Project Manager, Maryna Andarala as Data Manager, and Afiah Zabre and Gabrielle Bonneville as Project Officers (study launch, data collection, coordination, data management, and statistical analyses). Special thanks to the Heart Failure Association of the ESC.
Funding
This work was supported by the following companies since the start of EORP and for the period of the HF III study: Abbott Vascular Int. (2018–2021), Amgen Cardiovascular (2016–2018), AstraZeneca AB (2017–2020), Bayer AG (2016–2018), Boehringer Ingelheim (2016–2019), Bristol Myers Squibb (2017–2019), Daiichi Sankyo Europe GmbH (2017–2020), Edwards Lifesciences (2016–2019), Novartis Pharma AG (2018–2020), Servier (2015–2021), and Vifor (2019–2021).
Conflict of interest: There are no conflicts of interest related to the ESC HF III Registry or to this manuscript. Outside of the submitted work, there are the following disclosures: L.H.L. declares grants or contracts from AstraZeneca, Vifor Pharma, Novartis, Boston Scientific, and Boehringer Ingelheim; consulting fees for trial design and/or development/implementation strategy from Merck, Vifor Pharma, AstraZeneca, Bayer, Pharmacosmos, MedScape, Sanofi, Lexicon, Myokardia, Boehringer Ingelheim, and Servier; payment or honoraria for lectures from Abbott, MedScape, Radcliffe, AstraZeneca, and Novartis. He also serves on the Board of the Heart Failure Association of the ESC, the Board of the Swedish Society of Cardiology, HF Working Group and is as a Fellow of the ESC. He reports holding stock in AnaCardio. M.G.C.L. declares grants or contracts from AstraZeneca and Vifor Pharma; consulting fees from Medtronic and Takeda; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Boehringer Ingelheim, Novartis, AstraZeneca, Vifor Pharma, CareDx, Astellas, Abbott, Medtronic, and Bayer; and support for attending meetings and/or travel from Novartis and Abbott. E.B.V. declares payments made to her for honoraria for lectures and/or educational materials/events from Berlin Chemie AG, and Johnson & Johnson; consulting fees for Egis Pharma. A.K.C. declares payments made to her for honoraria for lectures and/or educational materials/events from Angelini Pharma, AstraZeneca, Bayer, Bausch Health, Boehringer Ingelheim, KRKA, Pfizer, Polpharma, and Servier. S.D.A. declares grants for IITs from Vifor Int and Abbott; consulting fees for serving on Ad-hoc advisory board from Novartis, for ad-hoc consultancy from Cordio, Cytokinetics, Faraday Pharmaceuticals, GSK, Sensible Medical, Amgen, Bioventrix, CVRx, Sanofi, and Vectorious, for ad-hoc consultancy/ad-hoc advisory board from Astra Zeneca, Repairon, Novo Nordisk, and Brahms, and for serving on the advisory board from Actimed Therapeutics and HeartKinetics; he reports being named co-inventor of two patent applications on MR-proANP (DE 102007010834 & DE 102007022367) while not benefitting personally from the related issued patents; fees for Covid19 advisory board work from Abbott; serving on the Registry Steering Committee for Servier; doing trial committee work & consultancy for Cardior, trial steering committee work for Impulse Dynamics and Pfizer; trial steering committee work & consultancy for Bayer AG, Boehringer Ingelheim, V-Wave, Cardiac Dimensions, and Occlutech; and trial/registry steering committee work & consultancy for Vifor Int. O.C. reports support for attending ESC Congress from Servier. A.J.S.C.s declares having received honoraria and/or lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Edwards, Eli Lilly, Menarini, Novartis, Servier, Vifor, Abbott, Actimed, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, Impulse Dynamics, Respicardia, and Viatris. G.F. declares support for the present manuscript (e.g. funding, provision of study materials, medical writing, article processing charges, etc.) and grants or contracts from the European Commission; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bayer, Boehringer Ingelheim; participation in a Data Safety Monitoring Board or Advisory Board from Bayer; and leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid with the Heart Failure Association of the ESC and JACC Heart Failure. T.M. declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Abbott, Edwards, Boehringer and Ingelheim. M.M. reports personal fees of minimal amounts in the last 3 years for consulting from Actelion, Amgen, Livanova and Vifor pharma as member of Executive or Data Monitoring Committees of sponsored clinical trials; from AstraZeneca, Abbott Vascular, Bayer, Boehringer Ingelheim and Edwards Therapeutics for participation in advisory boards and/or speeches at sponsored meetings; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events for one