Early changes in renal function during rapid up-titration of guideline-directed medical therapy following an admission for acute heart failure
Abstract
Aim
In this subgroup analysis of STRONG-HF, we explored the association between changes in renal function and efficacy of rapid up-titration of guideline-directed medical therapy (GDMT) according to a high-intensity care (HIC) strategy.
Methods and results
In patients randomized to the HIC arm (n = 542), renal function was assessed at baseline and during follow-up visits. We studied the association with clinical characteristics and outcomes of a decrease in estimated glomerular filtration rate (eGFR) at week 1, defined as ≥15% decrease from baseline. Patients in the usual care group (n = 536) were seen at day 90. The treatment effect of HIC versus usual care was independent of baseline eGFR (p-interaction = 0.4809). A decrease in eGFR within 1 week occurred in 77 (15.5%) patients and was associated with more rales on examination (p = 0.004), and a higher New York Heart Association class at the corresponding visit. Following the decrease in eGFR at 1 week, lower average optimal doses of GDMT were prescribed during follow-up (p = 0.0210) and smaller reductions in N-terminal pro-B-type natriuretic peptide occurred (geometrical mean 0.81 in no eGFR decrease vs 1.12 in GFR decrease, p = 0.0003). The rate of heart failure (HF) readmission or death at 180 days was 12.3% in no eGFR decrease versus 18.5% in eGFR decrease (p = 0.2274) and HF readmissions were 7.8% versus 16.6% (p = 0.0496).
Conclusions
In the STRONG-HF study, HIC reduced 180-day HF readmission or death regardless of baseline eGFR. An early decrease in eGFR during rapid up-titration of GDMT was associated with more evidence of congestion, yet lower doses of GDMT during follow-up.
Introduction
Chronic kidney disease is one of the most frequent comorbidities in heart failure (HF) patients, and changes in renal function are common.1 The relationship between HF and renal function remains complex, as the disease itself, its compensatory mechanisms, state of congestion, as well as guideline-directed medical therapy (GDMT), all affect renal function.2 Conversely, decreases in renal function often result in down-titration or stopping of GDMT and withholding some lifesaving therapies which have proven long-term renoprotective effects.3, 4 It has recently been shown that after initiation of the sodium–glucose cotransporter 2 inhibitor (SGLT2i) dapagliflozin, an initial dip in estimated glomerular filtration rate (eGFR) was even associated with better outcomes compared with a similar decline in patients randomized to placebo.5 Data on changes in renal function following up-titration of GDMT are scarce. We recently showed that an intensive treatment strategy (high-intensity care [HIC]) of rapid up-titration of guideline-directed medication coupled with close follow-up after an acute HF admission reduced symptoms, improved quality of life, and reduced the risk of 180-day all-cause death or HF readmission compared with usual care.6 In a pre-specified subgroup analysis of the Safety, Tolerability, and Efficacy of Rapid Optimization, Helped by NT-proBNP Testing of Heart Failure Therapies (STRONG-HF) study the benefit of HIC on the combined outcome at 180 days was similar in patients with an eGFR above and below median (59.4 ml/min/1.73 m2).6 In the present study, we further explored the association between (changes in) renal function and clinical characteristics and outcomes in patients undergoing rapid up-titration of GDMT.
Methods
Study design
The design and main results of the STRONG-HF study have previously been reported.6-8 In brief, the STRONG-HF trial was a multinational, multicentre, open-label, randomized, parallel-group study that enrolled 1078 patients hospitalized for acute HF, who were randomized in a 1:1 ratio to early and rapid up-titration of GDMT (beta-blockers; angiotensin-converting enzyme inhibitors [ACEi] or angiotensin receptor blockers [ARB] or angiotensin receptor–neprilysin inhibitors [ARNI]; and mineralocorticoid receptor antagonists [MRA]) compared with usual care. Early and rapid up-titration of GDMT and close follow-up was safe and effective in reducing a combined endpoint of 180-day all-cause death or HF readmission compared with usual care.6 Adult patients up to an age of 85 years, were eligible for enrolment if they were admitted to the hospital within 72 h before screening, had a N-terminal pro-B-type natriuretic peptide (NT-proBNP) >1500 ng/L, and were not treated with full doses of GDMT. Patients randomized to HIC (n = 542) were up-titrated to half optimal doses at randomization and to full optimal doses at week 2, if this was deemed safe to do so based on physical examination, as well as laboratory values including potassium, eGFR, and NT-proBNP. Patients in the HIC group were seen at 1, 2, 3, and 6 weeks and 90 days after randomization. Patients in the usual care group were followed according to local practice and were seen by the study team at day 90. Patients assigned to both groups were contacted by telephone at 180 days to assess vital status and rehospitalizations as well as use of HF medications.
The study was approved by appropriate authorities and all sites obtained approval from the local ethics committees. All patients provided written informed consent. The study is registered on ClinicalTrials.gov (NCT03412201).9
Study outcomes
The study primary outcome was a combined endpoint of 180-day first HF readmission or all-cause death. Secondary endpoints for this analysis were the separate components of the combined endpoint, namely 180-day all-cause death, 180-day first readmission for HF as well as change in EQ-5D visual analogue scale (VAS) from baseline to day 90.
