Beyond loop diuretics: Unlocking the potential of co-diuretics in heart failure management
The opinions expressed in this article are not necessarily those of the Editors of the European Journal of Heart Failure or of the European Society of Cardiology. doi: 10.1002/ejhf.2852
This article refers to ‘The blunted loop diuretic response in acute heart failure is driven by reduced tubular responsiveness rather than insufficient tubular delivery. The role of furosemide urine excretion on diuretic and natriuretic response in acute heart failure’ by J. Biegus et al., published in this issue on pages 1323–1333.
Congestion, characterized by the accumulation of salt and water, lies at the heart of heart failure (HF), driving debilitating symptoms such as dyspnoea and hospitalization. Loop diuretics have long been the primary therapeutic agents for addressing fluid overload, with dialysis as the only alternative for congestion removal. However, the optimal management of patients on loop diuretics remains a matter of debate. Despite numerous randomized trials conducted over the past three decades, no significant differences have been observed when comparing different loop diuretic strategies. One prominent example is the DOSE trial,1 which found no substantial difference between repeated intravenous bolus and continuous infusions of furosemide. Recently, new approaches, including subcutaneous (SC) injections, have emerged for loop diuretic administration.
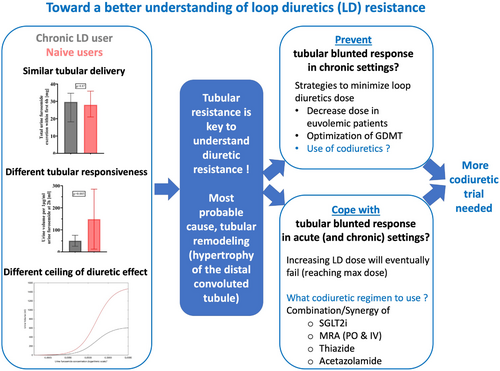
In this issue of the Journal, Biegus and colleagues present a mechanistic study that adds valuable insights to our understanding of loop diuretics' effects.2 The study included 50 patients admitted within 36 h of acute HF, comprising 28 furosemide-naïve individuals and 22 chronic furosemide users. The authors meticulously examined the association between previous furosemide use, diuretic response, and urine furosemide concentrations. Through this well-phenotyped cohort, the researchers standardized diuretic doses based on body weight and made notable observations. They found that chronic furosemide users exhibited urine furosemide excretion that was 30% lower compared to naïve patients (16.51 vs. 11.81 mg/6 h, p = 0.01). However, when accounting for estimated glomerular filtration rate (eGFR), a major determinant of excretion, furosemide excretion was similar across the groups (28.02 vs. 29.70 mg/6 h, p = 0.87). In contrast, the tubular response to administered loop diuretics was significantly higher in naïve patients compared to chronic users. Naïve patients exhibited three times higher urine volume per 1 μg/ml of urine furosemide at 2 h (148.6 ± 136.1 ml vs. 50.6 ± 56.1 ml, p = 0.005).
These findings significantly contribute to our understanding of the effects of loop diuretics. In patients with HF, particularly in the presence of significant congestion, the prevailing belief has been that insufficient tubular delivery hampers the effectiveness of loop diuretics due to absorption issues and reduced renal excretion. Decreased renal blood flow, impacting tubular delivery, has been suggested as a contributing factor.3 Moreover, the lower number or efficacy of furosemide transporters, such as organic anion transporter-1 or 2, in chronic loop diuretic users may further limit tubular delivery. The common approach to address significant congestion involves switching to high-dose intravenous or SC infusions of furosemide to increase tubular concentration. However, the study by Biegus and colleagues challenges this strategy, revealing that it is the reduced tubular responsiveness to loop diuretics, rather than inadequate tubular delivery, that plays a significant role. Dose–response curves reported by the authors clearly demonstrate that increasing loop diuretic doses reach a plateau in terms of urine volume and sodium excretion, indicating that higher doses cannot compensate for the diminished responsiveness. Moreover, high-dose loop diuretics pose the risk of direct adverse effects, such as ototoxicity, which are concentration-dependent. Thus, relying solely on increased furosemide doses cannot resolve the issue.