sponsored meeting supported by Abbott Vascular. M.P. declares speaker's fees from AstraZeneca, Boehringer Ingelheim and Novo Nordisk; payment for writing or reviewing the manuscript: Menarini, Servier; he serves on the ESC board. G.M.C.R. declares grants or contracts from Ricerca Corrente Ministero della Salute, Italy; and support for attending meetings and/or travel from Menarini, Vifor, AstraZeneca, Boehringer, Bayer, and Servier. F.R. declares not having received personal payments by pharmaceutical companies or device manufacturers in the last 3 years; the Department of Cardiology (University Hospital of Zurich/University of Zurich) reports research, educational and/or travel grants from Abbott, Amgen, AstraZeneca, Bayer, Berlin Heart, B. Braun, Biosense Webster, Biosensors Europe AG, Biotronik, BMS, Boehringer Ingelheim, Boston Scientific, Bracco, Cardinal Health Switzerland, Corteria, Daiichi, Diatools AG, Edwards Lifesciences, Fresenius, Guidant Europe NV (BS), Hamilton Health Sciences, Kaneka Corporation, Kantar, Labormedizinisches Zentrum, Medtronic, MSD, Mundipharma Medical Company, Novartis, Novo Nordisk, Orion, Pfizer, Quintiles Switzerland Sarl, Sahajanand IN, Sanofi, Sarstedt AG, Servier, SIS Medical, SSS International Clinical Research, Terumo Deutschland, Swiss National Foundation, Trama Solutions, V-Wave, Vascular Medical, Vifor, Wissens Plus, ZOLL. The research and educational grants do not impact on Prof. Ruschitzka's personal remuneration; remuneration for the time spent in the following consulting activities were made directly to the University of Zurich, and do not impact on Prof. Ruschitzka's personal remuneration, from AstraZeneca (IMC), Bayer, Boehringer Ingelheim, Citi Research, Klub Class, Novo Nordisk, Radcliffe Group, Stiftung Pfizer Forschungspreis, and Vifor; remuneration for following lectures were made directly to the University of Zurich, and do not impact on Prof. Ruschitzka's personal remuneration, from Abbott, Amgen, AstraZeneca (A+ Science AB), Bayer (At the Limits), Boehringer Ingelheim, Boston Scientific (CCE Services), Brigham and Women's Hospital Boston, C.T.I GmbH, Hôpitaux Universitaires des Genève (GECORE), Luzerner Kantonsspital, Sanofi-Aventis, Servier, Medscape (WebMD), Medtronic, Medworld, Novartis, Roche, Ruwag, Swiss Heart Failure Academy, The Hong Kong Heart Failure Society, Trama Solutions SL, Inselspital Bern, Charité–Universitätsmedizin Berlin (Medical Education Global Solutions), Romanian Society of Cardiology, ÖKG Österreichische Gesellschaft für Kardiologie; Support for attending meetings and/or travel from AstraZeneca (IMC/A+ Science AB), Boehringer Ingelheim, Centro Hospitaler de Vila Nova de Gaia, C.T.I. GmbH (Universitätsklinikum Düsseldorf), European Society of Cardiology, Novartis, Spektar Putovanja, Austrian Heart Failure Association, Heart Failure Association of the ESC; remuneration for following Advisory Boards were made directly to the University of Zurich and do not impact on Prof. Ruschitzka's personal remuneration, from Bayer: HF Expert Summit, Advisory Board Meeting; Roche: Advisory Board Meeting; IMC/AstraZeneca: Advisory Board Meeting; Amgen: Advisory Board Meeting; secretarial and administrative support of the HFA President/Past-President 2018–2020 of the ESC. G.S. reports grants or contracts from Vifor Pharma, Novartis, Boehringer Ingelheim, Boston Scientific, AstraZeneca, Pharmacosmos, Merck, Bayer, Cytokinetics, Horizon 2022 funding; consulting fees from TEVA, MIUR (Ministero dell'Istruzione, Università e Ricerca), Medical Education Global Solutions, Atheneum, Genesis, Vifor Pharma, and Agence Recherche (ANR); payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Servier, Cytokinetics, Medtronic, Dynamicom Education, Vifor Pharma, Roche, Translational Medicine Academy Foundation (TMA), Medical Education Global Solutions, AstraZeneca, and Novartis; support for attending meetings and/or travel from Boehringer Ingelheim; participation in a Data Safety Monitoring Board or Advisory Board for AstraZeneca, Uppsala Clinical Research Center (UCR), Servier, Edwards, and Vifor. A.P.M. received grants or contracts for participating in study committees from Novartis, and AstraZeneca. All other authors have nothing to disclose.
Appendix A1: EORP Oversight Committee
2016–2018: A. Vahanian, FR (Chair); A. Budaj, PL; N. Dagres, DE; N. Danchin, FR; V. Delgado, NL; J. Emberson, GB; O. Friberg, SE; C.P. Gale, GB; G. Heyndrickx, BE; B. Iung, FR; S. James, SE; A.P. Kappetein, NL; A.P. Maggioni, IT; N. Maniadakis, GR; K.V. Nagy, HU; G. Parati, IT; A-S. Petronio, IT; M. Pietila, FI; E. Prescott, DK; F. Ruschitzka, CH; F. Van de Werf, BE; F. Weidinger, AT; U. Zeymer, DE.