Baseline and change in renal function
Renal function was assessed per protocol at baseline, which was 2 days prior to anticipated discharge, and at day 90 in both study arms. In the HIC arm, renal function was additionally assessed at weeks 1, 2, 3, and 6 after randomization. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation. A decrease in eGFR was defined as ≥15% from baseline and was assessed at week 1, as this would be more than generally expected shortly after initiation of GDMT.10, 11
Signs and symptoms of congestion were assessed at weeks 1, 2, 3, and 6 after randomization in the HIC group. Congestion status (New York Heart Association [NYHA] class, orthopnoea, peripheral oedema, rales and jugular venous pressure) was evaluated by investigators through physical examination at corresponding time points to the decrease in eGFR (at weeks 1), as well as during follow-up. Changes in systolic blood pressure and pulse, as well as relative change in NT-proBNP were also evaluated at week 1.
Statistical analysis
Clinical variables were evaluated over baseline renal function by tertiles of eGFR as well as renal function changes (a ≥15% decrease in eGFR at week 1). Frequency (percentage) was used to summarize categorical variables while normally distributed continuous variables were summarized with mean ± standard deviations and non-normally continuous variables with geometric mean and associated 95% confidence interval.
All randomized patients, excluding those randomized in error, were included in analyses of outcomes through day 90. As previously described, analyses of day 180 outcomes excluded those patients enrolled at sites that did not follow patients to 180 days, with down-weighting of results for patients enrolled prior to changing the primary endpoint from 90 to 180 days.6 Cox proportional hazards regression models using a restricted cubic spline with three knots was used to model the treatment effect on the primary endpoint as a function of continuous baseline eGFR.
Multivariable predictors of eGFR change were selected from baseline characteristics previously shown to be associated with renal function change using backwards selection in the HIC group.12, 13 Missing covariates were multiply imputed using 10 imputation datasets. The association of clinical outcomes with eGFR change was analysed using a landmark approach beginning from the timepoint where the eGFR change was measured.14 Covariates for adjustment were chosen from previously known predictors using backwards selection in the usual care group. Plots of unadjusted Kaplan–Meier estimates for the primary endpoint based on specified cut-points of the change in eGFR at weeks 1 are included. Changes in EQ-VAS from baseline to day 90 were analysed using a linear regression model adjusting for baseline EQ-VAS and randomization stratification factors LVEF category (≤40/>40%) and region.
Comparison of signs and symptoms between the change in eGFR groups was done using the Cochran–Mantel–Haenszel test of general association. The frequency of adverse events between eGFR change groups at week 1 were analysed. Only events with an onset date equal to or greater than day 7 through 90 days post-randomization were included.
The average dose of the three medications (ACEi/ARB/ARNI, beta-blocker and MRA) relative to the optimal doses were computed for each patient. The trajectory of this average percentage optimal dose is displayed for a ≥15% decrease in eGFR at week 1. A comparison of the average percentage of optimal dose between those with a ≥15% decrease and those without at week 1 was conducted using a mixed model for repeated measures including group, visit, and group-by-visit interaction effects.
Two-sided p-values <0.05 were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline renal function
Baseline eGFR in the entire population was 64.8 ± 22.3 ml/min/1.73 m2, and 505 (46.9%) of patients had an eGFR <60 ml/min/1.73 m2. Patients with lower eGFR at baseline were older, more likely to be female and have a history of HF and atrial fibrillation (online supplementary Table S1). Additionally, patients with a lower eGFR had a higher NYHA class, higher NT-proBNP, and were less likely to be treated with ACEi/ARB/ARNI, yet more likely to receive beta-blockers at baseline. Patients with a lower eGFR were less likely to receive loop diuretics at baseline and were prescribed lower doses.
In the HIC arm, baseline eGFR was 64.3 ± 21.3 ml/min/1.73 m2, and 253 (50.1%) patients had an eGFR <60 ml/min/1.73 m2. Change in eGFR over time in the HIC arm is shown in Figure 1.
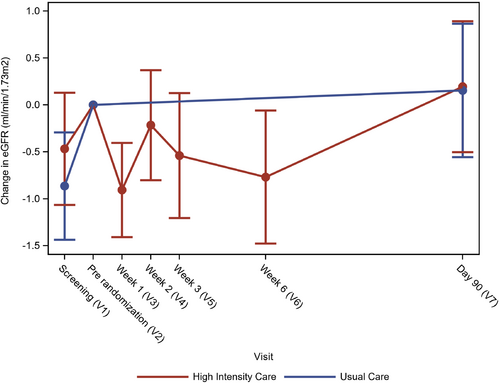
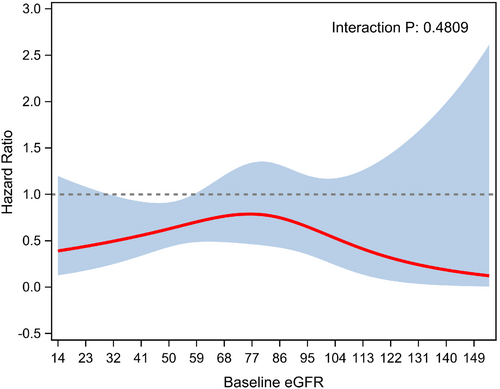
The treatment effect of HIC versus usual care was independent of baseline eGFR (p-interaction = 0.48; Figure 2).