The concept of blunted tubular response to loop diuretics and tubular remodelling
Biegus and colleagues' study breathes new life into the pathophysiological studies described almost four decades ago by Kaissling et al.4 In their seminal paper, Kaissling et al. revealed significant hypertrophy of the distal convoluted tubule, resulting in tubular remodelling that impairs the response to loop diuretics by affecting Na+ tubular transporters. This morphological alteration forms the basis for the lack of diuretic efficacy observed in patients chronically treated with loop diuretics.
Can tubular remodelling be prevented?
Minimizing the long-term dose of diuretics appears to be a logical approach to mitigate tubular remodelling. This approach holds several practical advantages, particularly when encountering challenges associated with declining renal function5 and low blood pressure,6 and when initiating HF drugs.7 However, the actual impact of long-term diuretic minimization has yet to be evaluated. The effectiveness of such an approach should be prospectively tested in clinical trials.
Co-diuretics as the key to success?
Given that blunted responsiveness to loop diuretics appears to be the primary issue, it is imperative to explore strategies that enhance responsiveness. While minimizing chronic loop diuretic use serves as a preventive measure, when confronted with a patient experiencing acute HF and unresponsiveness to loop diuretics, the most pragmatic approach may involve employing co-diuretics. Co-diuretics, which possess diuretic effects distinct from loop diuretics but can enhance overall diuretic response, have traditionally been regarded as last-resort options. Promising candidates include high-dose mineralocorticoid receptor antagonists (MRA), thiazides, and more recently, acetazolamide. Limited evidence suggests the diuretic effects of MRAs. In an open-label trial, spironolactone increased natriuresis in patients hospitalized for acute HF.8 Some indications of efficacy have been observed with intravenous forms of MRAs, such as canrenoate potassium, in loop diuretic-refractory patients.9 Additionally, the safety profile of relatively high-dose intravenous MRAs appears favourable.10 Regarding thiazide use, the CLOROTIC trials recently demonstrated that hydrochlorothiazide led to significant weight loss, diuresis, and weight reduction compared to placebo at 72 and 96 h. However, hydrochlorothiazide was associated with a higher incidence of impaired renal function, while rates of hypokalaemia were similar between groups.11 Finally, the ADVOR trial revealed that acetazolamide exhibited superior efficacy in achieving successful decongestion compared to placebo, maintaining excellent safety with comparable rates of adverse events, worsening kidney function, hypokalaemia, and hypotension between the groups.12 Although evidence exists for the acute efficacy of co-diuretics, their use in chronic settings remains largely unexplored. It remains unknown whether utilizing co-diuretics to reduce loop diuretic doses may result in less long-term tubular remodelling and preserved tubular response.
Advancing our understanding
- The synergistic effects and safety of combined thiazide/acetazolamide use in acute settings.
- The synergistic effects and safety of thiazides or acetazolamide in patients concurrently treated with sodium–glucose cotransporter 2 inhibitors in acute settings.
- The role of acetazolamide and thiazides in chronic/ambulatory settings.
- The role of canrenoate in acute settings.
- The impact of long-term co-diuretic use to reduce loop diuretic doses on long-term loop diuretic responsiveness.
Addressing these numerous questions could serve as the foundation for equally numerous clinical trials (Figure 1).
Another approach to advancing our understanding may lie in field experience. The Heart Failure Association (HFA) has recently advocated for a more systematic approach to quantifying natriuresis.13 The use of natriuresis measurements is likely to enhance our comprehension of diuretic response, particularly when diuresis assessment alone is challenging. This approach could provide valuable field and clinical experience to address some of the aforementioned questions. However, despite its endorsement by the HFA, systematic implementation of this approach remains limited.
The future promises exciting discoveries as many aspects of diuretic therapy in HF remain to be unraveled. Let us hope that within the next few years, we will decipher the optimal utilization of diuretics, the age-old and fundamental treatment for HF.
Conflict of interest: N.G. reports honoraria from AstraZeneca, Bayer, Boehringer, Lilly, Novartis.