2018–2020: C.P. Gale, GB (Chair); B. Beleslin, RS; A. Budaj, PL; O. Chioncel, RO; N. Dagres, DE; N. Danchin, FR; J. Emberson, GB; D. Erlinge, SE; M. Glikson, IL; A. Gray, GB; M. Kayikcioglu, TR; A.P. Maggioni, IT; K.V. Nagy, HU; A. Nedoshivin, RU; A-P. Petronio, IT; J.W. Roos-Hesselink, NL; L. Wallentin, SE; U. Zeymer, DE.
2020–2022: B.A. Popescu, RO (Chair); D. Adlam, GB; A.L.P. Caforio, IT; D. Capodanno, IT; Ovidiu Chioncel, RO; M. Dweck, GB; D. Erlinge, SE; Laurent Fauchier, FR; Marek Gierlotka, PL; M. Glikson, IL; Tina Hansen, DK; J. Hausleiter, DE; B. Iung, FR; M. Kayikcioglu, TR; P. Ludman, GB; L. Lund, SE; A.P. Maggioni, IT; Julien Magne, FR; S. Matskeplishvili, RU; B. Meder, DE; Julinda Mehilli, DE; K.V. Nagy, HU; A. Nedoshivin, RU; D. Neglia, IT; A.A. Pasquet, BE; Eva Prescott, DK; J.W. Roos-Hesselink, NL; F.J. Rossello, ES; S.M. Shaheen, EG; A. Torbica, IT.
2022–2024: B. Iung, FR (Chair); B. Popescu, RO (Past-Chairperson); D Adlam, UK; C Bouleti, FR; A Caforio, IT; D Capodanno, IT; L Fauchier, FR; M Gilard, FR; S James, SE; P Ludman, UK; J Magne, France; A Pasquet, BE; T Pilgrim, CH; J Rossello, ES; S Shaheen, EG; AvTimoteo, PO; A Torbica, IT; U Zeymer, DE.
Appendix A2: ESC HF III Registry Executive Committee (Board Members of the HFA of the ESC)
Lars H. Lund, SE (Chairperson); Marisa Crespo-Leiro, ES (Immediate Past Chair); Petar Seferovic, RS; Frank Ruschitzka, CH; Gerasimos Filippatos, GR; Alexandre Mebazaa, FR; Massimo Piepoli, IT; Andrew Coats, AU; Stefan Anker, DE; Theresa McDonagh, UK; Mitja Lainscak, SI; Giuseppe Rosano IT; Aldo Maggioni, IT (First Chair; EORP representative).
Appendix A3: ESC HF III Registry Steering Committee (National Coordinators)
Lars Lund, SE (Registry Chair); Ahmed Bennis, MA; Andrejs Erglis, LV; Andrzej Gackowski, PL; Alena Kurlianskaya, BY; Amina Rakisheva, KZ; Alex Simms, GB; Barnabas Gellen, FR; Bela Merkely, HU; Candida Fonseca, PT; Daniela Cassar Demarco, MT; Duska Glavas, HR; Eva Goncalvesova, SK; Eleonora Vataman, MD; Elizabeta Srbinovska Kostovska, MK; Erkin Mirrakhimov, KG; Gani Bajraktari, XK; Grigorios Giamouzis, GR; Artan Goda, AL; Gulnaz Dadashova, AZ; Hamayak Sisakian, AM; Hadi Skouri, LB; Heli Tolppanen, FI; Israel Gotsman, IL; Jean Beissel, LU; Jelena Celutkiene, LT; Jose Manuel Garcia Pinilla, ES; Larisa Dizdarevic-Hudic, BA; Lars Lysgaard Gullestad, NO; Leonid Voronkov, UA; Magdy Abdelhamid, EG; Micha T. Maeder, CH; Mitja Lainscak, SI; Morten Schou, DK; Massimo Francesco Piepoli, IT; Marija Polovina, RS; Milos Taborsky, CZ; Mikheil Tsverava, GE; Naima Hammoudi, DZ; Ovidiu Chioncel, RO; Petra Van Pol, NL; Plamen Gatzov, BG; Belma Pojskic, BA; Petar M. Seferovic, RS; Rudolf Berger, AT; Stefan Stoerk, DE; Timur Abdullaev, UZ; Tiina Uuetoa, EE; Vassilis Barberis, CY; Vyacheslav Mareev, RU; Wachter Rolf, DE; Walter Droogne, BE; Yuksel Cavusoglu, TR; Zumreta Kušljugić, BA.