Decrease in estimated glomerular filtration rate in the high-intensity care group
A decrease in eGFR ≥15% at week 1 was present in 77 (15.5%) patients. Baseline characteristics for patients with a decrease in eGFR ≥15% at week 1 are shown in Table 1. Briefly, patients with a decrease in eGFR ≥15% at week 1 had a lower blood pressure, higher NYHA class before hospital admission, and lower baseline creatinine (all p < 0.02). No differences in use or doses of GDMT at baseline were observed.
| Parameter | Patients without >15% decline in eGFR (n = 421) | Patients with ≥15% decline in eGFR (n = 77) | p-value |
|---|---|---|---|
| Age, years, mean (SD) | 62.3 (13.78) | 64.6 (12.92) | 0.1708 |
| Sex, n (%) | 0.6556 | ||
| Female | 169 (40.1) | 33 (42.9) | |
| Male | 252 (59.9) | 44 (57.1) | |
| Self-reported race, n (%) | 0.4043 | ||
| Black | 101 (24.0) | 11 (14.3) | |
| Caucasian | 312 (74.1) | 65 (84.4) | |
| Native American | 1 (0.2) | 0 | |
| Other | 6 (1.4) | 1 (1.3) | |
| Pacific Islander | 1 (0.2) | 0 | |
| Systolic blood pressure at baseline, mmHg, mean (SD) | 124.0 (13.60) | 120.1 (11.58) | 0.0184 |
| NT-proBNP, ng/L, geom. mean (95% CI) | |||
| At screening | 6032.5 (5712.2–6370.7) | 6553.8 (5749.6–7470.4) | 0.2417 |
| At baseline | 3163.4 (2982.3–3355.6) | 3504.5 (2997.7–4097.0) | 0.1884 |
| eGFR, ml/min/1.73 m2, mean (SD) | |||
| At screening | 63.7 (21.59) | 65.5 (21.18) | 0.5161 |
| At baseline | 63.7 (21.18) | 69.2 (22.82) | 0.0396 |
| History of atrial fibrillation or atrial flutter or present at screening, n (%) | 174 (41.3) | 32 (41.6) | 0.9702 |
| Geographical region, n (%) | 0.3996 | ||
| Europe | 303 (72.0) | 59 (76.6) | |
| Non-Europe | 118 (28.0) | 18 (23.4) | |
| Clinical history, n (%) | |||
| Stroke or transient ischaemic attack | 38 (9.0) | 13 (16.9) | 0.0373 |
| Severe liver disease | 1 (0.3) | 1 (1.6) | 0.1693 |
| Psychiatric or neurological disorder | 3 (0.7) | 3 (3.9) | 0.0188 |
| Malignancies | 13 (3.1) | 4 (5.2) | 0.3514 |
| Diabetes | 108 (25.7) | 28 (36.4) | 0.0540 |
| Diabetes control method | |||
| Insulin | 37 (8.8) | 8 (10.4) | 0.6569 |
| Diet only | 64 (15.2) | 26 (33.8) | 0.0001 |
| Oral antidiabetic agents | 72 (17.1) | 25 (32.5) | 0.0018 |
| Pulmonary embolism | 11 (2.6) | 2 (2.6) | 0.9938 |
| Acute coronary syndrome | 124 (29.5) | 27 (35.1) | 0.3247 |
| Coronary artery bypass surgery | 23 (5.5) | 3 (3.9) | 0.5698 |
| Percutaneous coronary intervention | 62 (14.7) | 10 (13.0) | 0.6898 |
| Angina Canadian Cardiovascular Society class 2 or higher | 59 (14.0) | 8 (10.5) | 0.4125 |
| Moderate or severe chronic obstructive pulmonary disease or asthma | 9 (2.1) | 2 (2.6) | 0.8008 |
| Sustained ventricular arrhythmia (with syncopal episodes in past 3 months) | 0 | 0 | |
| Cardiac resynchronization therapy | 3 (0.7) | 0 | 0.4575 |
| Automatic internal cardiac defibrillator | 3 (0.7) | 0 | 0.4575 |
| History of heart failure, n (%) | 361 (85.7) | 64 (83.1) | 0.5483 |
| NYHA class 1 month before hospital admission, n (%) | 0.0111 | ||
| I | 21 (5.4) | 8 (10.5) | |
| II | 117 (30.1) | 19 (25.0) | |
| III | 175 (45.0) | 24 (31.6) | |
| IV | 76 (19.5) | 25 (32.9) | |
| Ischaemic aetiology | 193 (46.0) | 41 (53.2) | 0.2385 |
| Left ventricular ejection fraction, %, mean (SD) | 36.71 (12.44) | 35.88 (13.01) | 0.5939 |
| Hospitalized for heart failure in the past year? n (%) | 105 (24.9) | 21 (27.3) | 0.6652 |
| Number of heart failure hospitalizations in the past year | 0.3 (0.66) | 0.4 (0.64) | 0.6986 |
| History of atrial fibrillation or atrial flutter, n (%) | 179 (42.5) | 33 (42.9) | 0.9558 |
| Type of atrial fibrillation or atrial flutter, n (%) | 0.1647 | ||
| Paroxysmal | 42 (23.7) | 9 (27.3) | |
| Permanent | 100 (56.5) | 22 (66.7) | |
| Persistent | 35 (19.8) | 2 (6.1) | |
| Local laboratory, mean (SD) | |||
| Haemoglobin, g/L | 136.2 (19.91) | 136.3 (21.65) | 0.9688 |
| Lymphocytes, % | 27.8 (10.05) | 26.9 (9.39) | 0.4620 |
| White blood cells, 109/L | 6.8 (1.91) | 7.3 (2.10) | 0.0353 |
| Glucose, mmol/L | 6.1 (2.48) | 6.1 (2.24) | 0.9575 |
| Creatinine, μmol/L | 107.7 (29.14) | 98.2 (31.03) | 0.0094 |
| Potassium, mmol/L | 4.3 (0.44) | 4.3 (0.52) | 0.8518 |
| Sodium, mmol/L | 140.1 (3.91) | 140.5 (4.64) | 0.3896 |
| Urea, mmol/L | 8.0 (3.35) | 8.2 (4.19) | 0.5171 |
| ALT, IU/L | 30.2 (44.38) | 28.9 (20.27) | 0.8005 |
| Total bilirubin, μmol/L | 17.3 (11.11) | 17.5 (11.79) | 0.8384 |
| Total cholesterol, mmol/L | 4.3 (1.11) | 3.8 (1.02) | 0.0022 |
| Signs and symptoms of congestion before randomization (at baseline), n (%) | |||
| NYHA class | 0.3634 | ||
| I | 26 (6.2) | 6 (7.8) | |
| II | 251 (59.6) | 39 (50.6) | |
| III | 140 (33.3) | 32 (41.6) | |
| IV | 4 (1.0) | 0 | |
| Oedema | 0.9039 | ||
| 0 | 247 (58.7) | 44 (57.1) | |
| 1+ | 148 (35.2) | 27 (35.1) | |
| 2+ | 25 (5.9) | 6 (7.8) | |
| 3+ | 1 (0.2) | 0 | |
| Rales | 0.1289 | ||
| No rales | 360 (85.7) | 62 (80.5) | |
| Rales <1/3 | 52 (12.4) | 15 (19.5) | |
| Rales 1/3–2/3 | 8 (1.9) | 0 | |
| Rales >2/3 | 0 | 0 | |
| Orthopnoea | 0.5742 | ||
| None | 251 (59.6) | 50 (64.9) | |
| 1 pillow (10 cm) | 155 (36.8) | 23 (29.9) | |
| 2 pillows (20 cm) | 14 (3.3) | 4 (5.2) | |
| >30° | 1 (0.2) | 0 | |
| JVP | 0.5175 | ||
| <6 cm | 330 (84.2) | 62 (88.6) | |
| 6–10 cm | 58 (14.8) | 8 (11.4) | |
| >10 cm | 4 (1.0) | 0 | |
| Oral heart failure medications taken before randomization, n (%) | |||
| ACEi/ARB/ARNI | 273 (64.8%) | 54 (71.1%) | 0.2938 |
| Beta-blockers | 147 (34.9%) | 20 (26.3%) | 0.1440 |
| Mineralocorticoid receptor antagonists | 400 (95.0%) | 68 (89.5%) | 0.0580 |
| Loop diuretic | 405 (96.2%) | 72 (94.7%) | 0.5504 |
| Oral heart failure medications optimal dose categories at visit 2 (post-randomization), n (%) | |||
| ACEi/ARB/ARNI | 0.6178 | ||
| None | 7 (1.7) | 1 (1.3) | |
| <1/2 Optimal dose | 81 (19.2) | 16 (20.8) | |
| 1/2 − < Full optimal dose | 324 (77.0) | 60 (77.9) | |
| ≥ Full optimal dose | 9 (2.1) | 0 | |
| Beta-blockers | 0.9246 | ||
| None | 8 (1.9) | 1 (1.3) | |
| <1/2 Optimal dose | 50 (11.9) | 11 (14.3) | |
| 1/2 − < Full optimal dose | 358 (85.0) | 64 (83.1) | |
| ≥ Full optimal dose | 5 (1.2) | 1 (1.3) | |
| Mineralocorticoid receptor antagonists | 0.9373 | ||
| None | 8 (1.9) | 1 (1.3) | |
| <1/2 Optimal dose | 1 (0.2) | 0 | |
| 1/2 − < Full optimal dose | 247 (58.7) | 47 (61.0) | |
| ≥Full optimal dose | 165 (39.2) | 29 (37.7) | |
| Loop diuretic dose, furosemide equivalence, mean (SD) | 61.7 (50.21) | 58.8 (39.34) | 0.6378 |
- ACEi, angiotensin-converting enzyme inhibitor; ALT, alanine transaminase; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; CI, confidence interval; eGFR, estimated glomerular filtration rate; JVP, jugular venous pressure; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation.