Appendix A4: ESC HF III Registry Investigators
Algeria: Algiers: S. Benkhedda, D. Djermane, M. Baouni, F. Benouareth, K. Mouzaoui, N. Dahimene, S. Mansouri, F. Kerkouri, Algiers: A. Chibane, M. Djouhri, S. Benferhat, L. Talbi, Algiers: Y. Bouhouita-Guermech, R. Benkouar, A. Boudrifa, H. Elnaajer, K. Bouasria, O. Kassoul, Y. Tir, Algiers: D-E. Nibouche, A. Sik, A. Bounah, Algiers: N. Hammoudi, G. Sofiane, Algiers: M. Bouame, A. Sayah, E. Tebbache, Bejaia: A. Kachenoura, Beni Messous: F. Daimellah, Blida: M.T.C. Bouafia, M. Bouafia, N. Dammene Debbih, W. Takdemt, Armenia: Gyumri: T. Manukyan, Yerevan: L. Tumasyan, A. Chilingaryan, A. Stepanyan, L. Tunyan, Yerevan: H. Hayrapetyan, K. Azaryan, M. Tadevosyan, H. Poghosyan, Yerevan: H. Sisakian, G. Martirosyan, L. Sahakyan, M. Hovhannisyan, S. Pepoyan, Belarus: Minsk: A. Kurlianskaya, D. Salauyou, A. Kozyrava, O. Shatova, T. Troyanova-Shchutskaia, Belgium: Aalst: M. Vanderheyden, A. Moya, H. Batjoens, Brussels: A-C. Pouleur, O. Gurne, E. Tshilanda, F. Severino, Genk: W. Mullens, M. Dupont, S. Christiaens, C. Claes, S. Lenaerts, Kortrijk: D. Derthoo, M. Dumoulein, S. Naessens, I. Senesael, M. De Coninck, Leuven: W. Droogne, E. Sels, V. Servaes, Liege: P. Troisfontaines, E. Hoffer, M. Melissopoulou, D. Malmendier, M. Massoz, S. Jacquet, V. Dinraths, Bosnia and Herzegovina: Sarajevo: E. Begic, A. Mujakovic, A. Subo, A. Selimagic, F. Custovic, D. Mackic, E. Dzambasovic-Karaselimovic, A. Cehajic, A. Djozic, A. Biscevic-Obradovic, F. Kadic, E. Hrvat, I. Kurbasic, M. Tuce, Sarajevo: A. Durak-Nalbantic, A. Dzubur, E. Jahic, S. Sokolovic, Z. Gljiva Gogic, K. Aganovic, F. Zvizdic, A. Begic, N. Resic, E. Hodzic, M. Halilcevic, M. Vucijak Grgurevic, N. Hadžibegic, N. Sabanovic Bajramovic, S. Miseljic, Tuzla: E. Smajic, Z. Kušljugic, L. Dizdarevic-Hudic, K. Kovacevic, D. Loncar, M. Kovacevic, M. Selimovic, U. Pajic, I. Hudic, A. Avdic, A. Bijedic, A. Brkic, D. Mršic, E. Becirovic, I. Bijedic, Zenica: B. Pojskic, J. Djelmic, M. Ejubovic, L. Pojskic, E. Ramic, M. Sammak, H. Selimovic, E. Stimjanin, M. Sut, H. Torlak, I. Bisco, D. Bogicevic, A. Brkovic, Brazil: Caetanópolis: R. Salgado, A. Silva, B. Azeredo, D. De Souza, I. Couri, L. Salgado, Bulgaria: Plovdiv: M. Tokmakova, D. Jovanovska, Sofia: N. Runev, B. Stoimenov, E. Manov, R. Pancheva, V. Kolev, Sofia: V. Mincheva, V. Tsanova, O. Eftimova, I. Gruev, M. Mintchev, V. Stoyanovski, T. Petrusheva, Croatia: Opatija: V. Persic, M. Barisic, D. Raljevic, Split: D. Glavas, D. Milicic, J.A. Borovac, T. Zaninovic Jurjevic, J. Sikic, V. Persic, K. Pesek, Zabok: K. Pesek, S. Roginic, N. Borsic, Zagreb: J. Sikic, T. Friscic, Z. Planinic, Cyprus: Nicosia: T. Christodoulides, S. Kakoulli, Nicosia: V. Barberis, T. Michaelidou, Egypt: Assiut: H. Hasan-Ali, M. Abdel Ghany, A. Halim, S. Dimitry, Aswan: P.P.S. Selwanos, M. Hassan, Benha: A-S.M. Sabry, Beni Suef: Y.A. Abdelhady Ahmed, T. Osman, M. Eshak, Cairo: M. El Sayed, K. Menyawi, A. Roshdy, W. El Kilany, A. Abdeltawab, A.R. El Sayed, Cairo: O. Botrous, A. El Bagoury, Cairo: A. Samir, H. Kandil, M. Hosny, M. Abdelghany, M. Abdelhamid, M.K. Shokry, S. Essam, A. Kamal, Giza: H. Ammar, A. Kamel, A. Magdy, H. Elnesr, M. Amin, Mansoura: M.M. Yousif, A.H. Eladawy, M.F. Areed, M.H. Abdelnabi, A.G. Mahmoud, M.F. Abdelrahman, Port