In multivariable analysis, a decrease in eGFR ≥15% at week 1 was independently associated with a history of diabetes, more advanced age, lower systolic blood pressure and higher baseline eGFR (Table 2). After adjustment for these variables, GDMT prescribed at randomization was not associated with a decrease in eGFR 1 week later.
| Predictor | OR for unit change of: | Univariable results | Multivariable results (excluding medication use) | Multivariable results (including medication use) at visit 2 (post-randomization) | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Age, years | 5 | 1.07 (0.97–1.17) | 0.1719 | 1.20 (1.06–1.35) | 0.0033 | 1.20 (1.06–1.35) | 0.0034 |
| Male sex | Yes vs. No | 0.89 (0.55–1.46) | 0.6558 | ||||
| Geographical region | Europe vs. Non-Europe | 1.28 (0.72–2.25) | 0.4009 | ||||
| NYHA class 1 month prior | III/IV vs. I/II | 1.17 (0.87–1.56) | 0.2960 | ||||
| History of diabetes | Yes vs. No | 1.65 (0.99–2.76) | 0.0565 | 1.85 (1.08–3.17) | 0.0262 | 1.84 (1.07–3.17) | 0.0273 |
| History of heart failure | Yes vs. No | 0.82 (0.42–1.58) | 0.5491 | ||||
| Angina class II or higher | Yes vs. No | 0.74 (0.34–1.61) | 0.4547 | ||||
| Moderate or severe COPD or asthma | Yes vs. No | 1.22 (0.26–5.76) | 0.8011 | ||||
| Ischaemic aetiology | Yes vs. No | 1.34 (0.82–2.18) | 0.2426 | ||||
| NYHA class (pre-randomization) | III/IV vs. I/II | 1.37 (0.83–2.25) | 0.2161 | ||||
| History of atrial fibrillation or atrial flutter present at screening | Yes vs. No | 1.01 (0.62–1.65) | 0.9702 | ||||
| Baseline systolic BP, mmHg | 5 | 0.89 (0.80–0.98) | 0.0193 | 0.88 (0.79–0.97) | 0.0131 | 0.87 (0.78–0.97) | 0.0124 |
| Baseline pulse, bpm | 5 | 1.00 (0.90–1.11) | 0.9664 | ||||
| Baseline respiratory rate, breaths/min | 2 | 0.97 (0.79–1.18) | 0.7385 | ||||
| JVP (pre-randomization) | ≥6 cm vs. <6 cm | 0.65 (0.30–1.40) | 0.2686 | ||||
| Oedema (pre-randomization) | 2+/3+ vs. 0/1+ | 1.28 (0.51–3.23) | 0.5957 | ||||
| Haemoglobin (pre-randomization), g/L | 5 | 1.00 (0.94–1.06) | 0.9687 | ||||
| Lymphocytes (pre-randomization), % | 2 | 0.98 (0.94–1.03) | 0.4970 | ||||
| Baseline eGFR, ml/min/1.73 m2 | 5 | 1.06 (1.00–1.12) | 0.0412 | 1.15 (1.07–1.24) | 0.0002 | 1.15 (1.07–1.23) | 0.0002 |
| Creatinine (pre-randomization), μmol/L | 5 | 0.93 (0.88–0.98) | 0.0074 | ||||
| Total bilirubin (pre-randomization), μmol/L | 2 | 1.00 (0.96–1.05) | 0.9450 | ||||
| Sodium (pre-randomization), mmol/L | 1 | 1.03 (0.97–1.09) | 0.3893 | ||||
| Medication use at visit 2 (post-randomization) | |||||||
| ACEi/ARB/ARNI | ≥ 1/2 vs. <1/2 Optimal dose | 0.93 (0.52–1.68) | 0.8163 | 1.10 (0.57–2.10) | 0.7778 | ||
| Beta-blockers | ≥ 1/2 vs. <1/2 Optimal dose | 0.87 (0.44–1.70) | 0.6751 | 0.93 (0.45–1.93) | 0.8515 | ||
| Mineralocorticoid receptor antagonists | ≥ 1/2 vs. <1/2 Optimal dose | 1.66 (0.21–13.29) | 0.6331 | 1.89 (0.23–15.76) | 0.5566 | ||
- ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; BP, blood pressure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; JVP, jugular venous pressure; NYHA, New York Heart Association; OR, odds ratio.
Decrease in estimated glomerular filtration rate and change in vital signs, biomarkers, congestion status and guideline-directed medical therapy
A decrease in eGFR at week 1 was not associated with changes in systolic blood pressure, or heart rate (Table 3). A decrease in eGFR was, however, significantly associated with an increase in NT-proBNP from baseline to the corresponding visit. Patients with a decrease in eGFR ≥15% at week 1 additionally had significantly more rales (p = 0.004), and a higher NYHA class at the corresponding visit (online supplementary Table S2). No difference in dose of loop diuretics was found in patients with a decrease in eGFR versus those without.
| Endpoint | Patients without >15% decline in eGFR (n = 421) | Patients with ≥15% decline in eGFR (n = 77) | Mean difference/group ratio (95% CI) | p-value |
|---|---|---|---|---|
| Change in systolic blood pressurea | −1.50 (0.57) | −1.56 (1.35) | −0.06 (−2.94, 2.82) | 0.9676 |
| Change in pulsea | −2.56 (0.46) | −1.55 (1.07) | 1.01 (−1.28, 3.29) | 0.3879 |
| Relative change in NT-proBNPb | 0.81 | 1.12 | 1.37 (1.16, 1.63) | 0.0003 |
- CI, confidence interval; eGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
- a Least square mean change from ANCOVA model adjusted for baseline value. Least square mean difference (95% CI) presented comparing the groups.
- b Geometric mean ratio representing the ratio of the post-baseline value over the baseline value from an ANCOVA model of the log-transformed NT-proBNP adjust for baseline log-transformed NT-proBNP; a value <1.0 represents a decrease from baseline. Group ratio represents the ratio of the ratios in the two groups; a value >1.0 represents a greater relative change in the group with an eGFR decline than in those without.
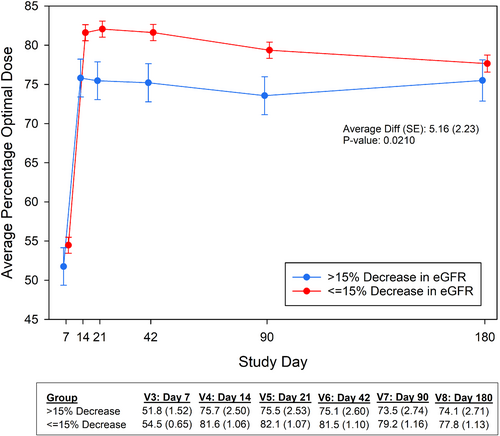
There was no association between a decrease in eGFR and achieved doses of GDMT at week 1. Figure 3 shows the trajectory of the average percentage of optimal dose during follow-up for a decrease in eGFR at week 1, showing significantly lower average of optimal doses of GDMT during follow-up for patients with an early decrease in eGFR (p = 0.021). At 6-month follow-up, there was no difference in average of optimal doses of GDMT between groups.
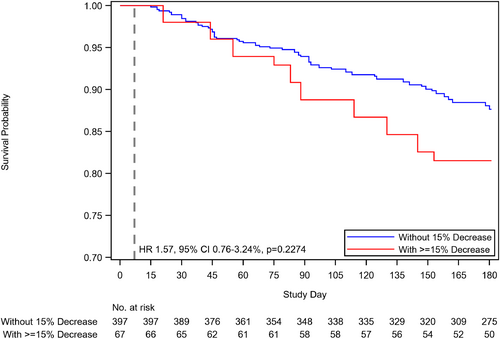
Decrease in estimated glomerular filtration rate and outcomes
A decrease in eGFR ≥15% at week 1 was not significantly associated with an increased risk of the combined outcome of HF readmission or all-cause death at 180 days (Table 4 and Figure 4). A decrease in eGFR at week 1 was, however, associated with a borderline statistically significant increased risk of HF rehospitalization at 180 days (p = 0.0496); significance was however lost after multivariable adjustment (p = 0.21). There was no significant association between a decrease in eGFR and 180-day all-cause death or EQ-VAS (Table 4).
| Endpoint | Patients without >15% decline in eGFR (n = 421) | Patients with ≥15% decline in eGFR (n = 77) | Unadjusted | Adjusted | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |||
| All-cause death or heart failure readmission by day 180a | 48/397 (12.3%) | 10/67 (18.5%) | 1.57 (0.76–3.24) | 0.2274 | 1.42 (0.68–2.97) | 0.3563 |
| All-cause death by day 180b | 25/398 (6.9%) | 3/67 (6.2%) | 0.89 (0.26–2.99) | 0.8515 | 0.50 (0.13–1.88) | 0.3076 |
| Heart failure readmission by day 180c | 32/397 (7.8%) | 9/67 (16.6%) | 2.24 (1.00–5.00) | 0.0496 | 1.70 (0.74–3.86) | 0.2082 |
| LS mean (SE) | LS mean (SE) | LS mean difference (95% CI) | p-value | LS mean difference (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| EQ-VAS change from baseline to day 90d | 11.08 (0.76) | 8.83 (1.74) | −2.24 (−5.94 to 1.45) | 0.2335 | −0.42 (−4.06 to 3.22) | 0.8208 |
- CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LS, least square; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; SE, standard error; VAS, visual analogue scale.
- Results restricted to subjects at sites where patients were followed to 180 days. Results for patients in cohort 1 are down-weighted proportional to half its sample size.
- Patients censored or who experienced the event by day 7 were excluded from the analyses. Risks computed from day 7.
- n/N (Kaplan–Meier estimates) are presented. HR from Cox proportional hazards model.
- Analyses of EQ-VAS exclude patients from countries where a linguistically available translation of the questionnaire was not available.
- a Adjusted for baseline diastolic blood pressure, baseline NT-proBNP, ischaemic aetiology, and oedema.
- b Adjusted for baseline creatinine, baseline haemoglobin, baseline urea, and baseline NT-proBNP.
- c Adjusted for body mass index, baseline diastolic blood pressure, baseline cholesterol, baseline potassium, baseline NT-proBNP, baseline left ventricular ejection fraction, and oedema.
- d All analyses adjusted for baseline EQ-VAS, region, and left ventricular ejection fraction category (≤40/>40%). Adjusted analyses further adjusted for age, baseline haemoglobin, baseline creatinine, baseline cholesterol, baseline NT-proBNP, hospitalized for in prior year, oedema, and NYHA class.
A decrease in eGFR at week 1 was associated with more adverse events; however, both cardiac and renal adverse events were not significantly different between groups (online supplementary Table S3). A decrease in eGFR at week 1 was also associated with more serious adverse events, which was driven by significantly more cardiac failure (p = 0.041) (online supplementary Table S4).