Said: A. Elbahry, Zagazig: A. Saad, M. Ali, Estonia: Tallinn: T. Uuetoa, S. Udalova, T. Anier, S. Viks, B. Veermäe, Finland: Helsinki: H. Tolppanen, K. Oksaharju, France: Bron: N. Mewton, A. Jobbé Duval, C. Boiteux, C. Bergerot, G. Baudry, L. Sebbag, M. Buisson, D. Mohamed-Said, F. El Harrane, M. Koenig, N. El-Jonhy, Créteil: A. Galat, D. Bodez, C. Chalard, F. Djelaili, S. Guendouz, S. Oghina, T. Damy, M. Idjellidaine, M. Kharoubi, N. Djefel, Y. Sellah, Douai: R. Pilato, Fort de France: J. Inamo, A. Monfort, N. Ozier-Lafontaine, Grenoble: D. Guijarro, T. Fourme, A. Monard, P. Bouheret, Grenoble: M. Salvat, A. Boignard, C. Augier, C. Casset, M. Maurin, E. Plan, D. Pollet, Kremlin-Bicêtre: P. Jourdain, E. Berthelot, M.T. Bailly, N. Hrynchyshyn, G. Leprun, Le Chesnay: B. Livarek, J-L. Georges, N. Baron, C. Charbonnel, J-B. Azowa, Le Mans: C. Bachelet, P. Cloitre, P. Poret, S. Braun, O. Zid, G. Kabalu, A. Denizet Mulocher, C. Bros, C. Dericbourg, C. Saint Andre, E. Lefebvre, J-F. Laine, G. Terrien, J-C. Amirault, D. Lecuyer, M. Rousseau, Paris: F. Beauvais, A. Cohen-Solal, D. Logeart, N. Bennacer, Poitiers: B. Gellen, Toulon: J-M. Tartiere, F. Challal, F. Fellini, R. Landes, A. Elkeurti, C. Genin, P. Armangau, V. Gardan, Toulouse: M. Galinier, O. Lairez, J. Roncalli, C. Biendel, C. Delmas, C. Delon, E. Cariou, P. Fournier, R. Itier, Greece: Athens: A. Karavidas, D. Balta, C. Mandila, E. Velaoras, I.N. Karavidas, Ioannina: L. Michalis, K. Naka, A. Bechlioulis, L. Lakkas, A. Rammos, I. Dimou, Thessaloniki: C. Karvounis, M-A. Bazbani, Hungary: Budapest: B. Merkely, A. Kosztin, Budapest: N. Nyolczas, D. Vagany, I. Juhasz, S. Papp, M. Szabo, E. Szogi, Hodmezovasarhely: A. Pálinkás, I. Szoko Csaszar, I. Juhasz Nagy, S. Rostásné Toth, Pecs: T. Habon, M. Rabai, R. Halmosi, R. Gal, L. Illes, S. Kovacsne Levang, Iraq: Baghdad: H. Ali Farhan, I.F. Yaseen, Israel: Haifa: E. Radzishevsky, Jerusalem: I. Gotsman, O. Ezra, Safed: T. Levinas, I. Nordkin, S. Benyamin, Tel Aviv: M. Laufer-Perel, A. Milwidsky, B. Sadeh, D. Wexler, O. Havakuk, Y. Arbel, M. Revivo, S. Sadon, Italy: Brescia: M. Metra, L. Italia, L. Rossi, Cassano Delle Murge: A. Passantino, R. Carbonara, C. Rizzo, R. La Gioia, Florence: M. Milli, F. Grossi, Foggia: N.D. Brunetti, A. Mallardi, M. Correale, L'Aquila: M. Penco, G. Patti, S. Romano, R. Petroni, E. Salustri, S. Minardi, Lumezzane: S. Scalvini, E. Zanelli, A. Olivares, Milan: P. Agostoni, M. Doldi, E. Salvioni, P. Gugliandolo, Milano: A. Frisinghelli, C. Calloni, Monserrato: C. Cadeddu Dessalvi, M. Deidda, A. Peccianti, D. Cocco, E. Garau, Naples: A. Cittadini, A. Salzano, F. Giallauria, G. Crisci, R. Lucci, A.M. Marra, V. Valente, R. D'assante, Naples: P. Perrone-Filardi, P. Gargiulo, S. Paolillo, L. Bardi, S. Dellaversana, Palermo: G. Novo, A. Ferlisi, D. Adorno, E. Bonni, E. Corrado, F. Sabatino, S. Novo, Piacenza: M.F. Piepoli, B. Matrone, G. Halasz, L. Moderato, V. Pelizzoni, Rome: M. Mancone, C. Miotti, E. Pagliaroli, M. Magnocavallo, San Vito al Tagliamento: D. Pavan, T. Durat, V. D'Onofrio, A. Gardin, G. Ganci, F. Martinis, N. Pezzutto, S. Maier, Trieste: G. Sinagra, M. Merlo, L. Restivo, F. Ramani, Udine: D. Miani, M. Baldassi, M. Driussi, Verona: F.L. Ribichini, L. Maritan, M. Cicoira, M. Lia, M. Setti, M. De Stefano, Kazakhstan: Aktobe: B. Zholdin, G. Mamedova, R. Iznatova, Y. Sim, Almaty: R. Tuleutayev, A. Nurmukhambetova, I. Yakupova, D. Urazbekov, Almaty: N. Aidargaliyeva, A. Akanova, A. Makhmudova, A. Tleules, G. Nurbekova, M. Akilbekova, S. Nurlybai, Almaty: A. Aizhan, Almaty: A. Rakisheva, A. Illyassova, Nur-Sultan: A. Dzholdasbekova, Z. Ismagulova, A. Kassenova, M. Alkenova, A. Aizhan, Kyrgyzstan: Bishkek: E. Mirrakhimov, K. Neronova, J. Esenbekova, Lithuania: Kaunas: D. Zaliaduonyte, J. Laukaitiene, J. Borkyte, A. Mazutaviciute, R. Norvilaite, Vilnius: J. Celutkiene, E. Paleviciute, J. Simonavicius, L. Gedvilaite, M. Laukyte, E. Lycholip, T. Simbelyte, Malta: Msida: R.G. Xuereb, S. Xuereb, W. Camilleri, M. Farrugia, J. Fleri Soler, L-L. Buttigieg, R. Xuereb, A.M. Moore, D. Cassar Demarco, C. Attard, M. Vella, I. Grech, R. Bonnici, R. Buhagiar, G. Buhagiar, T. Farrugia, Mexico: Culiacan, Sinaloa: M.O. De Los Rios Ibarra, A.Z. Baños Velasco, F.M. Vizcaíno Rios, Mexico: A. Mendez, A. Alvarez, C. Guizar, Queretaro: S. Leon Gonzalez, L. Resendiz-Barron, Moldova, Republic Of: Chisinau: E. Vataman, D. Bursacovschi, D. Lisii, E. Paraniuc, J. Cazacu, M. Dogot, N. Botnari, Chisinau: L. Grib, S. Filimon, E. Samohvalov, L. Purteanu, Chisinau: A. Grivenco, Chisinau: V. Revenco, I. Cabac-Pogorevici, I. Cojuhari, Morocco: Casablanca: A. Bennis, S. Chraibi, A. El Makhlouf, S. Ferhi, S. Soulami, N. Mouine, A. Moustaghfir, K. Adnan, C. Bencheqroun, Casablanca: G. Abidi, H. Elmousalami, S. Hajib, L. Bouzoubaa, M. Ghita, I. Nassiri, S. Abderrazak, Z. Benchaouia, G. Benhayoune, N. Boughaidi, D. Ghellab, Kenitra: A. Sourat, B. Menebhi, M. Bensaoud, A. El Ouazzani, A. Lachhab, A. Zakaria, F. El Idrani, H. Lachhab, Oujda: N. Elouafi, A. Hbali, G. Elmazani, I. Alla, Rabat: S. Ztot, M. Najat, B.E. El Younassi, I. Asfalou, L. Chami, M. Raissouni, Rabat: S. Abdelali, A. Benthami, A. Drissi Kacemi, S. Essadki, A. Louali, K. Bennis, Y. Lididi, R. Bouhouch, R. Mesbahi, S. Bouzidi Belmajdoub, Nigeria: Calabar: I. Ukpeh, R. Basake, E-O. Akpan, E. Emmanuel, O. Mbang, North Macedonia: Skopje: M. Gjerakaroska Radovikj, Skopje: E. Srbinovska Kostovska, I. Mitevska Peovska, L. Poposka, Norway: Lillehammer: G. Hogalmen, M. German, Oslo: L. Lysgaard Gullestad, C. Holt Bendz, O. Haugene, E. Bjorkelund, Poland: Katowice: K. Mizia-Stec, K. Bula, Krakow: J. Nessler, A. Gackowski, A. Siniarski, M. Kabat, Krakow: P. Rubis, A. Karabinowska, E. Dziewiecka, S. Wisniowska-Smialek, Lodz: M. Lelonek, A. Cieslak, A. Klaus, J. Szulc, K. Drazek, K.A. Nguyen, M. Kiedrowska, Lodz: A. Bielecka-Dabrowa, M. Pyziak Stepien, M. Rybak, P. Leczycki, P. Chrusciel, A. Wittczak, M. Banach, A. Chuda, A. Szwedzinska, M. Rembek Wieliczko, D. Mrozowska, F. Pawliczak, J. Lewek, M. Maciejewski, A. Cichocka-Radwan, A. Bikiewicz, K. Janikowski, Lublin: J. Szponar, A. Kujawa, A. Sutkowska, B. Cebulak, K. Pirog, J. Wieczorek, M. Suchecka, S. Goliszek, Opole: M. Gierlotka, E. Cierpiala, J. Bugajski, J. Plonka, K. Rekucki, Poznan: E. Straburzynska-Migaj, D. Budzynski, M. Gasiorowski, M. Dudek, Torun: G. Skonieczny, A. Dolacinska, A. Metzgier Gumiela, A. Stawicka, M. Rolirad, Warsaw: A. Kaplon-Cieslicka, M. Wawrzacz, K. Ozieranski, M. Kleszczewska, A. Tyminska, Warsaw: R.J. Gil, A. Pawlak, M. Wozniak, M. Pietraszek, A. Nazaruk, A. Bobel, A. Smolarczyk, D. Wiligorska, D. Ziecik, J. Latek, K. Byczkowska, Warszaw: P. Leszek, J. Urbanowski, N. Wiligorska, Zabrze: Z. Kalarus, T. Kukulski, A. Swiatkowski, M. Szulik, N. Kandora, Zielona Gora: P. Jesionowski, T. Zemleduch, A. Kasperowicz, K. Sosinska, Portugal: Faro: A. Camacho, R. Fernandes, H. Costa, Lisbon: C. Tavares Aguiar, A. Tralhão, A. Ventosa, C. Brízido, C. Strong, B. Rocha, G. Cunha, Lisbon: D. Brito, A. Nunes-Ferreira, I. Aguiar Ricardo, R. Santos, S. Pereira, T. Rodrigues, J. Agostinho, J. Brito, J. Rigueira, N. Cunha, P. António, P. Morais, D. Cravo, Lisbon: C. Fonseca, C. Rodrigues, I. Araujo, J. Presume, M. Proenca, S. Maltes, Porto: B. Moura, E. Barreira, P. Janeira, C. Ferrao, Porto: J. Silva-Cardoso, A. Sousa, B. Mena, X. Resende, M. Fonseca, M. Braga, M. Campelo, E. Moreira, P. Araújo, P. Diogo, R. Pinto, S. Amorim, B. Moura, Romania: Baia Mare: C. Pop, A. Iosip, Bucharest: C-J. Sinescu, N. Avram, D.G. Baltag, N. Samoila, Bucharest: M. Dorobantu, A. Scafa Udriste, A. Scarlatescu, N. Oprescu, O. Fronea Gheorghe, Bucharest: O. Chioncel, L. Antohi, O. Geavlete, Bucharest: G.A. Dan, I.C. Daha, S. Bari, C. Delcea, D.C. Ciuculete, I. Lupasteanu, C. Stanescu, A. Vijan, Craiova: C. Militaru, A. Giuca, G.C. Moise, E.G. Pascu, Galati: L. Grigorica, C. Corciova, Targu Mures: T. Benedek, M. Chitu, N. Rat, R. Hodas, D. Opincariu, I.S. Benedek, Timisoara: D. Lighezan, A.C. Florea, R. Buzas, V. Ivan, Russian Federation: Elektrostal: A. Molchanova, M. Piskareva, E. Bulgakova, E. Kudryashova, Kazan: A. Galyavich, L. Baleeva, Z. Galeeva, Krasnodar: A. Fendrikova, V. Skibitskiy, Z. Sokaeva, V. Babayan, N. Spiropulos, Moscow: E.V. Resnik, A.I. Kovaleva, M.S. Komissarova, V.A. Lazarev, Moscow: V. Larina, Moscow: Y. Belenkov, Y. Danilogorskaya, E. Zheleznykh, E. Privalova, I. Ilgisonis, V. Kaplunova, M. Kozhevnikova, G. Shakaryants, A. Shchendrygina, A. Yusupova, V. Zektser, Moscow: Z. Kobalava, E. Shavarova, L. Karapetyan, Moscow: G. Gendlin, A. Melekhov, I. Zakharova, M. Kuznetsova, M. Yunyaeva, Moscow: G. Arutyunov, A. Arutyunov, R. Muradyan, Moscow: S. Golitsyn, E. Gupalo, N. Mironova, Moscow: O. Drapkina, R. Myasnikov, T. Pavlunina, V. Dindikova, Y. Mareev, E. Andreenko, K. Krupychka, M. Kudryavtseva, O. Kulikova, Moscow: I. Orlova, J. Begrambekova, Nizhny Novgorod: N. Vinogradova, I. Fomin, Perm: N. Koziolova, A. Veclich, P. Karavaev, Rostov-On-Don: A. Chesnikova, O. Kolomatskaya, V. Gavrina, Ryazan: E. Smirnova, A. Karakiyan, I. Budanova, Saint-Petersburg: M. Sitnikova, A. Kuular, M. Trukshina, T. Lelyavina, Samara: D. Duplyakov, E. Zorina, R. Chernitsov, A. Sergeeva, Tomsk: A. Garganeeva, O. Tukish, K. Kopeva, V. Aleksandrenko, Ulyanovsk: A. Surminova, A. Tsareva, Vladivostok: T. Musurok, A. Garkusha, Serbia: Belgrade: A.D. Ristic, B. Ivanovic, D. Simic, M. Ašanin, P.M. Seferovic, J. Simic, V. Kovacevic, G. Krljanac, D. Matic, M. Mihailovic, M. Ostojic, M. Polovina, M. Tesic, D. Djikic, D. Simeunovic, I. Petrovic Djordjevic, I. Veljic, I. Milinkovic, K. Andjelkovic, A. Uscumlic, D. Sacic, Belgrade: M. Dekleva Manojlovic, S. Veljkovic, D. Stefanovic, J. Stevic, Belgrade: S. Hinic, M. Zdravkovic, A. Dokovic, J. Djokic, V. Mudrenovic, V. Popadic, S. Klasnja, Belgrade: S. Radovanovic, M. Radovanovic, V. Celic, A. Ilic, N. Blagojevic, D. Bosnjakovic, D. Toncev, Niš: S. Apostolovic, D. Stanojevic, S. Milutinovic, Niska Banja: B. Ilic, M. Deljanin Ilic, L. Nikolic, M. Stojanovic, D. Petrovic, D. Simonovic, S. Šaric, D. Hristov, Sremska Kamenica: I. Srdanovic, J. Dejanovic, T. Popov, S. Cemerlic Maksimovic, S. Dimic, S. Keca, V. Drljavic, D. Bogdanovic, I. Popov, J. Pavic Poljak, Slovakia: Bratislava: E. Goncalvesova, M. Luknár, Bratislava: P. Solik, Svidnik: M. Pytliak, P. Bojcík, Slovenia: Murska Sobota: M. Lainscak, N. Cmor, E. Dora, L. Majc Hodoscek, A. Vogrincic Cernezel, Trbovlje: B. Leskovar, T. Furlan, A. Milanovic, K. Vrbinc Vrtek, S. Poznic, V. Grilj, M. Režun, Spain: Alcazar de San Juan: V. Martinez Mateo, M.J. Fernandez Anguita, Cáceres: C. Ortiz Cortés, Denia: A. Valle Muñoz, H. Morillas Climent, J. Seller Moya, Figueres: S. Darnés Soler, S. Cudini, Granada: S. López Fernández, L. Jordán Martínez, F. Bermúdez Jiménez, La Coruna: M. Crespo Leiro, D. Couto Mallon, E. Barge Caballero, G. Barge Caballero, M.J. Paniagua Martin, P. Pardo Martinez, C. Naya Leira, C. Riveiro Rodriguez, M. Martinez Castro, P. Blanco Canosa, Z. Grille Cancela, Malaga: J.M. Garcia Pinilla, A. Robles Mezcua, A. Rodriguez Cordoba, C. Cruzado Alvarez, L. Morcillo Hidalgo, P. Marquez Camas, A.I. Perez Cabeza, P. Redondo-Gomez, M. Robles Mezcua, Ubeda: J.L. Bonilla Palomas, Sweden: Stockholm: L. Lund, M. Nygren, G. Savarese, C. Hage, E. Jonsson, E. Ottenblad, F. Granstrom, H. Lundberg, K. Karlsson, Syrian Arab Republic: Damascus: Y. Bani Marjeh, A. Abdin, F. Alhussein, F. Mgazeel, Turkey: Adana: F. Yavuz, Adiyaman: A. Karakus, Alanya Antalya: A. Coner, S. Akinci, Ankara: B. Demirkan, Antakya: O. Akkus, Antalya: A. Genc, Bursa: F.O. Arican Ozluk, Duzce: H. Harbalioglu, Eskisehir: Y. Cavusoglu, E. Babayigit, E. Sener, Gumushane: E.I. Yuce, Istanbul: H. Altay, Istanbul: Ö. Yildirimtürk, Izmir: C. Altin, Izmir: B. Kilicaslan, B. Unal, H. Acet, Izmir: N. Cetin, Kars: C. Burak, Kocaeli: D. Karacimen, Kocaeli: A. Agacdiken Agir, Y.U. Celikyurt, Mersin: A. Celik, E.E. Sahin, O. Sakarya, M. Demir, Mugla: O. Basaran, Samsun: A.E. Atas, Ukraine: Dnipro: O. Khaniukov, Ivano-Frankivsk: I. Vakaliuk, I. Drapchak, V. Sovtus, N. Tymochko, O. Prytuliak, Kharkiv: V. Tseluyko, N. Matviichuk, Kharkiv: M. Kopytsya, T. Storozhenko, Kharkiv: I. Rudyk, O. Medentseva, D. Babichev, Kiev: L. Voronkov, A. Liashenko, Kiev: I. Rudenko, K. Lazareva, N. Sishkina, A. Honcharuk, O. Vasylenko, Y. Antoniuk, Kiev: M. Dolzhenko, L. Hrubyak, L. Lobach, T. Simagina, Kiev: S. Kozhuhov, N. Dovganych, N. Thor, M. Danko, O. Yarynkina, O. Bazyka, Kiev: A. Parkhomenko, A. Stepura, D. Bilyi, O. Irkin, O. Dovhan, Kiev: V. Batushkin, D. Poddyachaya, Kiev: O. Zharinov, B. Todurov, I. Lischuk, Kiev: K. Rudenko, Vinnitsa: V. Zhebel, I. Pashkova, L. Sursaieva, Krivoy Rog: V. Potabashniy, V. Fesenko, O. Markova, O. Kniazieva, Zaporozhye: O. Berezin, O. Kremzer, United Kingdom: Bodelwyddan: M. Aldwaik, A. Bolger, R. Manley, V. Garvey, Uzbekistan: Tashkent: T. Abdullaev, S. Mirzarakhimova, A. Rasulov, A. Karimov, H. Gulomov, I. Tsoy, R. Kurbanova, R. Bekbulatova, Tashkent: U. Kamilova, D. Tagaeva.