Discussion
The key findings of the present study are that despite the fact that patients with poorer renal function at baseline were older and had more severe HF, the beneficial effects of rapid up-titration were maintained and independent of baseline eGFR. An early decrease in eGFR following rapid up-titration of GDMT was associated with more congestion (symptoms and increase in NT-proBNP). Following an early decrease in eGFR, average doses of GDMT relative to optimal doses over time (up to 90 days) were significantly lower. Patients with an early decrease in eGFR had numerically higher event rates, but the difference was not statistically significant.
Renal function and guideline-directed medical therapy in heart failure
Heart and kidney are closely related, and, simply put, failure of one results in suffering of the other. Chronic kidney disease is one of the most prevalent comorbidities in patients with HF (∼50%) and is associated with an increased risk of mortality.1 These HF patients with concomitant kidney disease not only have an increased risk of adverse outcome, they are also more likely to be treated with no or lower doses of life-saving GDMT.15 The reasons for under-treatment are probably multifactorial. First, for most GDMT there is strong evidence for efficacy and safety for an eGFR >30 ml/min/1.73 m2 in reducing the risk of all-cause mortality, or cardiovascular death and HF rehospitalization.3 For an eGFR <30 ml/min/1.73 m2 data are scarce and strong evidence is lacking, leading to caution and less use of these therapies in these patients, who are most likely at higher risk of poor outcomes and may derive greater benefit of GDMT. Second, most GDMT have an effect on renal function after initiation, which is most pronounced for ACEi/ARB/ARNI and SGLT2i. For all of these, an early decline or acute drop in eGFR shortly after initiation of the drug is observed.3, 16-19 This is however not associated with poor outcomes and does not diminish its treatment effect. Recently, the initial dip in eGFR after initiation of dapagliflozin was shown to be associated with better outcomes compared to a similar decline in patients treated with placebo.5 This suggests that the initial dip in eGFR might be a marker of beneficial response to the therapy. Data from the EMPEROR-Reduced trial confirmed that the initial decline in eGFR is not associated with poor outcomes.20 Similarly for ACEi/ARB in HF patients, post hoc analyses from randomized controlled trials have suggested that the beneficial effect of therapy might be even more pronounced in patients that experience a drop in eGFR compared to those who do not experience a drop in eGFR.19, 21 Furthermore, despite the early decline in eGFR, treatment with ARNI and SGLT2i have a renoprotective effect in the long term where a reduction in the rate of eGFR decline over time is observed.4, 22-27 When confronted with a decline in renal function in a patient with HF, it is therefore of the utmost importance to investigate the cause of this decline in renal function before halting lifesaving GDMT and distinguish whether this is due to the treatment itself or due to other causes, such as haemodynamic deterioration or congestion.3, 4, 11, 28 Finally, current guidelines, expert opinion papers and evidence from the STRONG-HF trial recommend rapid up-titration of GDMT, yet currently there are no data on the effect of rapid up-titration on renal function available.6, 29-31
Rapid up-titration of guideline-directed medical therapy and (changes in) renal function
This paper adds novel information to these important evidence gaps. First, we found that the beneficial effect of rapid up-titration was maintained, regardless of baseline eGFR. Second, we did not observe a direct relationship between an early decrease in renal function and use of GDMT at the preceding visits. However, we did find a significant association between a decrease in eGFR and lower doses of GDMT during follow-up. Per protocol, further up-titration of ACEi/ARB/ARNI and MRA was contraindicated in patients with an eGFR <30 ml/min/1.73 m2, however given the mean baseline eGFR of 64.8 ml/min/1.73 m2, an early decrease of 15% did not result in an eGFR <30 ml/min/1.73 m2 in the vast majority of patients. The hesitation to further up-titrate could result from the observed association between a decrease in eGFR and the suggestion of more congestion, i.e. more symptoms of congestion and an increase in NT-proBNP levels. The relation between renal function and congestion in HF is an intricate one, where increased venous pressure is the strongest predictor of a decrease in eGFR while decongestive treatment may also result in worsening renal function.32-35 From our data, we are unable to specifically determine the cause of the early decrease in renal function, whether this is the consequence of congestion or up-titration of GDMT. It could be hypothesized that patients with a decrease in eGFR are less well up-titrated and therefore have less improvement in NT-proBNP and more signs of congestion. On the other hand, these patients might have more severe HF, resulting in more signs and symptoms of congestion and therefore resulted in physicians prescribing lower doses of GDMT during follow-up.36 We did however not observe an association with loop diuretic use and doses which may have been expected in patients with more congestion, where higher doses of loop diuretics have previously additionally been suggested to impair the ability to up-titrate GDMT.37
Rapid up-titration, renal function and outcomes
The decrease in renal function was not significantly associated with the combined outcome of HF readmission and all-cause mortality; however, we did observe a borderline significantly increased risk of HF readmission and numerically more clinical events, as well as more (serious) adverse events in patients with an early decrease in eGFR. We found no significant association between a decrease in eGFR and the risk of 180-day all-cause death. As data on renal function early during follow-up were only available in the HIC arm of the STRONG-HF trial, we could however be underpowered to detect significant effects on outcome.
Nevertheless, the positive effect on outcomes of rapid up-titration was independent of baseline renal function, and the observed increased risk associated with a decline in renal function could also have been a consequence of sicker patients, i.e. with more congestion, with an associated higher event rate. Importantly, in contrast to adequate treatment with GDMT, decongestion in HF has not been shown to improve outcome, and therefore up-titration of GDMT should be a priority. Taken together these results may suggest that patients who have a small decline in eGFR during rapid up-titration of GDMT are less likely to receive full doses of GDMT due to care provider hesitation. At the same time these changes seem to be associated with more congestion and numerically more HF readmissions. These data call to question if care provider hesitation is justified, and if continued up-titration of GDMT should be encouraged despite a drop in eGFR.
Limitations
There are several inherent limitations to these analyses, in addition to those already mentioned in the overall STRONG-HF study. Given that subgroup analyses are performed, statistical power might be limited as the study was not specifically powered for these analyses. We were only able to study early changes in renal function in the HIC group, as renal function was not assessed at these time points in the usual care group. Therefore, the association with outcomes and comparison with a decrease in eGFR in the usual care group could not be investigated. Per protocol, assessment of renal function was included in the evaluation to determine possibility for up-titration and an eGFR <30 ml/min/1.73 m2 was considered a contraindication for up-titration of renin–angiotensin–aldosterone system inhibitors, possibly inducing bias. SGLT2i were not included in the treatment protocol of the STRONG-HF study. Finally, we were only able to describe associations, and causality cannot be proven. Our analysis should be considered hypothesis generating, providing the first data on the association between changes in renal function, rapid up-titration of GDMT and outcomes.
Conclusions
In patients enrolled in the STRONG-HF study, a strategy of rapid up-titration of GDMT following a hospitalization for acute HF was effective in reducing HF rehospitalizations and mortality regardless of baseline renal function. In the context of rapid up-titration of GDMT, an early decrease in eGFR was associated with less improvement in congestion and NT-proBNP decrease during follow-up. Additionally, an early decrease in eGFR was associated with lower doses of GDMT during follow-up. These findings suggest that an early decrease in eGFR in the context of rapid up-titration of GDMT should be evaluated carefully especially with respect to the congestion status of the patient, yet should not necessarily lead to discontinuation of GDMT.
Funding
Funding for the study was through a grant provided to the Heart Initiative by Roche Diagnostics International. The funder had no role in study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the manuscript for publication.
Conflict of interest: A.M. has received grants from Roche Diagnostics, Abbott Laboratories, 4TEEN4, and Windtree Therapeutics; honoraria for lectures from Roche Diagnostics, Bayer, and MSD; is a consultant for Corteria Pharmaceuticals, S-form Pharma, FIRE-1, Implicity, 4TEEN4, and Adrenomed; and is coinventor of a patent on combination therapy for patients having acute or persistent dyspnoea. B.D., C.E., M.B., M.N., K.T., G.C. are employees of Momentum Research, which has received grants for research from Abbott Laboratories, Amgen, Celyad, Cirius Therapeutics, Corteria Pharmaceuticals, Heart Initiative, Sanofi, Windtree Therapeutics, and XyloCor Therapeutics. B.D. and G.C. are directors of Heart Initiative, a non-profit organisation. M.Ad. has received speaker fees from Abbott Vascular and Medtronic. J.C. has received personal fees from Novartis, AstraZeneca, Boehringer Ingelheim, Roche Diagnostics, and Pfizer. A.C.S. has received honoraria for lectures or consultancy from AstraZeneca, Novartis, Vifor, Bayer, Merck, Sanofi, Abbott, and Boehringer Ingelheim. O.C. received grants from Servier. A.D. works for the Faculty of Medicine, Eduardo Mondlane University (Maputo, Mozambique), which received research grants from the Heart Initiative for their participation in this study. R.D. has received supporting fees for coordination of STRONG-HF trial activities. G.F. has received lecture fees or was a committee member for trials and registries sponsored by Bayer, Vifor, Boehringer Ingelheim, Medtronic, Servier and Amgen. C.S.P.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Bayer and Roche Diagnostics; has served as consultant or on the Advisory Board/ Steering Committee/Executive Committee for Actelion, Alleviant Medical, Allysta Pharma, Amgen, AnaCardio AB, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Darma Inc., EchoNous Inc, Eli Lilly, Impulse Dynamics, Intellia Therapeutics, Ionis Pharmaceutical, Janssen Research & Development LLC, Medscape/WebMD Global LLC, Merck, Novartis, Novo Nordisk, Prosciento Inc, Radcliffe Group Ltd., Redcardio Inc, ReCor Medical, Roche Diagnostics, Sanofi, Siemens Healthcare Diagnostics and Us2.ai; and serves as co-founder & non-executive director of Us2.ai. MP has received personal fees from Abbott Laboratories, AstraZeneca, Boehringer Ingelheim and Vifor Pharma. P.S.P. has received grants or research contracts from American Heart Association, Roche, Siemens, Ortho Diagnostics, Abbott, Beckman Coulter, and Siemens; consulting fees from Roche; honoraria from WebMD; and he has financial interest in The Heart Course. K.S. has received grants from Medtronic, Servier, and Amylam and honoraria from MSD, Novartis, and Sanofi. A.A.V. has received consultancy fees or research support from AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Cytokinetics, Myocardia, Merck, Novartis, Novo Nordisk, and Roche Diagnostics. All other authors have nothing to disclose.




